Overexpressing trehalase improves drought stress tolerance associated with enhanced stomatal sensitivity to abscisic acid.
Abstract
Introduction of microbial trehalose biosynthesis enzymes has been reported to enhance abiotic stress resistance in plants but also resulted in undesirable traits. Here, we present an approach for engineering drought stress tolerance by modifying the endogenous trehalase activity in Arabidopsis (Arabidopsis thaliana). AtTRE1 encodes the Arabidopsis trehalase, the only enzyme known in this species to specifically hydrolyze trehalose into glucose. AtTRE1-overexpressing and Attre1 mutant lines were constructed and tested for their performance in drought stress assays. AtTRE1-overexpressing plants had decreased trehalose levels and recovered better after drought stress, whereas Attre1 mutants had elevated trehalose contents and exhibited a drought-susceptible phenotype. Leaf detachment assays showed that Attre1 mutants lose water faster than wild-type plants, whereas AtTRE1-overexpressing plants have a better water-retaining capacity. In vitro studies revealed that abscisic acid-mediated closure of stomata is impaired in Attre1 lines, whereas the AtTRE1 overexpressors are more sensitive toward abscisic acid-dependent stomatal closure. This observation is further supported by the altered leaf temperatures seen in trehalase-modified plantlets during in vivo drought stress studies. Our results show that overexpression of plant trehalase improves drought stress tolerance in Arabidopsis and that trehalase plays a role in the regulation of stomatal closure in the plant drought stress response.
Trehalose is a nonreducing sugar linking two Glc units in an α,α-1,1 configuration and is implicated in osmoregulation and stress protection in many bacteria and fungi. The biosynthesis of trehalose in plants involves two consecutive enzymatic reactions mediated by trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphate phosphatase (TPP), with trehalose-6-phosphate (T6P) as an intermediate compound (Cabib and Leloir, 1958). The synthesized trehalose can be hydrolyzed into two Glc monomers by the enzyme trehalase. Most genomes of higher plants contain elaborate trehalose biosynthesis gene families (Lunn, 2007; Avonce et al., 2010). The genome of Arabidopsis (Arabidopsis thaliana) contains 11 TPS genes (AtTPS1–AtTPS11) and 10 TPP genes (AtTPPA–AtTPPJ; Leyman et al., 2001). The Arabidopsis TPS proteins carry a TPS- and a TPP-like domain and are divided into two classes based on their homology with the yeast (Saccharomyces cerevisiae) TPS (ScTps1) and TPP (ScTps2) proteins. Class I displays the highest similarity to ScTps1 and includes four proteins (AtTPS1–AtTPS4), but only AtTPS1 has demonstrated TPS activity (Blázquez et al., 1998; Van Dijck et al., 2002; Vandesteene et al., 2010). The class II proteins (AtTPS5–AtTPS11) are most similar to ScTps2, but they do not show any detectable TPS or TPP activity upon expression in yeast (Vogel et al., 2001; Ramon et al., 2009). All TPP proteins (AtTPPA–AtTPPJ) contain specific phosphatase boxes (Thaller et al., 1998) but do not share homology to class I and II TPS proteins. Expression studies in yeast revealed that they all have TPP activity, and promoter-GUS-GFP lines showed that each one has a tissue-specific expression pattern in Arabidopsis (Vandesteene et al., 2012). In contrast to the large TPS and TPP gene families in Arabidopsis, trehalase is encoded by a single gene, AtTRE1 (Müller et al., 2001a; Lunn, 2007). Among the vascular plants, only a few desiccation-tolerant resurrection plants, such as Selaginella lepidophylla and Myrothamnus flebellifolius, accumulate substantial amounts of trehalose (Zentella et al., 1999). In most other higher plants, only trace amounts of trehalose are detected, barely exceeding 0.15 mg g−1 dry weight, which implies that there is insufficient trehalose for it to act as a compatible solute during environmental stress (Vogel et al., 2001; Fernandez et al., 2010).
The intermediate of trehalose biosynthesis, T6P, has emerged as an essential sugar-signaling metabolite that regulates plant metabolism and influences many aspects of plant growth and development (Schluepmann et al., 2004; Lunn et al., 2006). T6P promotes biosynthetic processes in growing tissues in response to high Suc (Delatte et al., 2011b) and induces starch synthesis in the plastids via thioredoxin-mediated activation of AGPase (Kolbe et al., 2005). In developing tissues of Arabidopsis, T6P inhibits the activity of the SNF1-related kinase1 (SnRK1; Zhang et al., 2009), which is known to repress plant growth and to promote survival under stress (Baena-González et al., 2007). T6P, SnRK1, and the sugar-regulated transcription factor basic region leucine zipper transcription factor11 (bZIP11) have been postulated to be part of a regulatory loop, controlling Suc availability and utilization in plants (Delatte et al., 2011b). Furthermore, a strong correlation between T6P levels and abscisic acid (ABA) sensitivity has recently been proposed (Vandesteene et al., 2012).
The Arabidopsis trehalase, AtTRE1, can functionally replace the acid trehalase of yeast (ScAth1) when expressed in the yeast ath1Δ strain (Müller et al., 2001a; Frison et al., 2007). Based on in silico analysis, AtTRE1 is predicted to be localized to the extracellular space, mitochondria, or plastids (http://suba.plantenergy.uwa.edu.au/; Heazlewood et al., 2007). The protein is known to contain potential N-glycosylation sites, indicating that the enzyme might be secreted (Frison et al., 2007). GFP studies confirmed that AtTRE1 is an apoplastic enzyme anchored to the cell membrane through a putative N-terminal transmembrane span (residues 46–63; Frison et al., 2007). This finding suggests that endogenous trehalose has to be transported out of the cell in order to be degraded. Trehalase expression and activity are higher in flowers, green siliques, and developing seeds than in other parts of the plant, indicating a role for trehalase in flower development (Müller et al., 2001a). Transcriptional profiles and microarray data sets show that AtTRE1 expression is increased in vegetative tissues during senescence and is induced by auxin, cytokinin, sugar availability, and abiotic stresses such as drought (Müller et al., 2001b; Zimmermann et al., 2004; Brenner et al., 2005; Rolland et al., 2006). An important role for trehalase has been proposed during plant-microbe interactions in plants hosting trehalose-producing microorganisms, such as arbuscular mycorrhizal fungi, rhizobia, and the clubroot disease-inducing pathogen Plasmodiophora brassicae (Müller et al., 1994; Schubert and Wyss, 1995; Brodmann et al., 2002). Recently, an Attre1 knockout line was characterized (SALK_147073C; Alonso et al., 2003) and reported to have a 2-fold increase in T6P levels when grown on sorbitol (Delatte et al., 2011a).
Many attempts have been made to enhance the stress tolerance of model plants and crops by introducing TPS and TPP genes of yeast or bacterial origin. In general, the stress tolerance of these plants increased, although trehalose levels remained low. In most cases, the plants exhibited aberrant phenotypes such as stunted roots, lancet-shaped leaves, and growth retardation (Romero et al., 1997; Cortina and Culianez-Macia, 2005). The undesired abnormalities in these plants were ascribed to altered levels of T6P, perturbing the developmental processes influenced by this important signal metabolite (Eastmond et al., 2003; Schluepmann et al., 2003, 2004). Plants with improved stress tolerance but no obvious morphological defects were obtained by introducing bifunctional TPS-TPP constructs (Garg et al., 2002; Karim et al., 2007; Miranda et al., 2007), by placing the introduced TPS genes under the control of drought-inducible or tissue-specific promoters (Garg et al., 2002; Karim et al., 2007), or by overexpressing the plant endogenous TPS1 in Arabidopsis (Avonce et al., 2004) and rice (Oryza sativa; Li et al., 2011). It seems unlikely that the increased drought tolerance in these transgenic lines could be ascribed to trehalose acting as an osmoprotectant, since the trehalose levels never exceeded 1 mg g−1 fresh weight (Garg et al., 2002; Avonce et al., 2004; Cortina and Culianez-Macia, 2005). The improved tolerance seemed instead to correlate with a higher soluble carbohydrate content and an increased photosynthetic capacity (Garg et al., 2002).
The activity of the endogenous trehalase is readily detectable in plants, and treatment with validamycin A, a trehalose analog and competitive inhibitor of trehalase, led to a 20-fold increase in trehalose in leaves of Arabidopsis plants grown on 10 mm trehalose (Müller et al., 2001a). Together, these observations indicate that the endogenous trehalase activity is high enough to restrict the accumulation of trehalose, even in transgenic plants engineered to overproduce this sugar, and that genetic down-regulation of trehalase could be a good strategy for increasing the trehalose content (Vinocur and Altman, 2005). In this work, we present a novel approach for engineering drought tolerance in Arabidopsis plants by manipulating the expression and activity of the endogenous trehalase AtTRE1.
RESULTS
Isolation and Characterization of Arabidopsis Plants with Altered AtTRE1 Expression
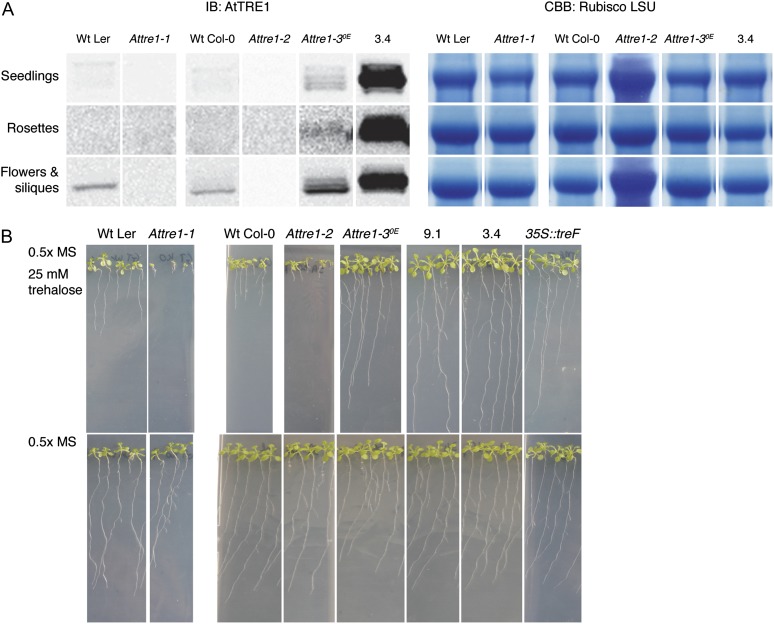
The AtTRE1 gene, encoding the only specific trehalase in Arabidopsis, was overexpressed in Arabidopsis plants of the Columbia-0 (Col-0) accession, and multiple homozygous 35S::AtTRE1 lines (2.3, 3.4, 4.1, 5.2, 6.5, 9.1, and 11.1) were obtained. Seedlings of the 9.1 and 3.4 lines show 292- and 139-fold increases in AtTRE1 expression, respectively, and were selected for further experiments based on their high trehalase transcript levels (Table I). A detailed expression study in rosette leaves of the 3.4 line revealed 52- to 178-fold increases in AtTRE1 transcripts (Supplemental Table S1). Lines from publicly available collections with transfer DNA (T-DNA) or transposon insertions in the AtTRE1 gene were screened to obtain plants that were homozygous for the insertion (Supplemental Fig. S1). One knockout line was found with no AtTRE1 transcripts detectable by quantitative reverse transcription-PCR (Table I): Attre1-1 (transposon insertion line GT_16843 from the Genetrap collection in the Landsberg erecta [Ler] accession; Sundaresan et al., 1995). The T-DNA insertion line Attre1-2 (SALK_147073C from the SALK collection in the Col-0 accession; Alonso et al., 2003) seemed to be a knockdown, since AtTRE1 transcription was decreased by a factor of 6 in seedlings (Table I) and by a factor of 7 to 14 in young rosette leaves, while it was hardly detectable in the oldest leaves (Supplemental Table S1). The Attre1-3OE line (SALK_151791 in Col-0) contains an insertion 304 bp upstream of the transcription start site and was unexpectedly found to be an overexpressing line, with AtTRE1 transcript levels that were 8 times increased in seedlings (Table I) and 3 to 11 times increased in the rosettes (Supplemental Table S1) compared with the wild type. The AtTRE1 (approximately 64-kD) protein was weakly detectable in wild-type Ler and Col-0 seedlings by immunoblotting with an anti-AtTRE1 antibody (Fig. 1A). At the same protein loading, a much stronger signal was observed in extracts from wild-type flowers and siliques, but no AtTRE1 protein was detected in wild-type rosettes (Fig. 1A). The absence of the AtTRE1 protein in wild-type rosettes is in conflict with previous immunoblots showing a faint signal of AtTRE1 in rosette leaves of bolted wild-type plants (Frison et al., 2007). The antibody used in that work was raised against two peptides of the AtTRE1 protein (residues 93–107 and 377–392), while our antibody was raised against one peptide (residues 94–107). It might thus be possible that our antibody, which recognizes a single epitope of AtTRE1 in close proximity to the transmembrane span, is less efficient in reaching its target than the antibody used by Frison et al. (2007).
Table I. Characterization of the trehalase-modified lines.
Relative AtTRE1 expression, trehalase-specific activity, trehalose, and T6P in seedlings and rosettes of the trehalase-modified lines are significantly different from the wild type (Ler and Col-0) as indicated: *P < 0.075, **P < 0.05, ***P < 0.01, ****P < 0.001, *****P < 0.0001 (Student’s t test). Values shown are means ± sd (n = 3; each replicate represents a pool of at least 12 seedlings or 10 rosettes). NA, Not applicable; ND, not determined.
| Genotype (Tissue) |
Relative AtTRE1 Expression (Seedlings) |
Trehalase Activity |
Trehalose (Seedlings) |
T6P (Seedlings) |
|
|---|---|---|---|---|---|
| Seedlings |
Rosettes |
||||
| nkat mg−1 protein | nmol g−1 fresh wt | ||||
| Wild-type Ler | 1.01 ± 0.2 | 0.13 ± 0 | 0.09 ± 0 | 26.4 ± 10.7 | 0.148 ± 0.03 |
| Attre1-1 | 0.00**** | 0.00**** | 0.00 ± 0** | 101.5 ± 14.5*** | 0.200 ± 0.03 |
| Wild-type Col-0 | 1.04 ± 0.4 | 0.10 ± 0 | 0.00 | 22.6 ± 3.8 | 0.143 ± 0.03 |
| Attre1-2 | 0.18 ± 0** | 0.00**** | ND | ND | ND |
| Attre1-3OE | 7.72 ± 1.0**** | 0.66 ± 0.1**** | 0.02 ± 0* | 9.3 ± 2.1*** | 0.100 ± 0.02 |
| 9.1 | 292.24 ± 12.2***** | 11.07 ± 1.1***** | 11.23 ± 3.4*** | 5.5 ± 2.6*** | 0.115 ± 0.01 |
| 3.4 | 138.67 ± 33.4*** | 12.12 ± 0.8***** | 10.71 ± 2.0*** | ND | ND |
| 35S::treF | NA | 23.09 ± 2.0***** | ND | ND | 0.094 ± 0 |
Figure 1.
Characterization of AtTRE1-modified lines. A, Immunoblot (IB) of AtTRE1 accumulation (approximately 64 kD) in protein extracts from seedlings, rosettes, flowers, and siliques and the corresponding Coomassie Brilliant Blue (CBB) stain showing the Rubisco large subunit as a constitutive control. B, Growth of trehalase mutants and wild-type (Wt) seedlings on 0.5× MS medium with and without 25 mm trehalose.
There was no detectable AtTRE1 protein in any of the tissues examined from the Attre1-1 knockout, while AtTRE1 protein accumulation in the Attre1-2 knockdown was limited to a hardly visible signal in the seedlings (Fig. 1A). The 35S::AtTRE1 lines 3.4 (Fig. 1A) and 9.1 (data not shown) had high levels of AtTRE1 protein in all three tissues, indicating constitutive overexpression throughout the plant. The Attre1-3OE line showed an AtTRE1 accumulation pattern that was qualitatively similar to wild-type plants but with considerably higher levels of the AtTRE1 protein in those tissues where it was expressed. This suggests that the spatiotemporal control of AtTRE1 expression in this line is still under the control of the endogenous promoter but that the level of expression is enhanced by the presence of the T-DNA insertion within the putative promoter region. A further point to note from the immunoblot analysis is the presence of multiple immunoreactive bands in the extracts of the AtTRE1-overexpressing lines (Fig. 1A).
Trehalase activity was higher in wild-type Ler seedlings than in wild-type Col-0 plantlets and was decreased by 31% in wild-type Ler rosettes, while staying below the background of the assay in rosette leaves of the wild-type Col-0 (Table I). No trehalase activity was detected in Attre1-1 and Attre1-2 mutants (Table I). Trehalase activity in the Attre1-3OE line was 6.5-fold increased in seedlings and detectable in rosettes, albeit at low levels (Table I). Seedlings of the 35S::AtTRE1 lines displayed 109- to 119-fold higher trehalase activities, and this magnitude of activity was also seen in the rosettes (Table I). Plants engineered to constitutively express the Escherichia coli cytosolic trehalase (35S::treF) had 227-fold higher trehalase activity than wild-type Col-0 (Table I).
Growth of the transgenic and wild-type seedlings was evaluated on one-half-strength Murashige and Skoog (0.5× MS) medium in the presence or absence of 25 mm trehalose. In the presence of this concentration of trehalose, the wild-type seedlings had stunted root growth and failed to develop rosette leaves (Fig. 1B), confirming the previous observations of Ramon et al. (2007). An even more pronounced growth inhibition was observed in Attre1-1 and Attre1-2 seedlings, while all of the AtTRE1 overexpressors were highly tolerant to the externally supplied trehalose. The roots and rosette leaves of the 35S::AtTRE1 lines 9.1 and 3.4 were noticeably larger than those of the Attre1-3OE seedlings, suggesting a positive correlation between enhanced growth on trehalose-containing medium and trehalase activity. However, the 35S::treF line, having the highest trehalase activity of all the lines tested, grew less well than the 35S::AtTRE1 seedlings. The apoplastic AtTRE1 enzyme presumably has direct access to the external trehalose in the medium and so will readily hydrolyze it to Glc. With the extracellular hydrolysis of trehalose, plants might also avoid taking up trehalose into the cells, where it has the potential to perturb their metabolism and signaling processes. In contrast, the cytosolic treF trehalase will only be able to hydrolyze trehalose that has been taken up into the cell. The intracellular trehalose can still perturb trehalose-sensitive processes, potentially counteracting the positive effects of the increased Glc supply.
Trehalose levels were determined in the different mutants. The Attre1-1 seedlings accumulated up to 101.5 nmol trehalose g−1 fresh weight, which was almost four times more than the levels found in wild-type Ler (26.4 nmol g−1 fresh weight; Table I). Trehalose contents in seedlings of the strong 35S::AtTRE1 line 9.1 and Attre1-3OE ranged from 5.5 to 9.3 nmol trehalose g−1 fresh weight and were both significantly lower than the 22.6 nmol trehalose g−1 fresh weight observed in wild-type Col-0. The Attre1-1 seedlings accumulated 0.20 nmol T6P g−1 fresh weight, which was somewhat higher than the 0.15 nmol T6P g−1 fresh weight seen in wild-type Ler (Table I). The trehalase-overexpressing lines showed a tendency to have slightly lower levels of T6P than wild-type Col-0 seedlings, but the differences were not statistically significant.
AtTRE1-Overexpressing Lines Are More Tolerant to Severe Drought Stress
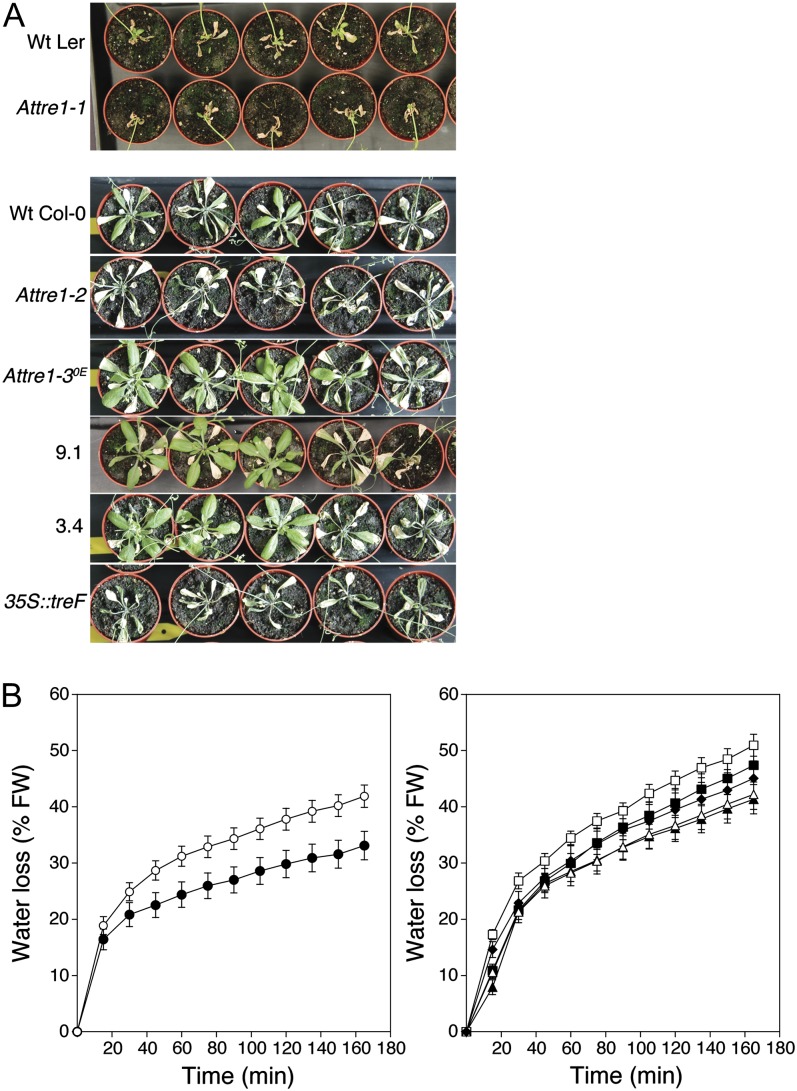
To test the effect of AtTRE1 on drought stress tolerance, our transgenic lines were tested in a severe drought stress experiment on soil-grown plants, as described in “Materials and Methods.” When the plants were 2 weeks old, water was gradually restricted in the same manner for all plants over a period of 20 d, and then plant were subsequently rewatered (Supplemental Fig. S2). After a period of 48 h, photographs and fresh weights were taken to examine the recovery of the plants, which is the ability to survive and rehydrate upon rewatering. Dry weights were assayed to study the growth performance of the plants during the drought stress period. As previously shown in the work of Meyre et al. (2001), we observed a difference in drought stress tolerance between the accessions Ler and Col-0. Col-0 plants acclimate to drought by increasing their root-to-shoot ratio and increasing their water use efficiency, while Ler plants exhibit more of a drought-escape strategy by flowering early and reallocating nutrients from the rosette to the flowers, which leads to a rapid yellowing of rosette leaves.
In the Col-0 background, two wild-type plants could survive out of five individuals, while four of the Attre1-3OE plants and three of the 35S::AtTRE1-overexpressing plants survived out of five (Fig. 2A; Table II). The 35S::treF and Attre1-2 plants were hypersensitive toward drought stress, since none of the individuals survived the drought treatment. The aboveground organs (rosette and inflorescence) of the surviving AtTRE1 overexpressors had almost twice the fresh weight of the surviving wild-type Col-0 control plants (Table II), which reflects the enhanced ability of the AtTRE1 overexpressors to recover after drought stress. Rosette dry weights of the Attre1-2 mutant were decreased, while inflorescence dry weights were increased, suggesting that this mutant invested more energy in flowering than wild-type Col-0 plants (Table II). No differences in dry weights were observed between the wild-type Col-0 and the AtTRE1 overexpressors Attre1-3OE and 3.4. The rosette dry weights of the overexpressor 9.1 were higher than the wild-type Col-0, indicating that this 35S::AtTRE1 line has an increased capacity to grow during dry conditions. Interestingly, the phenotypes in rosette growth of the Attre1-2 mutant and the AtTRE1 overexpressors are reversed under well-watered conditions (data not shown). The 35S::treF line grew worse than the wild-type Col-0 during drought (Table II) and nonstressed conditions (data not shown).
Figure 2.
A, Trehalase-modified plants after 20 d of water restriction, followed by a recovery period of 48 h upon rewatering. Five individual plants of each transgenic line were ordered from left to right according to their decreasing ability to survive upon drought stress. Wt, Wild type. B, Kinetics of water loss from detached leaves. Left panel, wild-type Ler (black circles) and Attre1-1 (white circles); right panel, wild-type Col-0 (black squares), Attre1-2 (white squares), Attre1-3OE (black triangles), 9.1 (white triangles), and 35S::treF (black diamonds). Water loss is expressed as the percentage of initial fresh weight (FW). Regression analysis (Student’s t test) showed significant differences between the wild-type Ler curve and the Attre1-1 curve (P < 0.0001) and between the wild-type Col-0 curve and the Attre1-2, Attre1-3OE, 9.1, and 35S::treF curves (P < 0.075). Values represent averages ± se (n = 3 batches containing three leaves each).
Table II. Characteristics of trehalase-modified plants after 20 d of water restriction, followed by a recovery period of 48 h upon rewatering.
The number of surviving plants out of five individuals, fresh weights of the surviving plants, dry weights of all the plants, and the number of newly appeared leaves in Ler plants are listed. Values are averages ± sd (n = 2–5). Significant differences are as indicated: *P < 0.075, **P < 0.05, ***P < 0.01 (Student’s t test; n = 5). NA, Not applicable (see text); ND, not determined.
| Genotype | Surviving Plants | Rosette Fresh Weight | Rosette Dry Weight | Flower Stem Fresh Weight | Flower Stem Dry Weight | Newly Appeared Leaves |
|---|---|---|---|---|---|---|
| g | ||||||
| Wild-type Ler | 5 | NA | 0.016 ± 0 | 0.110 ± 0.01 | ND | 4.2 ± 1.1 |
| Attre1-1 | 5 | NA | 0.019 ± 0.01 | 0.136 ± 0.04 | ND | 1.6 ± 0.5*** |
| Wild-type Col-0 | 2 | 0.110 ± 0.01 | 0.055 ± 0.01 | 0,030 ± 0.01 | 0.033 ± 0.01 | |
| Attre1-2 | 0 | NA | 0.044 ± 0.01* | NA | 0.049 ± 0.01*** | |
| Attre1-3OE | 4 | 0.183 ± 0.07 | 0.050 ± 0.01 | 0.055 ± 0.03 | 0.038 ± 0.01 | |
| 9.1 | 3 | 0.193 ± 0.04 | 0.077 ± 0.01** | 0.083 ± 0.01 | 0.027 ± 0.01 | |
| 3.4 | 3 | 0.211 ± 0.01 | 0.051 ± 0.02 | 0.093 ± 0.06 | 0.031 ± 0.02 | |
| 35S::treF | 0 | NA | 0.038 ± 0.01*** | NA | 0.029 ± 0.01 | |
All plants of the Ler accession survived the drought stress treatment; rosette leaves were mostly senescent, while the inflorescences were fully developed (Fig. 2A). Dry weights of the rosettes (and flower stem) were slightly increased in the Attre1-1 mutant compared with wild-type Ler, although not at a significant level (Table II). This tendency is more pronounced under well-watered conditions (data not shown). Attre1-1 plants formed significantly fewer leaves during the recovery period, most of which were cauline leaves on secondary inflorescences (Table II). Interestingly, the Attre1-1 line displayed decreased survival rates compared with wild-type Ler when slowing down the drying rate of the soil (data not shown).
Trehalase Is Strongly Expressed in the Stomata
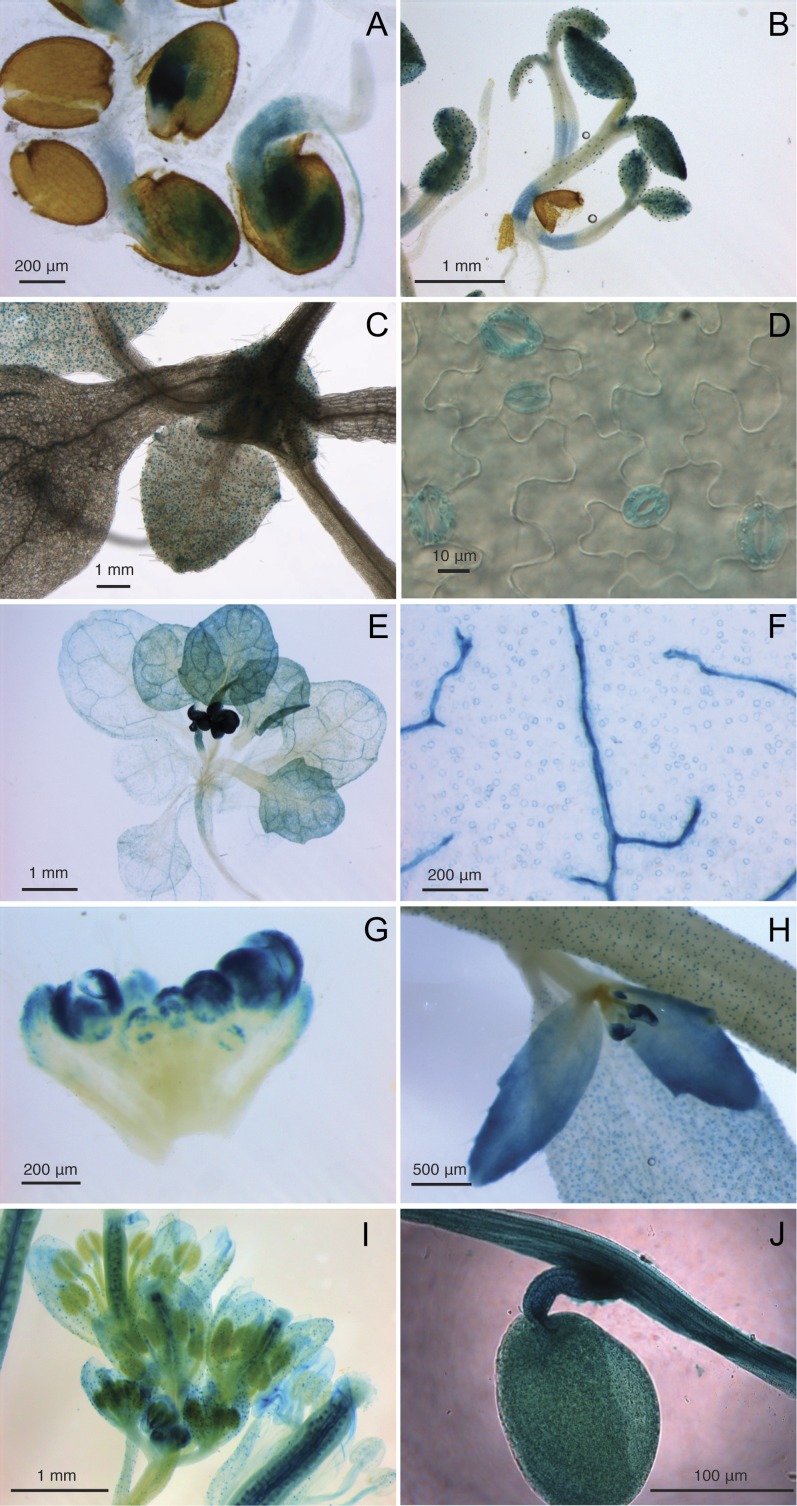
To investigate the spatiotemporal expression patterns of the AtTRE1 gene, promoter TRE1::GUS-GFP lines were constructed and subjected to GUS staining assays. During germination, GUS expression was mainly detected in the radicle at the early stages of testa rupture and root protrusion but shifted toward the hypocotyl and cotyledons with the onset of hypocotyl stretching (Fig. 3A). In 5-d-old seedlings, GUS staining was seen in the hypocotyl and shoot apical meristem and was very concentrated in the cotyledons, particularly in the stomatal guard cells (Fig. 3B). Very remarkable here was the sharp boundary between the basal part of the hypocotyl, with a diffuse GUS signal, and the apical part of the hypocotyl, with GUS expression limited to stomata. This observation might be explained by the fact that, at close proximity to the root, stomatal complexes are organized in a pattern reminiscent of the root epidermis pattern, which is clearly different from the epidermally specialized stomatal structures in the upper part of the plant (Berger et al., 1998). By the time the seedlings reached 10 d old, no GUS activity was apparent in the hypocotyl or cotyledons, but GUS remained strongly expressed in the stomata and hydathodes of young rosette leaves (Fig. 3, C and D). GUS expression in the rosettes of bolted plants was limited to the veins and stomatal guard cells in older leaves, while younger leaves displayed a stronger and more diffuse GUS signal that was similar to the one in older leaves (Fig. 3, E and F). Intense GUS staining was observed in flower buds at the onset of the generative stage (Fig. 3, E and G) and later in cauline leaves, in most parts of the flowering organ, especially the sepals, carpels, filaments, and in the stomata throughout the inflorescence and flower stem (Fig. 3, H and I). The abundant GUS signal in the carpels was due to GUS expression in the placenta and funiculus and to a lesser extent in the developing seeds (Fig. 3J).
Figure 3.
Histochemical localization of GUS activity in pTRE1::GUS-GFP lines during germination at the stages of root protrusion and hypocotyl stretching (A), in 5-d-old seedlings (B), in 10-d-old seedlings (C and D), in 5-week-old bolted plants (E), in the mature, second real leaf from a 5-week-old plant (F), in flower buds (G), in the flower stem, in cauline leaves, and in flower buds on secondary inflorescences (H), in the inflorescence (I), and in a developing seed at early maturation stage (J).
AtTRE1 Affects Transpiration and Stomatal Movements in Vitro
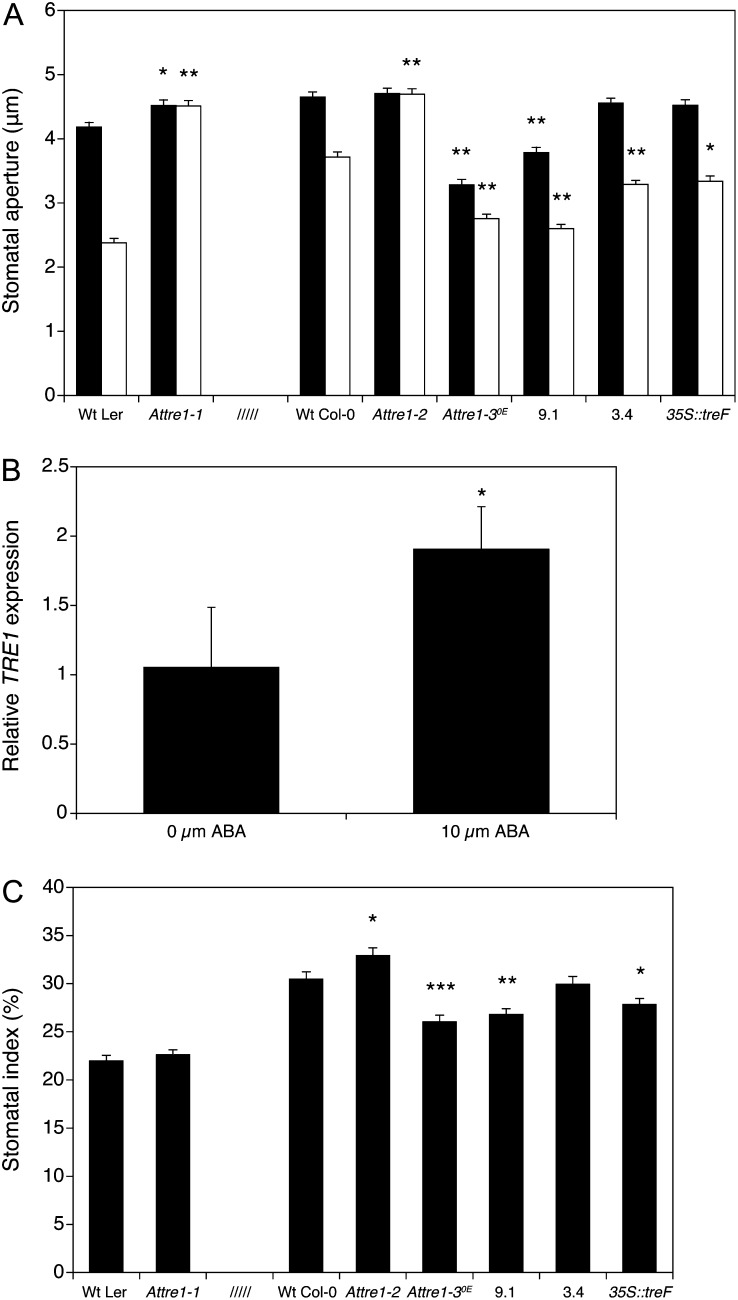
Given the specific AtTRE1 expression in stomatal guard cells, we investigated to what extent the AtTRE1-modified lines limit their transpirational water losses in response to water stress by excising the rosette leaves and measuring water loss over time. Water loss was significantly greater in the Attre1-1 and Attre1-2 mutants than in the corresponding wild-type Ler and Col-0 plants, whereas the AtTRE1-overexpressing lines Attre1-3OE and 9.1 showed lower water loss than the wild-type control (Fig. 2B). The 35S::treF line was indistinguishable from wild-type Col-0 up to about 90 min but had a slightly slower rate of water loss thereafter. These results support the idea that AtTRE1 regulates transpiration. ABA is a key regulator of stomatal closure, and several reports have found evidence of connections between trehalose metabolism and ABA signaling (Avonce et al., 2004; Gómez et al., 2010; Vandesteene et al., 2012). Therefore, we investigated whether the altered phenotype of the trehalase mutants under drought stress was correlated with any differences in stomatal responses upon treatment of detached leaves with 20 µm ABA in the light. In the absence of ABA, the Attre1-1 leaves had significantly larger stomatal apertures than wild-type Ler, but the Attre1-2 knockdown did not show any difference from the corresponding wild-type Col-0 controls (Fig. 4A). However, both the knockout and knockdown showed striking insensitivity to ABA, with no difference in stomatal aperture after 2 h of exposure to ABA, in marked contrast to the wild-type Ler and Col-0 plants, whose stomatal apertures were 43% and 20% lower, respectively, after ABA treatment. The Attre1-3OE and 35S::AtTRE1 9.1 overexpressing lines showed the opposite behavior to the knockout and knockdown, with stomatal apertures smaller than the wild type in the absence of ABA and decreasing even further than the wild type after ABA treatment. The other trehalase-overexpressing lines, 35S::AtTRE1 3.4 and 35S::treF, had similar stomatal apertures to wild-type Col-0 in the absence of ABA but showed more pronounced stomatal closure than the control plants after ABA treatment. These results suggest that increased trehalase activity enhances the sensitivity of the guard cells to exogenous ABA and in some cases restricts stomatal opening even in the absence of external ABA. Moreover, AtTRE1 appears to be essential for ABA-induced closure of guard cells, since both the Attre1-1 and Attre1-2 mutants were unable to close their stomata in response to ABA addition. Inhibition of seed germination is another classical response to ABA treatment. Interestingly, neither the AtTRE1 mutants nor the treF overexpressor seemed to interfere with the ABA responsiveness in seeds, since seed germination in the presence of ABA was similarly affected in both the transgenic and wild-type lines (data not shown).
Figure 4.
Leaf stomatal features. A, Stomatal pore sizes without ABA (black bars) and with 20 µm ABA (white bars). Stomatal apertures of the trehalase-modified lines within each treatment are significantly different from the wild type (Wt Ler and Wt Col-0) at P < 0.01 (*) and P < 0.0001 (**) by Student’s t test. Values represent averages ± se (n = 80). B, Relative AtTRE1 expression in wild-type seedlings treated for 60 min with 10 µm ABA is significantly increased compared with the solvent control (0 µm ABA) at P < 0.05 (*) by Student’s t test. Values represent averages ± sd (n = 3; each replicate represents a pool of at least 20 seedlings). C, Stomatal index of the trehalase-modified lines is significantly different from the wild-type index (Wt Ler and Wt Col-0) at P < 0.05 (*), P < 0.001 (**), and P < 0.0001 (***) by Student’s t test on data following the arcsine transformation. Values are means ± se (n = 60).
To investigate whether AtTRE1 expression is induced by ABA, wild-type Col-0 seedlings grown in axenic liquid cultures were incubated for 60 min in the presence of 10 µm ABA (diluted from a stock solution in methanol) or an equivalent amount of methanol as a solvent control. AtTRE1 expression was almost 2-fold higher in the ABA-treated seedlings than the controls (Fig. 4B), suggesting that AtTRE1 expression is both induced by ABA and modulates downstream responses to ABA.
Altering Trehalase Activity Affects the Stomatal Index and the Leaf Temperature of Water-Stressed Plants
Stomatal conductance is a major factor determining the rate of water loss via transpiration and is dependent on both the number of stomata and the extent to which they are opened. The stomatal index of the trehalase overexpressors was determined to assess whether changes in the numbers of stomata could account for their enhanced capacity to recover after drought stress. The Attre1-3OE, 35S::AtTRE1 9.1, and 35S::treF plants had a 9% to 15% lower stomatal index than the corresponding wild-type Col-0 plants (Fig. 4C) and favor a decreased water loss through the stomata upon drought stress. The Attre1-2 knockdown showed a small but significant increase of 7% in stomatal index compared with wild-type Col-0, whereas the Attre1-1 mutant had the same value as the wild-type Ler control. The reciprocal changes in stomatal index resulting from overexpression or loss of trehalase activity in the Col-0 background suggest that trehalose metabolism could influence epidermal cell division and differentiation into guard cells. This is supported by GUS staining observations using 3-week-old, plate-grown seedlings of the promoter TRE1::GUS-GFP lines, where AtTRE1 expression was clearly visible in developing stomatal guard cells (Supplemental Fig. S3).
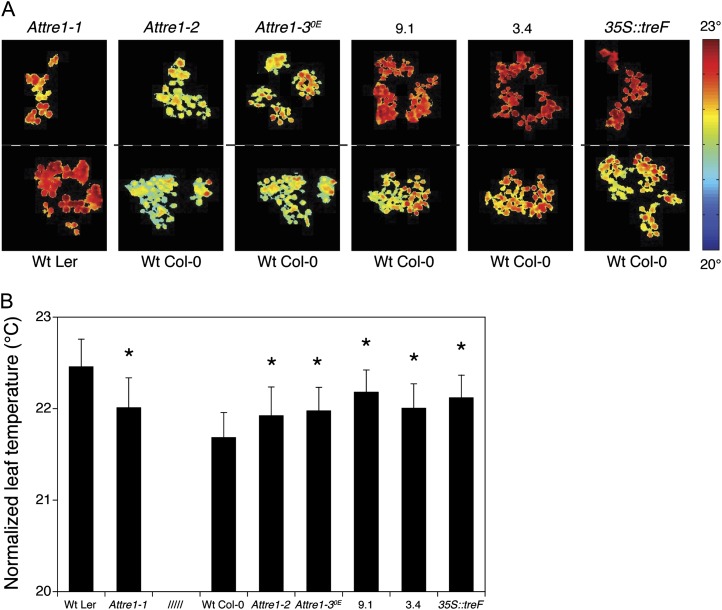
Thermal imaging is a powerful in vivo technique for detecting and analyzing mutants with altered rates of transpiration due to differences in stomatal density and/or stomatal aperture. Plants with lower stomatal conductance have lower rates of transpiration, leading to higher leaf temperatures, and vice versa for seedlings with a higher stomatal conductance. Seedlings of the various lines were grown under well-watered conditions for 8 d and then subjected to drought for 4 d before analyzing their leaf surface temperature by infrared imaging (Fig. 5A). Leaves of the Attre1-1 knockout mutant were 0.44°C colder than wild-type Ler (Fig. 5B), which is entirely consistent with the increased stomatal aperture (Fig. 4A) and greater water loss (Fig. 2B) observed in detached leaves from the mutant. In contrast, drought-stressed Attre1-2 seedlings displayed slightly higher leaf temperatures than the corresponding wild-type Col-0 plants. Assuming that the transpiration rate is dependent on the leaf size (von Caemmerer and Farquhar, 1981), the leaf area of the first pair of true leaves was measured in this mutant. The leaf area of the Attre1-2 line showed an increase of 25% compared with the wild-type Col-0 and could be held responsible for the lower transpiration rate in this mutant. Moreover, infrared assays with adult plants suggested that severe rather than mild drought stress might be necessary to overrule the effect of an increased growth rate in Attre1-2 plants, leading to a stomatal response similar to that of the Attre1-1 knockout (data not shown). All of the trehalase-overexpressing lines had higher leaf temperatures than wild-type Col-0, with the temperature difference ranging from 0.29°C to 0.50°C. This is consistent with the lower stomatal aperture and stomatal index observed in these plants (Fig. 4, A and C) as well as their increased ABA sensitivity, leading to lower rates of transpiration when the plants are drought stressed. Altogether, these data support the hypothesis that the trehalase plays a significant role in controlling stomatal conductance and that overexpression of either the endogenous trehalase (AtTRE1) or a heterologous form (E. coli treF) reduces water loss during drought stress in Arabidopsis plants.
Figure 5.
Transpiration of trehalase-modified seedlings restricted in water for 4 d. A, False-color infrared images of the drought-stressed plantlets. B, Normalized leaf temperatures of the transgenic seedlings as calculated from the quantification of three infrared images (approximately 3,000 square pixels) as shown in A are significantly different from wild-type (Wt) Ler and Col-0 at P < 0.0001 (*; Z score). Values are weighted means ± sd.
DISCUSSION
Overexpression of AtTRE1 Improves Drought Tolerance in Arabidopsis
Trehalose functions as a stress protectant and osmoprotectant in a wide variety of organisms (Elbein et al., 2003). Engineering trehalose metabolism in plants by introducing microbial TPS and TPP genes has been reported to improve plant survival and growth under abiotic stress conditions (Garg et al., 2002; Avonce et al., 2004; Miranda et al., 2007). Although 4-fold increases in trehalose content were obtained in Arabidopsis and up to 10-fold increases in rice, the absolute amounts of trehalose remained rather low and seemed unlikely to be making much contribution to osmoregulation or stress protection. The limited accumulation of trehalose was ascribed to the high endogenous trehalase activity in the plants. T6P, the intermediate of the trehalose biosynthesis, is a recognized key signaling molecule involved in regulating many metabolic and developmental processes in plants (Schluepmann et al., 2004; Kolbe et al., 2005; Lunn et al., 2006; Zhang et al., 2009) and has been proposed to play a role in the acquisition of abiotic stress tolerance in insects and fungi (Mizoguchi et al., 1996; Cheong et al., 2003). Therefore, it has been suggested that the improved drought tolerance of plants expressing microbial forms of TPS and/or TPP could be linked to changes in the level of T6P, leading to downstream effects such as the accumulation of soluble carbohydrates (Garg et al., 2002; Schluepmann et al., 2003). Nevertheless, these proposals remain largely speculative, and we cannot rule out the possibility that trehalose has a signaling or regulatory function as well.
To address this possibility, we investigated the effects of altering trehalase activity in Arabidopsis plants. As expected, loss of the endogenous trehalase in the Attre1-1 mutant led to a significant increase in the amount of trehalose, whereas lines that overexpressed AtTRE1 had lower trehalose contents (Table I). It is striking, however, that the trehalose accumulation in the Attre1-1 mutant was no more than 4-fold greater compared with wild-type plants. Similar results were obtained in Nicotiana tabacum plants transformed with a double gene construct to overexpress both a TPS and a TPP of microbial origin, which accumulated only 3-fold higher trehalose levels, even when the endogenous trehalase was inhibited by treatment with 1 mm validamycin A (Goddijn et al., 1997). These data indicate that endogenously synthesized trehalose might be broken down by another glycoside hydrolase, such as the broad-specificity acid α-glucosidase reported in some legume species (García et al., 2005). Alternatively, trehalose might be secreted out of the plant, perhaps from the roots into the soil, providing nutrients for beneficial bacteria and fungi (Fernandez et al., 2012). A further possibility is that trehalose exerts strong feedback inhibition on its own biosynthesis above a certain threshold.
There were no statistically significant changes in the levels of T6P when altering the trehalase activity in Arabidopsis plants (Table I), so any phenotypic differences seem most likely to be linked to the altered trehalose content. Curiously, decreasing the level of trehalose by overexpression of trehalase led to improved survival and growth of the plants under drought stress conditions, while the Attre1-1 knockout and Attre1-2 knockdown with elevated trehalose levels appeared to be more sensitive to drought stress. In one sense, these results were counterintuitive, given the commonly held view that trehalose functions as a compatible solute and stress protectant in many organisms. However, the low absolute concentrations of trehalose found in either wild-type or genetically engineered plants almost certainly rule out a role for this sugar as a quantitatively important compatible solute unless its distribution is highly localized in specific cell types. Unfortunately, currently available techniques for measuring trehalose are not sensitive enough to determine its distribution between different cells in plant tissues.
Since genetic modification of the trehalose biosynthesis pathway in plants led to differences in growth (Schluepmann et al., 2004; Lunn et al., 2006), it is important to consider whether the altered drought stress performances of the trehalase-modified lines were due to specific drought responses rather than differences in growth. This is surely the case for the AtTRE1 overexpressors, since the reduced growth seen in nonstress conditions (data not shown) was not reflected but was reversed during drought stress (Table II). The 35S::treF-overexpressing line grew slower than the wild-type Col-0 during normal (data not shown) and dry conditions (Table II) but was more sensitive toward drought. Although the Attre1-1 knockout grew better in well-watered conditions (data not shown), it is hard to conclude whether the slightly, but not significantly, increased growth rate affected the overall performance of this mutant during drought stress (Table II). The same indecisiveness applies for the Attre1-2 line. This knockdown displayed increased growth rates during well-watered conditions (data not shown) and mild drought stress (seedlings; Fig. 5A) but invested less energy in rosette growth and more energy in flowering than the wild-type Col-0 during severe drought stress (adult plants; Table II).
To investigate the function of trehalase during drought stress, it was important to know where and when trehalase is expressed in Arabidopsis plants. Analysis of the pTRE1::GUS-GFP promoter-reporter lines revealed strong expression in sink organs such as young rosette leaves, flower buds, and ripening siliques (Fig. 3, E, F, and I). This expression pattern is similar to that of the Arabidopsis TPS1 gene (van Dijken et al., 2004), suggesting that there may be factors in common regulating the expression of these two genes. T6P is proposed to link the growth of developing tissues to the carbohydrate status of the plant via regulation of the SnRK1 protein kinase (Schluepmann et al., 2003; Lunn et al., 2006; Zhang et al., 2009). In such tissues, trehalose could be an unwanted, perhaps deleterious, by-product of T6P signaling, and so the presence of high trehalase activity would help to prevent its accumulation. The AtTRE1 gene is also highly expressed during seed germination, budding, flowering, and seed maturation (Fig. 3, A and G–J). Whatever the functions of AtTRE1 are during these developmental stages, they do not appear to be essential, because the Attre1-1 knockout and Attre1-2 knockdown germinate, flower, and set seed under benign controlled growth conditions. However, the marked phenotypes of the AtTRE1-overexpressing and knockout lines under drought stress conditions (Fig. 2A) strongly indicate a role for trehalase and trehalose metabolism in abiotic stress tolerance.
Trehalase Affects the Stomatal Index and ABA-Induced Stomatal Closure
The pTRE1::GUS-GFP promoter-reporter lines showed strong expression of AtTRE1 in stomatal guard cells (Fig. 3, D and F). Stomatal closure can be studied in wild-type plants by an ABA treatment of detached leaves, floated on a KCl-containing buffer in the light (Fig. 4A). Expression of the Arabidopsis AtTRE1 gene is essential for this response, as the stomata in leaves from the Attre1-1 knockout and Attre1-2 knockdown showed no effect of ABA treatment on stomatal aperture (Fig. 4A). In contrast, the stomata in leaves from the AtTRE1 overexpressors were found to be hypersensitive to ABA (Fig. 4A). The higher leaf temperature of drought-stressed AtTRE1 overexpressors is indicative of a lower stomatal conductance and transpiration rate and could be explained by the increased stomatal sensitivity to endogenous high ABA levels, which would be expected under abiotic stress conditions. Interestingly, the Attps1-12 mutant, lower in TPS activity and T6P content than wild-type plants, was also found to have a smaller stomatal aperture (Gómez et al., 2010). Although trehalose was not measured, the decreased rates of T6P synthesis in the mutant suggest that it probably has lower levels of trehalose, like the trehalase overexpressors, which also showed reduced stomatal aperture. Thus, potential changes in the level of trehalose might be a contributing factor to the stomatal phenotypes of the Attps1-12 mutant, the Attre1 mutants, and the AtTRE1 overexpressors. Taken together, these data indicate that AtTRE1 and trehalose metabolism fulfill an important role in the control of water loss through the stomata upon drought stress in Arabidopsis plants.
Potassium ions, together with both inorganic (e.g. chloride) and organic (e.g. malate) anions, account for the majority of osmotic changes associated with stomatal opening and closing, but other solutes can also be important under certain conditions. When the levels of light are high enough, Suc accumulation plays a dominant role in raising the osmotic potential of the guard cells driving stomatal opening. The precise mechanism for modulating sugar levels during stomatal movement is not fully understood, but it may involve the transport of Suc into and out of the guard cells as well as the synthesis, redistribution, and catabolism of Suc within the guard cells (Outlaw and Manchester, 1979; MacRobbie, 1998). At present, we have insufficient information to assess whether perturbing trehalase activity and trehalose metabolism in the guard cells affects all types of stomatal movements or if the effects are specifically linked to sugar-related mechanisms of stomatal opening and closing.
Loss of AtTRE1 expression in the Attre1-2 knockdown led to an increased stomatal index, while overexpression of trehalase in the same Col-0 background generally decreased the stomatal index (Fig. 4C). These differences in stomatal number probably contribute to changes in stomatal conductance in the mutants, which are indicated by the differential rates of water loss from detached leaves (Fig. 2B) and the differences in leaf surface temperature (Fig. 5A) shown by the various lines. Stomatal number is determined by the rates of epidermal cell division and mechanisms triggering differentiation into guard cells and suppression of differentiation in the surrounding epidermal cells. Histochemical assays with pTRE1::GUS-GFP seedlings showed an intense AtTRE1 expression in the developing stomatal guard cells, suggesting that trehalase could play a role in guard cell differentiation. TPS1, the only known enzyme that synthesizes T6P in Arabidopsis (Vandesteene et al., 2012), is reported to interact with the cell cycle-dependent kinase CDKA;1 that likely acts in the stomatal pathway, with tubulin, and with the kinesin KCA1 (Bergmann and Sack, 2007; Geelen et al., 2007), implicating trehalose metabolism in control of the cell cycle. However, the physiological significance of this connection and the role of AtTRE1, if any, are unclear.
Arabidopsis Plants That Overexpress the E. coli treF Are More Sensitive to Drought Stress
Experiments with detached leaves showed that the Attre1-3OE and 35S::AtTRE1 9.1 plants, which overexpress the endogenous apoplastic trehalase (AtTRE1), had smaller stomatal apertures than wild-type Col-0 leaves in the absence of ABA (Fig. 4C) and lower rates of water loss (Fig. 2B). In contrast, the 35S::treF plants, engineered to overexpress the heterologous cytosolic trehalase (treF) from E. coli, had essentially the same stomatal aperture as wild-type Col-0 plants in the absence of ABA and only slightly lower rates of water loss, despite having even higher trehalase activity than the AtTRE1-overexpressing lines (Table I). In addition, the 35S::treF plants showed no increase in drought stress tolerance, unlike the AtTRE1 overexpressors (Fig. 2A). On the other hand, all of the trehalase-overexpressing lines showed enhanced stomatal sensitivity to exogenous ABA (Fig. 4A). These differences suggest that the intracellular location of the overexpressed enzyme could be a critical factor in determining which aspects of stomatal function are affected and whether this leads to improved drought stress tolerance in the plants.
AtTRE1 Is Required for ABA-Induced Stomatal Closure
The degree of stomatal closure observed in the different AtTRE1 overexpressors does not seem to be dependent on the amount of AtTRE1 protein accumulated in the leaves. For example, the Attre1-3OE line had less AtTRE1 than the 35S::AtTRE1 lines but seemed highly sensitive toward ABA (Figs. 1A and 4A). This finding suggests that the endogenous AtTRE1 promoter in Attre1-3OE might be activated upon ABA detection. This was confirmed by the observed increase in AtTRE1 expression of wild-type seedlings upon ABA addition (Fig. 4B), which also agrees with results from microarray analyses of Arabidopsis plants treated with ABA (Zimmermann et al., 2004). Analysis of the AtTRE1 promoter region revealed a DNA-binding site for MYB4 (Supplemental Fig. S4), a transcription factor acting in response to environmental stresses (Chen et al., 2002). Moreover, a W-box promoter motif for MYB102 and WRKY transcription factors (Supplemental Fig. S4), known to be involved in ABA signaling upon dehydration and osmotic stress (Denekamp and Smeekens, 2003; Ren et al., 2010), was also detected in the AtTRE1 promoter (ATHENA; O’Connor et al., 2005). Moreover, since MYB4 and MYB102 are both members from the R2R3-type MYB family, known to control the identity and fate of many plant cells (Stracke et al., 2001), these transcription factors could possibly induce AtTRE1 expression during developmental processes such as guard cell differentiation (Supplemental Fig. S3).
Interestingly, Arabidopsis microarrays also showed that AtTRE1 expression is light dependent, with a strong increase in AtTRE1 transcripts during extended night (Zimmermann et al., 2004), which indicates that AtTRE1 expression might be induced in the dark when stomatal closure is required.
Frison et al. (2007) suggested that the additional higher molecular mass bands of AtTRE1 seen on immunoblots may represent posttranslationally modified forms of the Arabidopsis trehalase (Fig. 1A). To reach its apoplastic location, the AtTRE1 protein is likely to be transported via the endomembrane system and then secreted by vesicular exocytosis; therefore, it may be subject to glycosylation at one or more sites (Frison et al., 2007). Within the AtTRE1 protein, four residues (Ser-71, Thr-128, Ser-463, and Ser-530) and one hotspot (residues 191–213) are predicted with high confidence to be phosphorylated, and a further 38 residues have potential to be phosphorylated (Heazlewood et al., 2008). Moreover, Ser-195, which is part of the phosphorylation hotspot, falls within the RXXS/T motif, which is a characteristic target site for the SNF1-related protein kinases SnRK2.2, SnRK2.6/Ost1, and SnRK2.3 (Furihata et al., 2006). This suggests that phosphorylation by this class of protein kinases might be another factor contributing to the appearance of multiple AtTRE1 forms on the immunoblots. Phosphorylation of the AtTRE1 protein seems most likely to occur while the protein is being synthesized or in transit within the cell, as there appears to be little phosphorylation of proteins once they are secreted into the apoplast (Durek et al., 2010).
CONCLUSION
The results presented here unequivocally demonstrate the importance of trehalase in stomatal function in Arabidopsis and provide a number of important leads for investigating the transcriptional and posttranslational regulation of trehalase expression and activity in guard cells. Furthermore, we have shown that manipulating the expression of the endogenous plant trehalase can have a beneficial effect on the plant’s response to water deficiency and that this is a promising strategy to engineer improvements in drought stress tolerance in crop species.
MATERIALS AND METHODS
Plant Material
To obtain 35S::AtTRE1 overexpressors, the AtTRE1 coding sequence (At4g24040) with a 2× hemagglutinin-tagged sequence was amplified using TRE1-BglII and TRE1-SmaI primers (for primer sequences, see Supplemental Table S1). This PCR product was cloned as a BglII-SmaI restriction fragment into a modified pCB302 minibinary expression vector (Hwang and Sheen, 2001). Arabidopsis (Arabidopsis thaliana) Col-0 plants were transformed by floral dip with Agrobacterium tumefaciens C58C1 carrying the expression vector. Seven independent homozygous lines were selected, which contained single insertions based on their 3:1 (resistant:susceptible) segregation patterns on glufosinate ammonium (50 mg L−1). The Escherichia coli treF gene was amplified with treF-F and treF-R primers (Supplemental Table S1). The obtained reaction product was inserted as an EcoRI restriction fragment into the pGreen binary vector (Hellens et al., 2000) between the cauliflower mosaic virus 35S promoter and terminator. A. tumefaciens-transformed Col-0 plants were selected by BASTA spraying. From the T2 plant 35S::treF 10.3, 138 out of 138 progeny were resistant to BASTA. The pTRE1::GUS-GFP lines were created by cloning a region of approximately 2 kb upstream of the AtTRE1 transcription start site plus the 5′-untranslated region with pTRE1-attB primers in the pHGWFS7 vector (Karimi et al., 2007), using the Gateway technology (Invitrogen) according to the manufacturer’s instructions. A. tumefaciens-transformed Col-0 plants were selected on hygromycin B (50 mg L−1), and three independent homozygous lines with single insertions were obtained. The Attre1-1 (GT_16843 in the Ler accession; Sundaresan et al., 1995), Attre1-2 (SALK_147073C in the Col-0 accession; Alonso et al., 2003), and Attre1-3OE (SALK_151791 in the Col-0 accession; Alonso et al., 2003) mutant seeds were ordered from the Nottingham Arabidopsis Stock Centre and checked for homozygosity and single T-DNA insertion on kanamycin (50 mg L−1). The Attre1-1 line was genotyped by PCR using the Ds3 primer together with the gene-specific primers TRE1-LP1 and TRE1-RP1. The following primers were used to screen the Attre1-3OE line: Lba1, TRE1-LP2, and TRE1-RP2. Seeds from the Attre1-2 line were genotyped using the Lba1 primer combined with TRE1-LP3 and TRE1-RP3 primers. The Attre1-2 mutant has been described earlier by Delatte et al. (2011a).
Plant Growing Conditions
Seeds were vapor-phase sterilized and cold treated at 4°C for 72 h in the dark. Unless stated otherwise, plants were grown for seedling experiments in a 16/8-h day/night cycle (21°C, 60% humidity, 100 µE m−2 s−1) on vertically oriented plates with 0.5× MS medium (4.3 g L−1), MES (0.5 g L−1), and Suc (1%, w/v) and solidified with Phytagar (8 g L−1; Duchefa). For trehalose sensitivity assays, seedlings were grown for 3 weeks on 0.5× MS medium (4.3 g L−1) containing MES (0.5 g L−1) with or without 25 mm trehalose and solidified with Phytagar (8 g L−1). For measuring AtTRE1 expression in seedlings upon ABA addition (10 µm), vapor-sterilized seeds were sown directly into liquid cultures of 0.5× MS medium (4.3 g L−1) with MES (0.5 g L−1) and Suc (1%). Flasks with equal amounts of seeds were incubated for 11 d in a 16/8-h day/night cycle (21°C, 60% humidity, 100 µE m−2 s−1) with continuous shaking (80 rpm). For experiments with soil-grown plants, these were generally grown in a growth chamber in a 16/8-h day/night cycle (21°C, 60% humidity, 100 µE m−2 s−1) unless described otherwise.
Quantitative Real-Time PCR
For measuring AtTRE1 expression, three replicates of at least 20 10-d-old, soil-grown (characterization) and 11-d-old, in vitro-cultured (ABA induction assay) seedlings were frozen in liquid nitrogen and ground to a fine powder with a mortar and pestle. Total RNA isolation, complementary DNA synthesis, and quantitative PCR were performed as described by Vandesteene et al. (2010). AtTRE1 expression levels were normalized to UBQ10 (At4g05320). Primer sequences of AtTRE1 (TRE1-fw and TRE1-rv) and UBQ10 (UBQ10-fw and UBQ10-rv) are listed in Supplemental Table S2.
Immunoblot Analysis
The following samples were harvested and frozen in liquid nitrogen: (1) at least 20 10-d-old seedlings grown on 0.5× MS medium; (2) 10 4-week-old rosettes from soil-grown plants; and (3) a pool of flowers/siliques originating from at least 10 different 6-week-old plants grown in soil. The frozen tissues were ground to a fine powder with a mortar and pestle and extracted in an equal volume of ice-cold extraction buffer (25 mm Tris-HCl, pH 7.6, 150 mm NaCl, 15 mm MgCl2, 15 mm EGTA, pH 8, 60 mm β-glycerophosphate, 0.1% Nonidet P-40, 0.1 mm Na3VO4, 1 mm NaF, 1 mm dithiothreitol, 0.1 mm benzamidine, and 5% ethylene glycol) containing protease inhibitors (Complete EDTA-free; Roche). Protein concentrations were determined according to Bradford (1976). For each sample, aliquots containing 20 µg of protein were analyzed by SDS-PAGE on duplicate NuPAGE NOVEX Bis-Tris Mini Gels (Invitrogen). One gel was stained with 0.25% Coomassie Brilliant Blue as a sample loading control, and proteins from the other gel were analyzed by immunoblotting. For detection of the AtTRE1 protein, we used as primary antibody an affinity-purified polyclonal anti-AtTRE1 antibody raised in rabbit against a peptide comprising amino acid residues 94 to 107 of the AtTRE1 protein (1:1,000; GenScript) and as secondary antibody a horseradish peroxidase-conjugated donkey anti-rabbit antibody (1:3,000 dilution in blocking buffer; GE Healthcare).
Trehalase Activity
Three replicates of at least 20 10-d-old seedlings and 10 rosettes from 4-week-old plants were frozen in liquid nitrogen and homogenized with a mortar and pestle. Aliquots (100 mg) of the tissue powder were extracted in 1 mL of ice-cold extraction buffer (0.1 m MES-KOH, pH 6, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1% [w/v] polyvinylpyrrolidone, and 1 mm dithiothreitol). The suspension was cleared by centrifugation (18,000g, 4°C, 10 min), and the supernatant was dialyzed (Standard RC Dialysis Tubing: Spectra/Por 3; Spectrum) overnight at 4°C against 10 mm MES-KOH, pH 7, containing 50 µm CaCl2. Trehalase activity was determined as described previously (Pernambuco et al., 1996) by incubating aliquots of the dialyzed extract with 250 mm trehalose and by measurement of the released Glc using Glc oxidase-peroxidase (Dialab).
Metabolite Measurements
Metabolite measurements were executed with three replicates of 12 seedlings each harvested 11 d after germination and grown at 22°C under continuous light (110 µE m−2 s−1). Plant material was immediately frozen in liquid nitrogen and homogenized with a mortar and pestle. Aliquots (15–20 mg) of the frozen tissue powder were extracted with chloroform-methanol as described by Lunn et al. (2006). Trehalose was quantified fluorimetrically as described by Mollo et al. (2011). T6P and other phosphorylated compounds were determined by liquid chromatography coupled to liquid tandem mass spectrometry as described by Lunn et al. (2006).
Drought Assay
Drought tolerance tests were performed in individual pots filled with a mixture of soil (20 g) and vermiculite (8 g). For each transgenic line and wild-type control, five plants were grown for 2 weeks in controlled, well-watered conditions (relative soil water content [RWCsoil] of 80%). During the following 20 d, the amount of water was gradually and equally reduced in the pots until the symptoms of drought stress were strongly pronounced, which occurred at an RWCsoil of 22%. To achieve an identical drying-out process among the different plants, pot weights were monitored and synchronized on a daily basis. At the end of the drought stress period, plants were rewatered. Photographs were taken and plant fresh and dry weights were assessed after a recovery period of 48 h.
Histochemical and Histological Analysis
For GUS staining assays, in vitro-grown seedlings and bolted plants were sampled 2, 5, 10, and 35 d after germination, while flowers of soil-grown plants were harvested from 6-week-old plants. GUS staining was performed according to Beeckman and Engler (1994). For the staining of rosette leaves, vacuum infiltration was applied for 2 h. After the staining step, plant material was cleared in 85% lactic acid (Sigma-Aldrich) and mounted on glass microscopy slides. Samples were examined with an M165C binocular microscope (Leica) and by differential interference contrast microscopy (BX51; Olympus).
Leaf Detachment, Stomatal Aperture, and Stomatal Counts
For stomatal aperture bioassays and leaf detachment studies, plants were grown in well-watered conditions. The first fully expanded leaves from 3-week-old plants were detached and used in both assays. For determining the stomatal aperture, leaves were floated in a stomata-opening buffer (10 mm MES-KOH, pH 6.15, 10 mm KCl) for 2 h under continuous light. To test the response to ABA, 20 and 0 µm (control) ABA was added to the buffer, and leaves were incubated 2 h more. Peels of abaxial epidermis were mounted on a microscopy slide with double-sided sticky tape, moistened with a few drops of assay solution, and covered with a slip. The microscopy slides were examined within 30 min after preparation with a BX51 light microscope (Olympus). Photographs of epidermal sections were taken from three separate leaves and from 10 different areas per leaf. Stomatal apertures were measured using ImageJ software. The stomatal index was determined from the same photographs used for measuring the stomatal aperture. The stomatal index (%) of a given leaf area was calculated as the ratio of the total number of stomatal guard cells to the total number of epidermal cells (sum of guard cells and pavement cells) multiplied by 100.
Thermal Imaging
Seedlings were grown in individual pots filled with a mixture of soil (20 g) and vermiculite (8 g) under humid conditions (RWCsoil of 80%) for 8 d and subsequently subjected to drought by not watering for 4 d (RWCsoil of approximately 70%). The 12-d-old plantlets were photographed in the same growth cabinet using a B400 camera (FLIR Systems). Three infrared photographs of approximately 3,000 square pixels were taken for each of the transgenic lines. All images contained an aluminum blackbody as a reference, a pot of transgenic seedlings, and a pot of wild-type seedlings. The RWCsoil did not vary more than 1.25% between two pots in one photograph. The emissivity of the aluminum body was set to 1. Images were analyzed with ImageJ, and leaf areas and temperatures were calculated in MATLAB (MathWorks). The leaf temperature data were normalized against the emissivity of the aluminum body and the leaf temperature of the respective wild type.
All experiments have been performed at least three times.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Genomic organization of GT_16843, SALK_147073C, and SALK_151791.
Supplemental Figure S2. The decrease in RWCsoil during the time course of the drought stress experiment.
Supplemental Figure S3. GUS activity in developing stomatal guard cells of the pTRE1::GUS-GFP lines.
Supplemental Figure S4. DNA-binding motifs in the AtTRE1 promoter region.
Supplemental Table S1. Relative AtTRE1 expression levels in rosette leaves of 3-week-old, trehalase-modified Col-0 plants.
Supplemental Table S2. Primers used in this study.
Acknowledgments
We thank Nico Van Goethem (KU Leuven) for assistance with preparation of the figures. We thank Gustavo Gudesblat (Ghent University) for his expertise with stomata assays. We thank Nathalie Wuyts (Université Catholique de Louvain) for developing software to analyze infrared images (FlirImageProcessor for ImageJ).
Glossary
- ABA
abscisic acid
- TPS
trehalose-6-phosphate synthase
- TPP
trehalose-6-phosphate phosphatase
- T6P
trehalose-6-phosphate
- Col-0
Columbia-0
- T-DNA
transfer DNA
- 0.5× MS
one-half-strength Murashige and Skoog
- Ler
Landsberg erecta
- RWCsoil:
relative soil water content
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Avonce N, Leyman B, Mascorro-Gallardo JO, Van Dijck P, Thevelein JM, Iturriaga G. (2004) The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol 136: 3649–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avonce N, Wuyts J, Verschooten K, Vandesteene L, Van Dijck P. (2010) The Cytophaga hutchinsonii ChTPSP: first characterized bifunctional TPS-TPP protein as putative ancestor of all eukaryotic trehalose biosynthesis proteins. Mol Biol Evol 27: 359–369 [DOI] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Beeckman T, Engler G. (1994) An easy technique for the clearing of histochemically stained plant tissue. Plant Mol Biol Rep 12: 37–42 [Google Scholar]
- Berger F, Linstead P, Dolan L, Haseloff J. (1998) Stomata patterning on the hypocotyl of Arabidopsis thaliana is controlled by genes involved in the control of root epidermis patterning. Dev Biol 194: 226–234 [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Sack FD. (2007) Stomatal development. Annu Rev Plant Biol 58: 163–181 [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Santos E, Flores CL, Martínez-Zapater JM, Salinas J, Gancedo C. (1998) Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J 13: 685–689 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brenner WG, Romanov GA, Köllmer I, Bürkle L, Schmülling T. (2005) Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J 44: 314–333 [DOI] [PubMed] [Google Scholar]
- Brodmann A, Schuller A, Ludwig-Müller J, Aeschbacher RA, Wiemken A, Boller T, Wingler A. (2002) Induction of trehalase in Arabidopsis plants infected with the trehalose-producing pathogen Plasmodiophora brassicae. Mol Plant Microbe Interact 15: 693–700 [DOI] [PubMed] [Google Scholar]
- Cabib E, Leloir LF. (1958) The biosynthesis of trehalose phosphate. J Biol Chem 231: 259–275 [PubMed] [Google Scholar]
- Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA, et al. (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14: 559–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S. (2003) CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 15: 1833–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina C, Culianez-Macia FA. (2005) Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci 169: 75–82 [Google Scholar]
- Delatte TL, Schluepmann H, Smeekens SC, de Jong GJ, Somsen GW. (2011a) Capillary electrophoresis-mass spectrometry analysis of trehalose-6-phosphate in Arabidopsis thaliana seedlings. Anal Bioanal Chem 400: 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte TL, Sedijani P, Kondou Y, Matsui M, de Jong GJ, Somsen GW, Wiese-Klinkenberg A, Primavesi LF, Paul MJ, Schluepmann H. (2011b) Growth arrest by trehalose-6-phosphate: an astonishing case of primary metabolite control over growth by way of the SnRK1 signaling pathway. Plant Physiol 157: 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp M, Smeekens SC. (2003) Integration of wounding and osmotic stress signals determines the expression of the AtMYB102 transcription factor gene. Plant Physiol 132: 1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durek P, Schmidt R, Heazlewood JL, Jones A, MacLean D, Nagel A, Kersten B, Schulze WX. (2010) PhosPhAt: the Arabidopsis thaliana phosphorylation site database. An update. Nucleic Acids Res 38: D828–D834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Li Y, Graham IA. (2003) Is trehalose-6-phosphate a regulator of sugar metabolism in plants? J Exp Bot 54: 533–537 [DOI] [PubMed] [Google Scholar]
- Elbein AD, Pan YT, Pastuszak I, Carroll D. (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13: 17R–27R [DOI] [PubMed] [Google Scholar]
- Fernandez O, Béthencourt L, Quero A, Sangwan RS, Clément C. (2010) Trehalose and plant stress responses: friend or foe? Trends Plant Sci 15: 409–417 [DOI] [PubMed] [Google Scholar]
- Fernandez O, Vandesteene L, Feil R, Baillieul F, Lunn JE, Clément C. (2012) Trehalose metabolism is activated upon chilling in grapevine and might participate in Burkholderia phytofirmans induced chilling tolerance. Planta 236: 355–369 [DOI] [PubMed] [Google Scholar]
- Frison M, Parrou JL, Guillaumot D, Masquelier D, François J, Chaumont F, Batoko H. (2007) The Arabidopsis thaliana trehalase is a plasma membrane-bound enzyme with extracellular activity. FEBS Lett 581: 4010–4016 [DOI] [PubMed] [Google Scholar]
- Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. (2006) Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA 103: 1988–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García NA, Iribarne C, López M, Herrera-Cervera JA, Lluch C. (2005) Physiological implications of trehalase from Phaseolus vulgaris root nodules: partial purification and characterization. Plant Physiol Biochem 43: 355–361 [DOI] [PubMed] [Google Scholar]
- Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ. (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99: 15898–15903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen D, Royackers K, Vanstraelen M, Inzé D, Van Dijck P, Thevelein JM, Leyman B. (2007) Trehalose-6-P synthase: AtTPS1 high molecular weight complexes in yeast and Arabidopsis. Plant Sci 173: 426–437 [Google Scholar]
- Goddijn OJM, Verwoerd TC, Voogd E, Krutwagen RWHH, de Graaf PTHM, van Dun K, Poels J, Ponstein AS, Damm B, Pen J. (1997) Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol 113: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez LD, Gilday A, Feil R, Lunn JE, Graham IA. (2010) AtTPS1-mediated trehalose 6-phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J 64: 1–13 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Durek P, Hummel J, Selbig J, Weckwerth W, Walther D, Schulze WX. (2008) PhosPhAt: a database of phosphorylation sites in Arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res 36: D1015–D1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazlewood JL, Verboom RE, Tonti-Filippini J, Small I, Millar AH. (2007) SUBA: the Arabidopsis subcellular database. Nucleic Acids Res 35: D213–D218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J. (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413: 383–389 [DOI] [PubMed] [Google Scholar]
- Karim S, Aronsson H, Ericson H, Pirhonen M, Leyman B, Welin B, Mäntylä E, Palva ET, Van Dijck P, Holmström KO. (2007) Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol Biol 64: 371–386 [DOI] [PubMed] [Google Scholar]
- Karimi M, Depicker A, Hilson P. (2007) Recombinational cloning with plant Gateway vectors. Plant Physiol 145: 1144–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe A, Tiessen A, Schluepmann H, Paul M, Ulrich S, Geigenberger P. (2005) Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci USA 102: 11118–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyman B, Van Dijck P, Thevelein JM. (2001) An unexpected plethora of trehalose biosynthesis genes in Arabidopsis thaliana. Trends Plant Sci 6: 510–513 [DOI] [PubMed] [Google Scholar]
- Li HW, Zang BS, Deng XW, Wang XP. (2011) Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 234: 1007–1018 [DOI] [PubMed] [Google Scholar]
- Lunn JE. (2007) Gene families and evolution of trehalose metabolism in plants. Funct Plant Biol 34: 550–563 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible W-R, Carillo P, Hajirezaei MR, Stitt M. (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC. (1998) Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond B Biol Sci 353: 1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Leonardi A, Brisson G, Vartanian N. (2001) Drought-adaptive mechanisms involved in the escape/tolerance strategies of Arabidopsis Landsberg erecta and Columbia ecotypes and their F1 reciprocal progeny. J Plant Physiol 158: 1145–1152 [Google Scholar]
- Miranda JA, Avonce N, Suárez R, Thevelein JM, Van Dijck P, Iturriaga G. (2007) A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 226: 1411–1421 [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K. (1996) A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA 93: 765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollo L, Martins MCM, Oliveira VF, Nievola CC, Figueiredo-Ribeiro Rd CL. (2011) Effects of low temperature on growth and non-structural carbohydrates of the imperial bromeliad Alcantarea imperialis cultured in vitro. Plant Cell Tissue Organ Cult 107: 141–149 [Google Scholar]
- Müller J, Aeschbacher RA, Wingler A, Boller T, Wiemken A. (2001a) Trehalose and trehalase in Arabidopsis. Plant Physiol 125: 1086–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Boller T, Wiemken A. (2001b) Trehalose becomes the most abundant non-structural carbohydrate during senescence of soybean nodules. J Exp Bot 52: 943–947 [DOI] [PubMed] [Google Scholar]
- Müller J, Xie Z-P, Staehelin C, Mellor RB, Boller T, Wiemken A. (1994) Trehalose and trehalase in root nodules from various legumes. Physiol Plant 90: 86–92 [Google Scholar]
- O’Connor TR, Dyreson C, Wyrick JJ. (2005) Athena: a resource for rapid visualization and systematic analysis of Arabidopsis promoter sequences. Bioinformatics 21: 4411–4413 [DOI] [PubMed] [Google Scholar]
- Outlaw WH, Jr, Manchester J. (1979) Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol 64: 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernambuco MB, Winderickx J, Crauwels M, Griffioen G, Mager WH, Thevelein JM. (1996) Glucose-triggered signalling in Saccharomyces cerevisiae: different requirements for sugar phosphorylation between cells grown on glucose and those grown on non-fermentable carbon sources. Microbiology 142: 1775–1782 [DOI] [PubMed] [Google Scholar]
- Ramon M, De Smet I, Vandesteene L, Naudts M, Leyman B, Van Dijck P, Rolland F, Beeckman T, Thevelein JM. (2009) Extensive expression regulation and lack of heterologous enzymatic activity of the class II trehalose metabolism proteins from Arabidopsis thaliana. Plant Cell Environ 32: 1015–1032 [DOI] [PubMed] [Google Scholar]
- Ramon M, Rolland F, Thevelein JM, Van Dijck P, Leyman B. (2007) ABI4 mediates the effects of exogenous trehalose on Arabidopsis growth and starch breakdown. Plant Mol Biol 63: 195–206 [DOI] [PubMed] [Google Scholar]
- Ren X, Chen Z, Liu Y, Zhang H, Zhang M, Liu Q, Hong X, Zhu J-K, Gong Z. (2010) ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J 63: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Romero C, Bellés JM, Vayá JL, Serrano R, Culiáñez-Macià FA. (1997) Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 201: 293–297 [DOI] [PubMed] [Google Scholar]
- Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M. (2003) Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6849–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S. (2004) Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol 135: 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert A, Wyss P. (1995) Trehalase activity in mycorrhizal and nonmycorrhizal roots of leek and soybean. Mycorrhiza 5: 401–404 [Google Scholar]
- Stracke R, Werber M, Weisshaar B. (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4: 447–456 [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Springer P, Volpe T, Haward S, Jones JDG, Dean C, Ma H, Martienssen RA. (1995) Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev 9: 1797–1810 [DOI] [PubMed] [Google Scholar]
- Thaller MC, Schippa S, Rossolini GM. (1998) Conserved sequence motifs among bacterial, eukaryotic, and archaeal phosphatases that define a new phosphohydrolase superfamily. Protein Sci 7: 1647–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesteene L, López-Galvis L, Vanneste K, Feil R, Maere S, Lammens W, Rolland F, Lunn JE, Avonce N, Beeckman T, et al. (2012) Expansive evolution of the trehalose-6-phosphate phosphatase gene family in Arabidopsis. Plant Physiol 160: 884–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesteene L, Ramon M, Le Roy K, Van Dijck P, Rolland F. (2010) A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis. Mol Plant 3: 406–419 [DOI] [PubMed] [Google Scholar]
- Van Dijck P, Mascorro-Gallardo JO, De Bus M, Royackers K, Iturriaga G, Thevelein JM. (2002) Truncation of Arabidopsis thaliana and Selaginella lepidophylla trehalose-6-phosphate synthase unlocks high catalytic activity and supports high trehalose levels on expression in yeast. Biochem J 366: 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijken AJ, Schluepmann H, Smeekens SC. (2004) Arabidopsis trehalose-6-phosphate synthase 1 is essential for normal vegetative growth and transition to flowering. Plant Physiol 135: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinocur B, Altman A. (2005) Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol 16: 123–132 [DOI] [PubMed] [Google Scholar]
- Vogel G, Fiehn O, Jean-Richard-dit-Bressel L, Boller T, Wiemken A, Aeschbacher RA, Wingler A. (2001) Trehalose metabolism in Arabidopsis: occurrence of trehalose and molecular cloning and characterization of trehalose-6-phosphate synthase homologues. J Exp Bot 52: 1817–1826 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. (1981) Some relationships between the biochemistry of photosynthesis and the gas-exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- Zentella R, Mascorro-Gallardo JO, Van Dijck P, Folch-Mallol J, Bonini B, Van Vaeck C, Gaxiola R, Covarrubias AA, Nieto-Sotelo J, Thevelein JM, et al. (1999) A Selaginella lepidophylla trehalose-6-phosphate synthase complements growth and stress-tolerance defects in a yeast tps1 mutant. Plant Physiol 119: 1473–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RA, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149: 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]