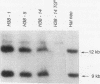
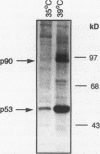
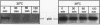Abstract
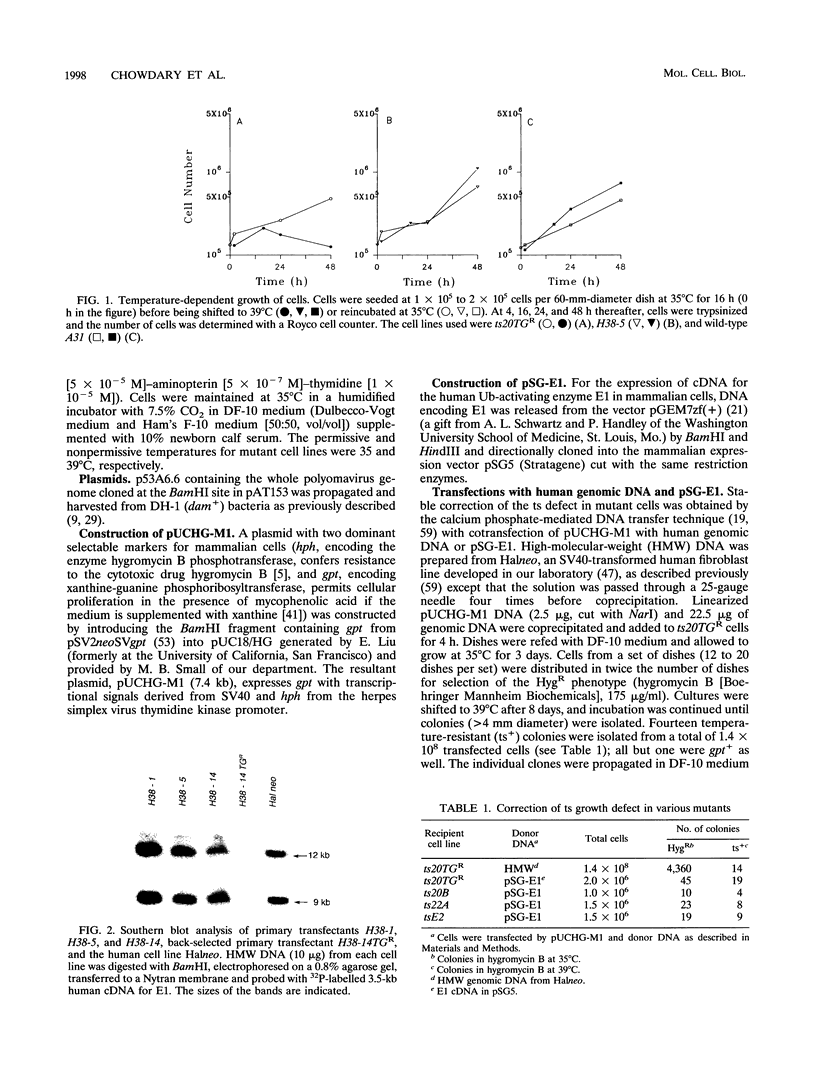
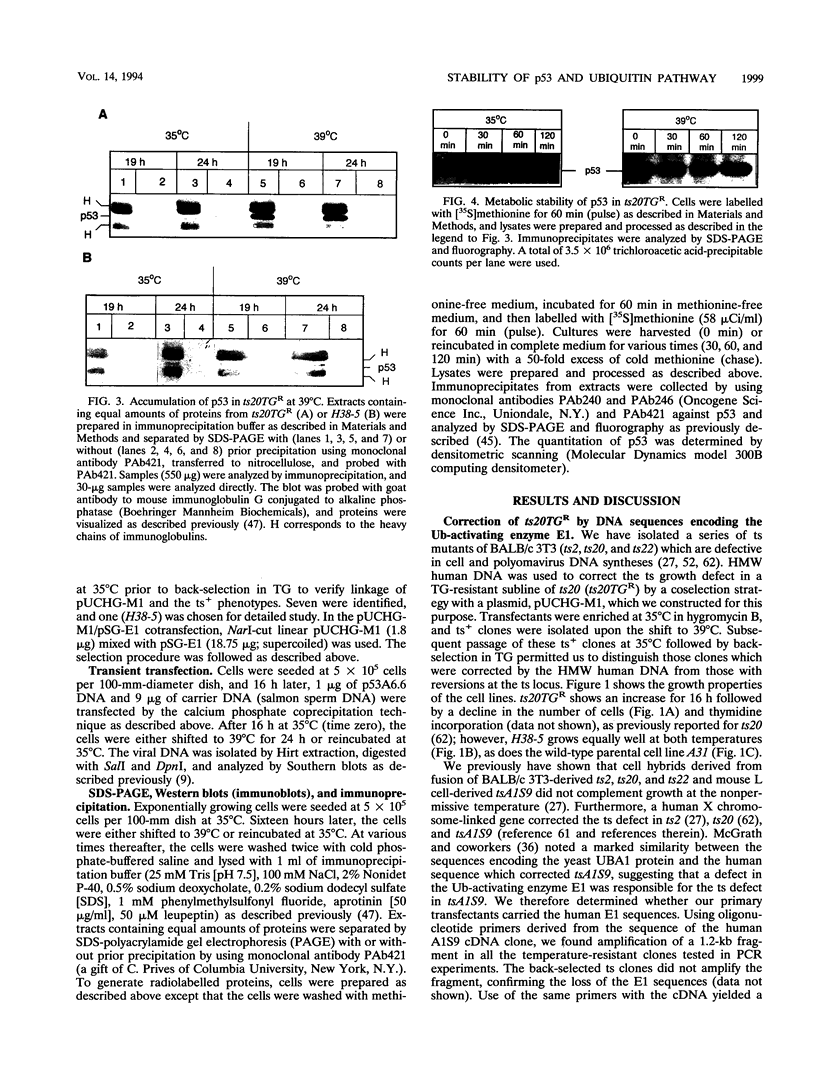
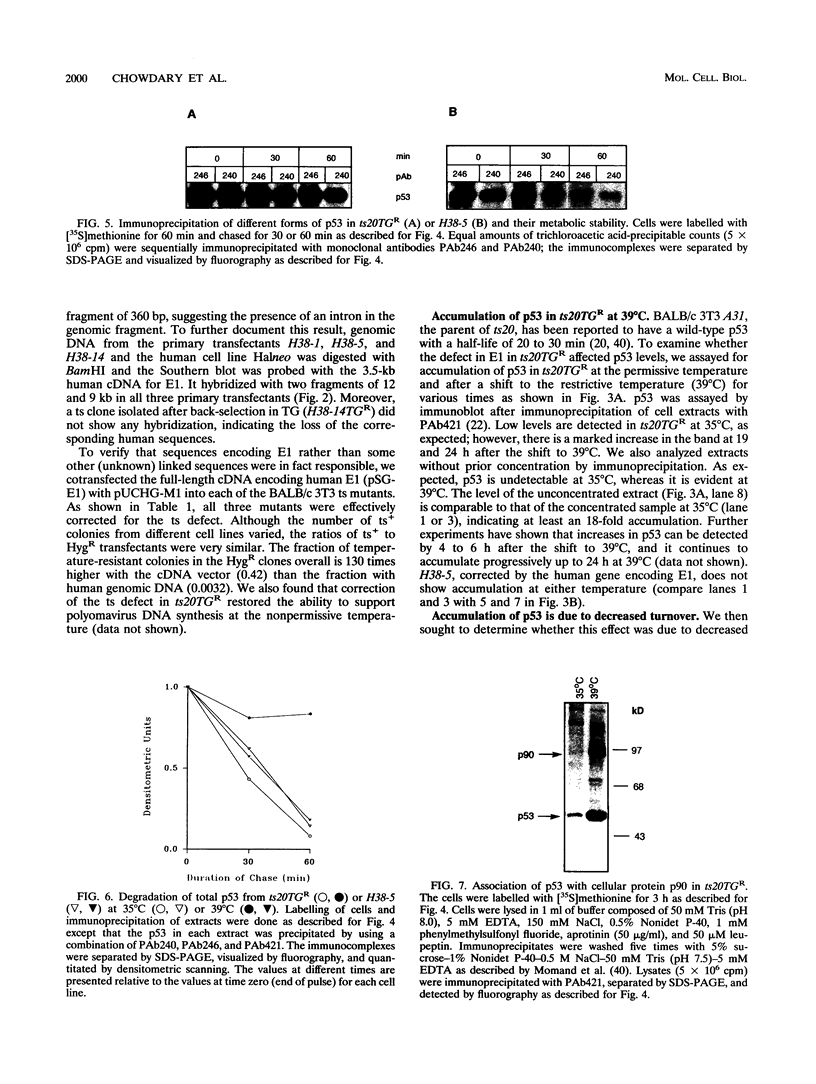
The wild-type p53 gene product plays an important role in the control of cell proliferation, differentiation, and survival. Altered function is frequently associated with changes in p53 stability. We have studied the role of the ubiquitination pathway in the degradation of p53, utilizing a temperature-sensitive mutant, ts20, derived from the mouse cell line BALB/c 3T3. We found that wild-type p53 accumulates markedly because of decreased breakdown when cells are shifted to the restrictive temperature. Introduction of sequences encoding the human ubiquitin-activating enzyme E1 corrects the temperature sensitivity defect in ts20 and prevents accumulation of p53. The data therefore strongly indicate that wild-type p53 is degraded intracellularly by the ubiquitin-mediated proteolytic pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agoff S. N., Hou J., Linzer D. I., Wu B. Regulation of the human hsp70 promoter by p53. Science. 1993 Jan 1;259(5091):84–87. doi: 10.1126/science.8418500. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Barak Y., Oren M. Enhanced binding of a 95 kDa protein to p53 in cells undergoing p53-mediated growth arrest. EMBO J. 1992 Jun;11(6):2115–2121. doi: 10.1002/j.1460-2075.1992.tb05270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargonetti J., Friedman P. N., Kern S. E., Vogelstein B., Prives C. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell. 1991 Jun 14;65(6):1083–1091. doi: 10.1016/0092-8674(91)90560-l. [DOI] [PubMed] [Google Scholar]
- Bernard H. U., Krämmer G., Röwekamp W. G. Construction of a fusion gene that confers resistance against hygromycin B to mammalian cells in culture. Exp Cell Res. 1985 May;158(1):237–243. doi: 10.1016/0014-4827(85)90446-x. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., DiGiuseppe J. A., Bercovich B., Orian A., Richter J. D., Schwartz A. L., Brodeur G. M. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):139–143. doi: 10.1073/pnas.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. R., Murphy L. C. Level of ubiquitinated histone H2B in chromatin is coupled to ongoing transcription. Biochemistry. 1990 May 22;29(20):4752–4757. doi: 10.1021/bi00472a002. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison K. S., Gwozd T., Prendergast J. A., Paterson M. C., Ellison M. J. A site-directed approach for constructing temperature-sensitive ubiquitin-conjugating enzymes reveals a cell cycle function and growth function for RAD6. J Biol Chem. 1991 Dec 15;266(35):24116–24120. [PubMed] [Google Scholar]
- Fakharzadeh S. S., Trusko S. P., George D. L. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991 Jun;10(6):1565–1569. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Goebl M. G., Yochem J., Jentsch S., McGrath J. P., Varshavsky A., Byers B. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science. 1988 Sep 9;241(4871):1331–1335. doi: 10.1126/science.2842867. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Goldberg A. L., Pardee A. B. Energy requirement for degradation of tumor-associated protein p53. Mol Cell Biol. 1984 Mar;4(3):442–448. doi: 10.1128/mcb.4.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P. M., Mueckler M., Siegel N. R., Ciechanover A., Schwartz A. L. Molecular cloning, sequence, and tissue distribution of the human ubiquitin-activating enzyme E1. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):258–262. doi: 10.1073/pnas.88.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992 Nov 13;71(4):543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Frey A. B., Levine A. J. Immunological evidence for the association of p53 with a heat shock protein, hsc70, in p53-plus-ras-transformed cell lines. Mol Cell Biol. 1987 Aug;7(8):2863–2869. doi: 10.1128/mcb.7.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupp T. R., Meek D. W., Midgley C. A., Lane D. P. Regulation of the specific DNA binding function of p53. Cell. 1992 Nov 27;71(5):875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- Jha K. K., Siniscalco M., Ozer H. L. Temperature-sensitive mutants of BALB/3T3 cells. III. Hybrids between ts2 and other mouse mutant cells affected in DNA synthesis and correction of ts2 defect by human X chromosome. Somatic Cell Genet. 1980 Sep;6(5):603–614. doi: 10.1007/BF01538640. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- La Bella F., Ozer H. L. Differential replication of SV40 and polyoma DNAs in Chinese hamster ovary cells. Virus Res. 1985 Jun;2(4):329–344. doi: 10.1016/0168-1702(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Lin J. Y., Simmons D. T. The ability of large T antigen to complex with p53 is necessary for the increased life span and partial transformation of human cells by simian virus 40. J Virol. 1991 Dec;65(12):6447–6453. doi: 10.1128/jvi.65.12.6447-6453.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Manfredi J. J., Prives C. Binding of p53 and p105-RB is not sufficient for oncogenic transformation by a hybrid polyomavirus-simian virus 40 large T antigen. J Virol. 1990 Nov;64(11):5250–5259. doi: 10.1128/jvi.64.11.5250-5259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Jentsch S., Varshavsky A. UBA 1: an essential yeast gene encoding ubiquitin-activating enzyme. EMBO J. 1991 Jan;10(1):227–236. doi: 10.1002/j.1460-2075.1991.tb07940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. p53 mutations: gains or losses? J Cell Biochem. 1991 Jan;45(1):22–29. doi: 10.1002/jcb.240450108. [DOI] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A. Temperature-dependent switching between "wild-type" and "mutant" forms of p53-Val135. J Mol Biol. 1990 Dec 5;216(3):481–484. doi: 10.1016/0022-2836(90)90371-R. [DOI] [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhasi-Kimhi O., Michalovitz D., Ben-Zeev A., Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986 Mar 13;320(6058):182–184. doi: 10.1038/320182a0. [DOI] [PubMed] [Google Scholar]
- Radna R. L., Foellmer B., Feldman L. A., Francke U., Ozer H. L. Restriction of human adenovirus replication in Chinese hamster cell lines and their hybrids with human cells. Virus Res. 1987 Nov;8(4):277–299. doi: 10.1016/0168-1702(87)90001-3. [DOI] [PubMed] [Google Scholar]
- Reihsaus E., Kohler M., Kraiss S., Oren M., Montenarh M. Regulation of the level of the oncoprotein p53 in non-transformed and transformed cells. Oncogene. 1990 Jan;5(1):137–145. [PubMed] [Google Scholar]
- Resnick-Silverman L., Pang Z., Li G., Jha K. K., Ozer H. L. Retinoblastoma protein and simian virus 40-dependent immortalization of human fibroblasts. J Virol. 1991 Jun;65(6):2845–2852. doi: 10.1128/jvi.65.6.2845-2852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Seto E., Usheva A., Zambetti G. P., Momand J., Horikoshi N., Weinmann R., Levine A. J., Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. L., Ozer H. L. Temperature-sensitive mutants of Balb/3T3 cells: description of a mutant affected in cellular and polyoma virus DNA synthesis. Cell. 1976 Feb;7(2):289–295. doi: 10.1016/0092-8674(76)90028-3. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stürzbecher H. W., Chumakov P., Welch W. J., Jenkins J. R. Mutant p53 proteins bind hsp 72/73 cellular heat shock-related proteins in SV40-transformed monkey cells. Oncogene. 1987 May;1(2):201–211. [PubMed] [Google Scholar]
- Tevethia M. J., Pipas J. M., Kierstead T., Cole C. Requirements for immortalization of primary mouse embryo fibroblasts probed with mutants bearing deletions in the 3' end of SV40 gene A. Virology. 1988 Jan;162(1):76–89. doi: 10.1016/0042-6822(88)90396-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wiebel F. F., Kunau W. H. The Pas2 protein essential for peroxisome biogenesis is related to ubiquitin-conjugating enzymes. Nature. 1992 Sep 3;359(6390):73–76. doi: 10.1038/359073a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Gannon J. V., Lane D. P. Monoclonal antibody analysis of p53 expression in normal and transformed cells. J Virol. 1986 Aug;59(2):444–452. doi: 10.1128/jvi.59.2.444-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacksenhaus E., Sheinin R. Molecular cloning, primary structure and expression of the human X linked A1S9 gene cDNA which complements the ts A1S9 mouse L cell defect in DNA replication. EMBO J. 1990 Sep;9(9):2923–2929. doi: 10.1002/j.1460-2075.1990.tb07483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G. C., Donegan J., Ozer H. L., Hand R. Characterization of a ts mutant of BALB/3T3 cells and correction of the defect by in vitro addition of extracts from wild-type cells. Mol Cell Biol. 1984 Sep;4(9):1815–1822. doi: 10.1128/mcb.4.9.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. Y., Abate M., Rice P. W., Cole C. N. The ability of simian virus 40 large T antigen to immortalize primary mouse embryo fibroblasts cosegregates with its ability to bind to p53. J Virol. 1991 Dec;65(12):6872–6880. doi: 10.1128/jvi.65.12.6872-6880.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]