A transcription factor connects sugar, abscisic acid, and GA pathways through glucose levels and signaling.
Abstract
Asr (for ABA, stress, ripening) genes are exclusively found in the genomes of higher plants, and the encoded proteins have been found localized both to the nucleus and cytoplasm. However, before the mechanisms underlying the activity of ASR proteins can be determined, the role of these proteins in planta should be deciphered. Results from this study suggest that ASR is positioned within the signaling cascade of interactions among glucose, abscisic acid, and gibberellins. Tobacco (Nicotiana tabacum) transgenic lines with reduced levels of ASR protein showed impaired glucose metabolism and altered abscisic acid and gibberellin levels. These changes were associated with dwarfism, reduced carbon dioxide assimilation, and accelerated leaf senescence as a consequence of a fine regulation exerted by ASR to the glucose metabolism. This regulation resulted in an impact on glucose signaling mediated by Hexokinase1 and Snf1-related kinase, which would subsequently have been responsible for photosynthesis, leaf senescence, and hormone level alterations. It thus can be postulated that ASR is not only involved in the control of hexose uptake in heterotrophic organs, as we have previously reported, but also in the control of carbon fixation by the leaves mediated by a similar mechanism.
Sugars supply necessary carbon and energy for the vast majority of metabolic processes in plants. Moreover, they act as signaling molecules in a wide number of physiological processes, such as growth, development, and senescence (Rolland et al., 2006; Baena-González and Sheen, 2008; Hanson and Smeekens, 2009; Eveland and Jackson, 2012). To date, the most studied sugar signaling factors include HEXOKINASE1, SNF1-RELATED KINASE (SnRK1), TOR KINASE, and C/S1 bZip factors (Baena-González and Sheen, 2008; Hanson and Smeekens, 2009; Eveland and Jackson, 2012). Sugar signaling pathways interact extensively with hormone pathways, and these interactions are partly responsible for the control of basic physiological processes (Rolland et al., 2006; Eveland and Jackson, 2012). Studying the nature of these interactions and the mechanisms of control involved in them will lead to a better understanding of these physiological processes. In this vein, awareness of the cross talk between sugars and hormones has arisen from studies with mutants of the hormonal pathways and/or sugar responses. For instance, a key link between sugars and abscisic acid (ABA) is exemplified by ABA INSENSITIVE4, which encodes an Apetala2 domain-containing transcription factor that binds a COUPLING ELEMENT1-like element present in many ABA and sugar-regulated promoters and is required in the Glc-mediated developmental arrest during vegetative morphogenesis (Arenas-Huertero et al., 2000; Dekkers et al., 2008). Despite this, there is relatively scarce information available on transcription factors regulating sugar-hormone cross talk.
The Asr (for ABA, stress, ripening) gene family is widely distributed in higher plants (Carrari et al., 2004; Frankel et al., 2006; Fischer et al., 2011) and constitutes group 7 of LATE EMBRYOGENESIS ABUNDANT proteins (Battaglia et al., 2008). It has been demonstrated that the ASR1 protein is present in the nucleus (Çakir et al., 2003; Wang et al., 2003; Kalifa et al., 2004a) and possesses a zinc-dependent DNA-binding activity (Kalifa et al., 2004a; Rom et al., 2006). In addition, fractionation assays and functional analyses suggest that the protein also localizes to the cytosol, where it has chaperone-like activity (Kalifa et al., 2004a; Konrad and Bar-Zvi, 2008; Urtasun et al., 2010; Dai et al., 2011; Hsu et al., 2011; Ricardi et al., 2012). Although the involvement of Asr genes in various abiotic and biotic stresses has been described (Amitai-Zeigersona et al., 1995; Jeanneau et al., 2002; Kalifa et al., 2004b; Yang et al., 2005; Liu et al., 2010; Dai et al., 2011; Henry et al., 2011; Hsu et al., 2011; Virlouvet et al., 2011; Jha et al., 2012; Arenhart et al., 2013), the exact molecular mechanism underlying their function remains unknown. Nevertheless, it has been demonstrated that the grape (Vitis vinifera) ASR VvMSA (for MATURATION-, STRESS-, ABA-INDUCED PROTEIN) protein interacts with a DROUGHT RESPONSE ELEMENT BINDING transcription factor in the nucleus (Saumonneau et al., 2008).
Several Asr orthologous and paralogous genes have been found to be transcriptionally regulated by ABA and sugars (Amitai-Zeigersona et al., 1995; Rossi et al., 1998; Çakir et al., 2003; Kalifa et al., 2004a; Liu et al., 2010; Chen et al., 2011; Henry et al., 2011; Virlouvet et al., 2011). Furthermore, a model for VvMSA transcriptional regulation at the convergence of Glc and ABA signaling cascades through HEXOKINASE1 and SnRK1 has recently been proposed (Saumonneau et al., 2012). Although the physiological role of Asr genes has proven elusive, VvMsa has been clearly demonstrated to recognize specific binding sites in the promoter/enhancer region of the hexose transporter VvHt1 (Çakir et al., 2003). In tubers of transgenic potato (Solanum tuberosum) plants, the levels of expression of Asr1 negatively correlated with tuber Glc content and mRNA levels of the hexose transporter Ht2 (Frankel et al., 2007). Recently, it has been reported that the overexpression of ASR1 in maize (Zea mays) has a large impact on vegetative biomass and that this phenotype correlates with changes in the branched-chain amino acid biosynthesis (Virlouvet et al., 2011). The molecular studies mentioned above suggest that ASR1 could play a regulatory role in the cross talk between primary carbon metabolism and hormone signaling. To gain insight into the physiological role of ASR1, we generated and comprehensively evaluated transgenic tobacco (Nicotiana tabacum) plants with altered expression levels of Asr1. Our results indicate that ASR1 regulates leaf Glc levels and carbon partitioning. Moreover, both hormone levels and sensitivity analyses suggest that this protein is not only involved in Glc signaling but also in Glc-ABA and Glc-GA cross talk in photosynthetically active leaves. These combined data are discussed in the context of current models of sugar and hormone sensing and their influence on whole-plant carbon partitioning.
RESULTS
Generation and Selection of Tobacco Transgenic Lines
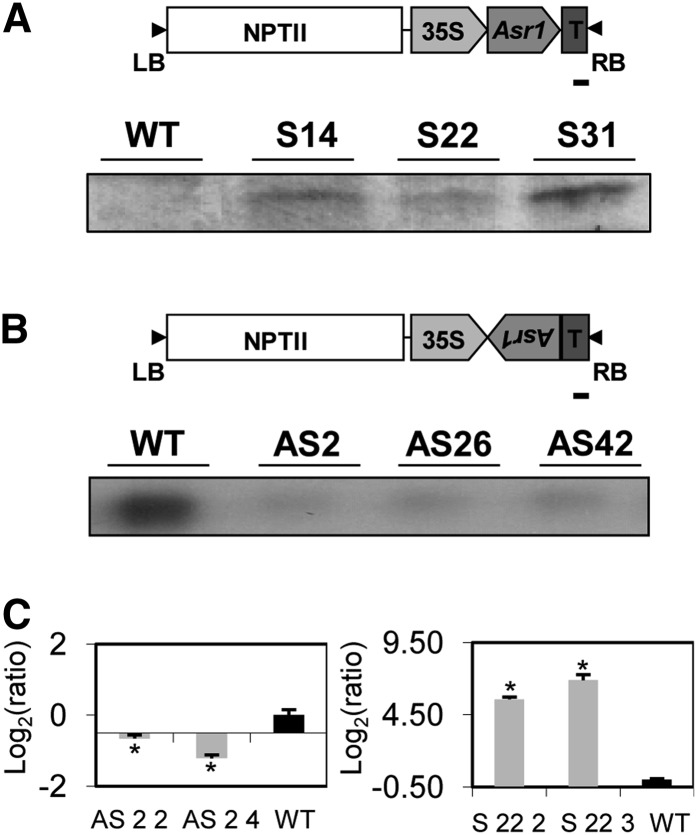
Fifty and thirty-four independent shoots of T0 antisense and sense lines, respectively, were obtained after selection in kanamycin-containing medium and transferred to the greenhouse. These lines were screened on the basis of the levels of Asr1 expression by means of northern blot (data not shown). For further analyses, three lines from each construct were selected (namely, overexpressors S14, S22, and S31 and silenced lines AS2, AS26, and AS42) and propagated in vitro in order to obtain six plants per line, which were subsequently analyzed by western (Fig. 1A) or northern (Fig. 1B) blots of fully expanded leaves. Progeny tests of the six transgenic lines showed segregation ratios of 3:1 (resistance:susceptibility to kanamycin), suggesting a single-copy insertion of the transgene. In addition, two transgenic lines (one overexpressor, S 22, and one silenced, AS 2) were selfed to T4 homozygous generation; two siblings per line in T1 generation were selected through progeny tests for further experiments in order to avoid possible maternal effects. Thus, siblings S 22 2 and S 22 3 derive from S 22, and siblings AS 2 2 and AS 2 4 derive from AS 2. When these lines were analyzed by quantitative real-time PCR (qRT-PCR), significant reductions and increments in Asr1 mRNA levels were confirmed in fully expanded leaves of antisense and sense plants, respectively, compared with wild-type controls (Fig. 1C).
Figure 1.
Asr1 expression in tobacco transgenic lines. Western- (A) and northern-blot (B) analyses of three overexpressing (S) and three silenced (AS) transgenic lines (T0). Schemes of the constructs used for plant transformation are shown in the top side of each figure. Bars = 100 bp. C, qRT-PCR expression analyses in source leaves from T4 homozygous lines (left, antisense plants; right, overexpressing plants). Asterisks indicate statistically significant differences by the permutation test (P < 0.05). n = 3 to 5. WT, Wild type.
Phenotypic Characterization of Asr1 Transgenic Lines
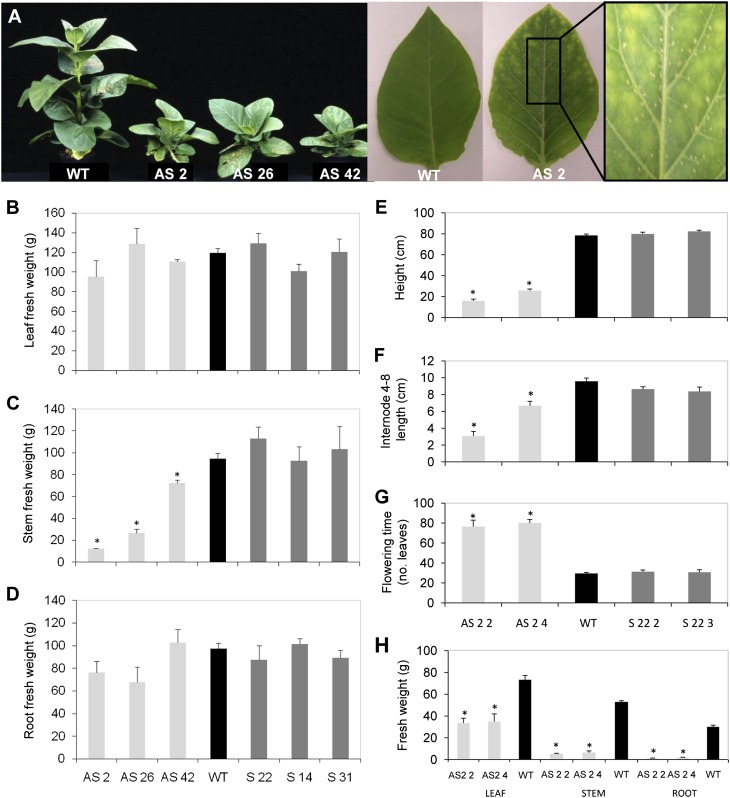
Selected transgenic lines were grown alongside wild-type controls and characterized in detail phenotypically. The three silenced lines showed a severe stunted phenotype (Fig. 2A), which was already evident at plantlet stage (data not shown). This phenotype is reflected in a significant decrease in stem fresh matter accumulation after 6 weeks of growth (Fig. 2C). In homozygous silenced lines, average internode lengths were shorter; consequently, plant height and stem matter accumulation were again significantly reduced (Fig. 2, E, F, and H). Also, these plants showed a dramatic reduction in fresh matter accumulation in leaves and roots (Fig. 2H), which was not observed in T0 silenced lines (Fig. 2, B and D), and an extended flowering time (Fig. 2G). By contrast, overexpressing lines showed no differences in any of the measured traits (Fig. 2).
Figure 2.
Phenotypic characterization of the Asr1 transgenic lines. A, Visual phenotype of 6-week-old plants of three independent T0 silenced transgenic lines (left). Expanded seventh leaves from a wild-type (WT) plant and a T4 homozygous silenced plant (AS 2 4; right). Fresh matter accumulated in leaves (B), stems (C), and roots (D) of independent T0 transgenic lines. Phenotypic characterization of T4 homozygous lines: plant height (E), internode 4 to 8 length (F), flowering time measured as the number of total leaves of the plant at the time of the setting of flower buds (G), and matter accumulation in leaves, stems, and roots (H). Data represent the means ± se of measurements from five to eight plants per line. Asterisks indicate statistically significant differences by Student’s t test (P < 0.05). AS, Silenced plants; S, overexpressing plants. [See online article for color version of this figure.]
Fully expanded leaves from silenced lines were also characterized by chlorotic and necrotic spots that appeared early during leaf expansion (Fig. 2A). When they were assayed for the oxidation of diaminobenzidine (DAB) by the hydrogen peroxide (H2O2) content of the cells, the typical insoluble brownish precipitate was stronger in silenced lines (Supplemental Fig. S1). Since this phenotype could be related to leaf senescence, ASR1 protein levels were assessed by western blot in leaves of wild-type plants. Older leaves, whose senescence is more advanced, accumulated higher levels of the ASR1 protein (data not shown).
Photosynthetic Parameters Are Altered in Asr1-Silenced Lines
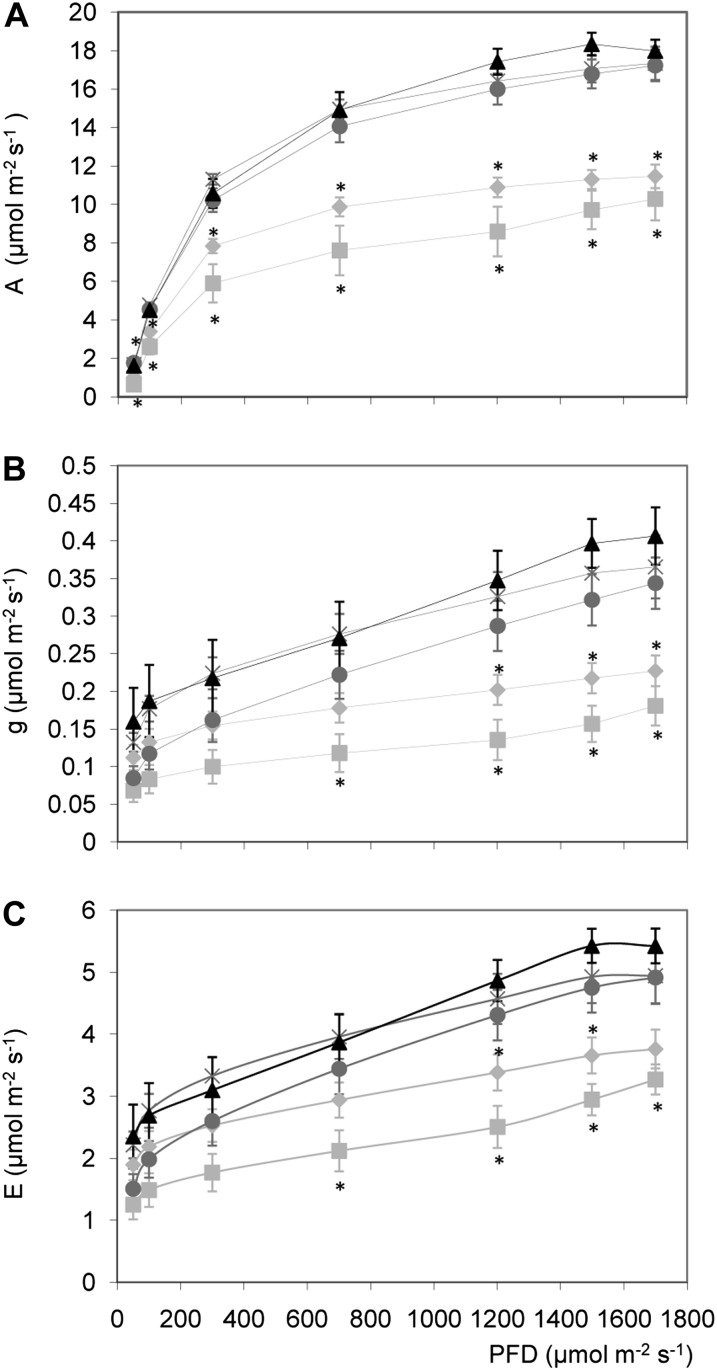
We reasoned that the dramatic reduction in fresh matter accumulation along with the early chlorosis in expanded leaves observed in the silenced Asr1 lines (Fig. 2) could be related to a reduction in CO2 assimilation. Indeed, this parameter was significantly reduced in homozygous silenced plants at all tested photosynthetically active radiations (PARs; Fig. 3A). However, transpiration rate and leaf conductance were reduced only at high irradiances (Fig. 3, B and C). These results correlated with weaker intensities of the bands corresponding to the large and small subunits of Rubisco in leaves from these plants, which also seem to have a different protein pattern (Supplemental Fig. S2). On the other hand, homozygous overexpressing lines were invariant compared with wild-type plants in all measured parameters (Fig. 3).
Figure 3.
Effect of altered Asr1 expression on photosynthetic parameters of T4 plants. A, CO2 assimilation rate or A; B, stomatal conductance or G; and C, transpiration rate or E, measured at the indicated photon flux density (PFD) in fully expanded leaves of T4 plants at vegetative stage. Light-gray squares, AS 2 2; light-gray rhombuses, AS 2 4; black triangles, wild type; dark-gray circles, S 22 2; dark-gray crosses, S 22 3. The data represent the mean ± se of measurements from four to six plants per line. Asterisks indicate statistically significant differences by Student’s t test (P < 0.05).
Changes in Leaf Carbohydrate Contents
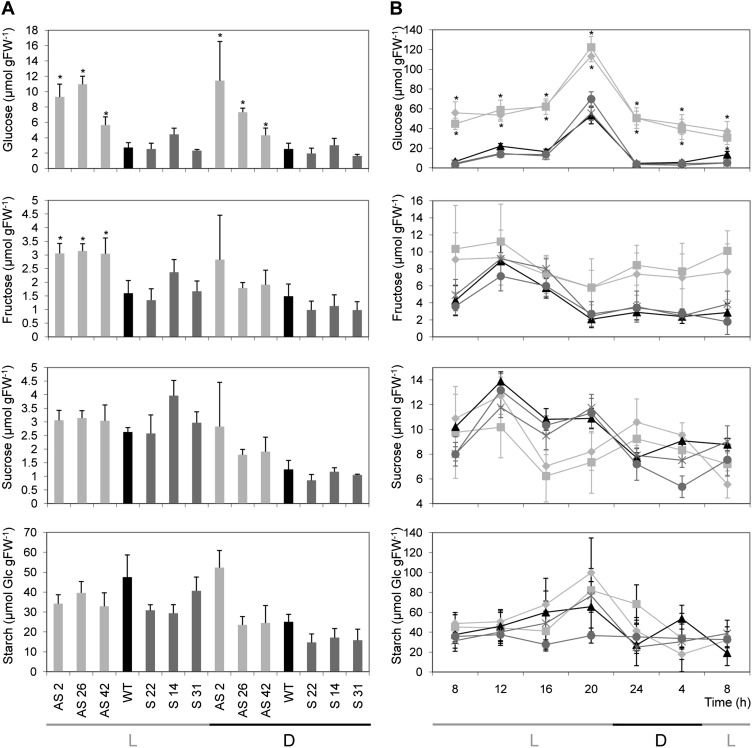
Since Asr1-silenced plants showed a reduction in CO2 assimilation and Asr genes have been related to carbon transport and metabolism in sink tissues, sugar contents were measured in leaves and phloem sap. Silenced plants showed significantly higher levels of leaf Glc during the middle of the light (1 pm) and dark (5 am) periods, while Fru was only increased during the light period (Fig. 4A). In the experiment performed with homozygous lines, the diurnal curve of leaf sugars showed that silenced plant leaves accumulated between 3 and 10 times more Glc than wild-type and overexpressing lines (Fig. 4B). However, no other variations in sugar contents were observed either in the silenced or overexpressing lines (Fig. 4, A and B).
Figure 4.
Leaf carbohydrate contents in Asr1 transgenic lines. A, Carbohydrate contents in source leaves of T0 plants at a light time point (1 pm) and a dark time point (5 am). B, Diurnal changes in carbohydrate contents in leaves from T4 homozygous plants. At each time point, samples were taken from mature source leaves and data represent the means ± se of measurements from four to six plants per line. L, Light period; D, dark period; FW, fresh weight. Light-gray squares, AS 2 2; light-gray rhombuses, AS 2 4; black triangles, wild type (WT); dark-gray circles, S 22 2; dark-gray crosses, S 22 3. Asterisks indicate statistically significant differences relative to wild-type controls by Student’s t test (P < 0.05).
A significant reduction in Suc level was found in the phloem sap of homozygous silenced plants (Supplemental Fig. S3). Remarkably, a strong deterioration was observed in leaves of silenced plants after the 17 h ex vivo, whereas wild-type and overexpressing leaves exhibited no visible changes (Supplemental Fig. S3). On the other hand, a decrease of Suc was detected in the phloem sap of the S 22 3-overexpressing line. Hexoses were detected in phloem sap (data not shown). However, this would suggest that there was some contamination from the vacuolar compartment of compromised, non-phloem cells during sampling, as detailed by Turgeon and Wolf (2009) and Liu et al. (2012), since tobaccos are Suc apoplasmic loaders (von Schaewen et al., 1990; Sonnewald et al., 1991).
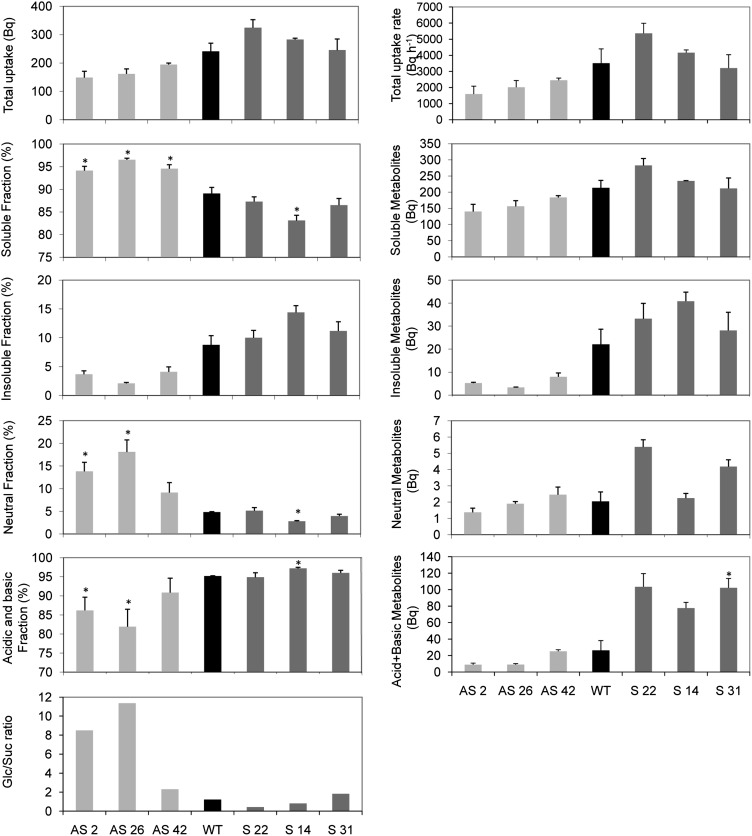
[U-14C]Glc Assimilation in Leaves
In order to study whether Glc assimilation in leaves could be affected by Asr1, we incubated leaf discs of transgenics and wild-type plants with [14C]Glc in the dark (Fig. 5). The total [14C]Glc uptake was invariant in silenced and overexpressing lines (Fig. 5). However, distribution of radioactivity between soluble and insoluble metabolite fractions differed significantly after 225 min of incubation (and also after 60 and 120 min; data not shown), with percentages of soluble 14C being significantly higher in discs from silenced leaves (Fig. 5). The insoluble fraction, which is mainly composed of starch, membrane components, and proteins, revealed a minor, albeit nonsignificant, decrease in the silenced plants (Fig. 5). In the overexpressing lines, the opposite effects were observed (Fig. 5). Within the soluble fraction, the neutral fraction (mostly soluble sugars) was also significantly increased in silenced plants (Fig. 5). Moreover, the combined acid and basic fractions, which together are composed by major components of the tricarboxylic acid cycle, were decreased in the three silenced plants compared with wild-type controls (Fig. 5). In addition, neutral fractions were separated in thin-layer chromatography (TLC) plates alongside authentic radioactive sugar standards. This analysis revealed up to 11-fold increases in Glc-to-Suc ratios in silenced plants, whereas these ratios were unaltered or marginally reduced in the overexpressing lines (Fig. 5). When taken together, these results thus suggest that Glc metabolism is impaired in silenced plants.
Figure 5.
Glc assimilation in Asr1 transgenic leaves (T0). Leaf discs were cut from 6-week-old plants at the onset of the light period and incubated with [U-14C]Glc. Soluble and insoluble fractions were measured in a scintillation counter. Soluble fractions were subsequently separated by ion-exchange chromatography. Total [U-14C]Glc uptake and soluble, insoluble, neutral, acid, and basic fractions are shown on the left. Total uptake rate, absolute soluble, insoluble, neutral, acid, and basic metabolites are shown on the right. Sugars from neutral fractions were separated on TLC plates, and Glc-to-Suc ratios are presented at bottom left. Data represent the mean ± se of measurements from three plants per line. Asterisks indicate statistically significant differences by Student’s t test (P < 0.05). WT, Wild type.
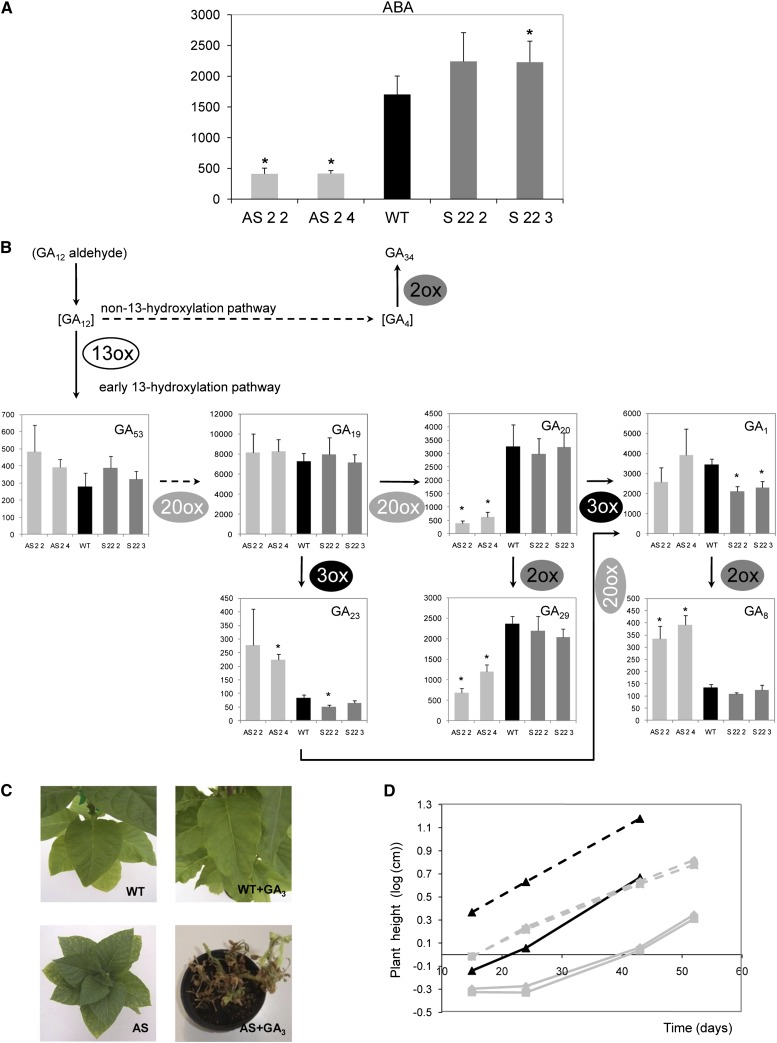
ABA and GA Metabolisms Are Altered in Asr1 Plants
Regulation of the Asr gene family at the mRNA levels by ABA is well documented in tomato (Solanum lycopersicum; Amitai-Zeigersona et al., 1995; Rossi et al., 1998), grape (Çakir et al., 2003), and maize (Virlouvet et al., 2011). It is also known that Glc modulates genes involved in ABA biosynthesis and signaling (Rolland et al., 2006). Therefore, we next measured ABA contents by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) in source leaves. In homozygous silenced plants, ABA levels were significantly reduced (more than or equal to 3 times; Fig. 6A). Conversely, overexpressing plants showed a marginal increase in the levels of this hormone; however, this was only statistically significant in line S 22 3 (Fig. 6A).
Figure 6.
ABA and GA metabolism in Asr1 tobacco plants and GA3 sensitivity of Asr1-silenced plants. ABA (A) and GA (B) levels were measured by LC-MS/MS in source leaves from 6-week-old plants. ABA and GA data are expressed in ng g dry weight−1 and pg g dry weight−1, respectively, and represent the means ± se of measurements from five independent plants per line. Asterisks indicate statistically significant differences compared with wild-type (WT) controls by Student’s t test (P < 0.05). Species in brackets were not detected. GA34 was convincingly detected, but levels were below the reliable level of quantification. Reconstruction of GA pathway was done based by Yamaguchi (2008) and MacMillan (1997). Continuous and dashed lines indicate single and multiple reactions, respectively. 13ox, 13-Oxidase; 20ox, 20-oxidase; 3ox, 3-oxidase; 2ox, 2-oxidase. Exogenous GA3 (10 μm) was applied to young seedling as described in “Materials and Methods.” Pictures of GA3-treated (right) and untreated (left) silenced transgenics (T4) and control plants (C). Plant height along growth (D). Gray squares, AS 2 2; gray triangles, AS 2 4; black triangles, wild type; continuous lines, untreated plants; dashed lines, treated plants. [See online article for color version of this figure.]
We also reasoned that the stunted phenotype of the silenced lines could be due to altered GA levels and decided to quantify these hormones in expanded leaves. Out of the 20 GA standards (GA8/29/23/3/1/6/5/19/13/20/44/34/53/51/7/4/24/15/9/12) used, only GA8/29/23/1/19/20/53 could be reliably identified and quantified in our samples (Fig. 6B). GA34 was convincingly detected, but the level was below the reliable level of quantification. A reconstruction of the most probable pathway of GA metabolism in tobacco leaves was carried out based on the pathways published by Yamaguchi (2008) and MacMillan (1997) (Fig. 6B). GA1 was the only growth-active GA that could be detected and quantified (Fig. 6B). Surprisingly, silenced homozygous plants did not show alterations in the levels of this GA (Fig. 6B). However, both its immediate precursor GA20 and its degradation product GA8 turned out to be significantly reduced and increased, respectively, in silenced plants (Fig. 6B). GA29, which derives from GA20, is also reduced in silenced plants (Fig. 6B). In contrast, overexpressing plants showed a marginal, though significant, decrease in GA1 (Fig. 6B). Except for GA23, which showed increased and decreased levels in the antisense line AS 2 4 and in the overexpressing line S 22 2, respectively, there were no changes in the levels of other active GA precursors (GA53 and GA19; Fig. 6B). For a more comprehensive analysis, product-to-substrate ratio for each oxidation step was calculated. Silenced lines exhibited significantly different values; while C20 oxidation steps GA19>GA20 and GA23>GA1 (catalyzed by the GA 20-oxidase) were lower than in wild-type controls, the C3 oxidation reactions GA20>GA1 and GA19>GA23 (catalyzed by the GA 3-oxidase) displayed 5-fold increased ratios (Supplemental Table S1). These results suggest that both GA 20-oxidase and GA 3-oxidase activities are altered in silenced plants.
Sensitivity to GA3
Given that GA1, the only growth-active GA that was detected, is unaltered in the stunted silenced plants and that the levels of ASR1 protein were increased in leaves of wild-type plants following a GA3 treatment (data not shown), we decided to investigate if the shorter internode lengths of the silenced plants were a consequence of an alteration in GA sensitivity.
For this purpose, the fold change in stem growth curve was used as a measure of the response to GA3. Although clear phenotypic differences were observed after 53 d of GA3 treatment between the silenced and wild-type plants (Fig. 6C), slopes of GA3-treated silenced plants were similar to those of the wild-type controls, thus suggesting similar extent of GA3 sensitivity between the genotypes (Fig. 6D).
Soluble sugars of treated wild-type plants were altered in comparison to untreated wild-type plants (Supplemental Table S2). However, silenced lines did not show any difference between treatments (Supplemental Table S2). Interestingly, similar results were observed for biomass accumulation: silenced treated plants showing no differences but wild-type plants accumulating higher levels of biomass (Supplemental Table S2). In addition, GA3 caused a delay in flowering time of the wild-type plants (Supplemental Table S2). However, in the silenced treated plants, this trait could not be investigated due to death of the treated plants before flowering.
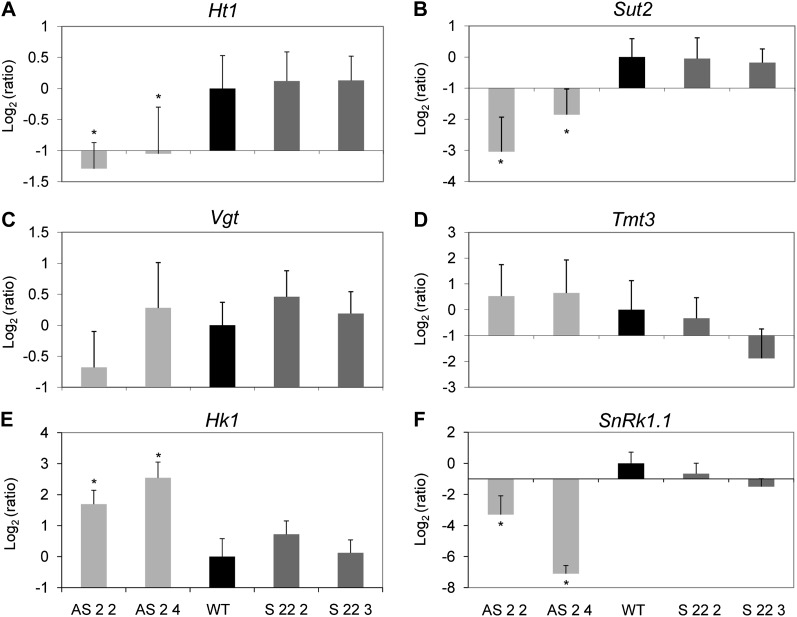
Asr1 Cross Talks at Sugar Transport and Signaling by Regulating mRNA Levels
It has been described that a grape ASR protein binds to conserved motifs in the promoter/enhancer region of the VvHt1 monosaccharide transporter (Çakir et al., 2003). In order to test a possible connection between Asr1 and sugar transporters in tobacco, we evaluated the mRNA levels of four putative sugar transporters, namely, Ht1 (ortholog to VvHt1), Sut2 (for Suc transporter2; Meyer et al., 2000; Hackel et al., 2006), Tmt3 (for Tonoplast monosaccharide transporter3; Wormit et al., 2006), and Vgt (for Vacuolar Glc transporter, although it seems to be localized to the chloroplast membrane; Büttner, 2007). Silenced homozygous plants showed lower mRNA levels of Ht1 and Sut2 than wild-type plants (Fig. 7, A and B). However, no differences were observed in the mRNA levels of Tmt3 and Vgt in these plants (Fig. 7, C and D). To gain further insight into the Glc signaling pathway, we also measured the mRNA levels of Hexokinase1 and SnRk1.1 (the catalytic subunit of SnRK1), two key genes in the Glc signaling pathway. Silenced homozygous plants had higher mRNA levels of Hexokinase1 and lower mRNA levels of SnRk1.1 (Fig. 7, E and F). By contrast, overexpressing plants were invariant with regard to the mRNA levels of any of these genes (Fig. 7).
Figure 7.
Expression analyses of sugar sensors and transporters in Asr1 tobacco lines. mRNA levels of sugar transporters Ht1 (A), Sut2 (B), Vgt (C), and Tmt3 (D) and sugar sensors Hexokinase1 (E) and SnRk1.1 (F) were quantified by qRT-PCR in leaves of T4 lines relative to wild-type (WT) plants. Asterisks indicate statistically significant differences by the permutation test (P < 0.05). n = 3 to 5. Ratio = (Etarget)ΔCp target(MEAN control – MEAN sample) ((Etarget)ΔCp target(MEAN control – MEAN sample))–1 (Pfaffl 2001).
DISCUSSION
Silencing Asr1 in tobacco resulted in dramatic alterations in all measured physiological parameters. By contrast, Asr1 overexpression did not generate significant changes under controlled growth conditions. Previous studies have shown that the grape ASR ortholog, VvMSA, regulates the expression of the hexose transporter ht1 gene (Çakir et al., 2003). Interestingly, the Asr1 transcript is expressed in the companion cells of the phloem of tomato leaves (Maskin et al., 2008). Likewise, ht1 is expressed in the phloem region of vascular bundles of grape leaves, petioles, and fruits (Vignault et al., 2005). Intriguingly, Asr1 silencing led to reduced mRNA levels of the tobacco ht1 ortholog and high leaf Glc levels (Figs. 4 and 7A). We also found that Glc metabolism is impaired in the silenced plants (Fig. 5). According to the proposed role of transporters such as HT1 (Lalonde et al., 2003; Vignault et al., 2005; Slewinski, 2011), the decreased expression of ht1 could explain the increased levels in Glc, since a failure of phloem cells to retrieve hexoses would result in sugar accumulation in the apoplast. It is conceivable that Glc could spread to other cell types, such as parenchyma and mesophyll cells. This hypothesis is in agreement with the phenotypic alterations observed in silenced plants, which are associated with high levels of Glc in mesophyll cells (see below). Expression of the Sut2 gene was also downregulated in silenced plants (Fig. 7B). Sut2 is a Suc transporter localized to the sieve elements of the phloem whose expression is regulated by Suc and whose function in leaf tissues has not been elucidated (Barker et al., 2000; Meyer et al., 2004; Hackel et al., 2006; Kühn, 2011). Sut2 down-regulation could be due to a direct regulation by ASR1, considering that proteins of sieve elements are synthesized in companion cells (Liesche et al., 2011). Even though carbon assimilation is lower in silenced plants, total leaf Suc levels are unaltered (Figs. 3 and 4). Hence, the low levels of Suc in phloem sap could be explained by the decrease in Suc loading into the phloem in order to maintain normal leaf Suc levels.
The effect of Glc as signal molecule is partly mediated by HEXOKINASE1, which among other functions, is involved in the inhibition of photosynthetic gene transcription (Moore et al., 2003) and the development of senescence (Dai et al., 1999). Another pivotal protein in sugar sensing and signal transduction is SnRK1, a global regulator of plant metabolism that controls energy-conserving processes and the remobilization of alternative energy sources (Ghillebert et al., 2011). SnRK1 regulation seems to rely on the overall energy status of the cell, independently of specific sugar molecules, and both Suc and Glc have a repressive effect on SnRK1 target genes (Baena-González and Sheen, 2008). It seems reasonable to assume that SnRK1 and HEXOKINASE1 pathways are connected. However, precise details concerning the nature of this connection are presently unknown (Cho et al., 2012).
Extensive interactions between Glc and hormone pathways have been reported (Rolland et al., 2006; Eveland and Jackson, 2012). However, the knowledge of the mechanisms underlying these interactions remains incomplete. It is known that in some cases, HEXOKINASE1 regulates the response to certain hormones, such as cytokinins and auxins (Moore et al., 2003). Our experiments showed that silenced plants have decreased ABA levels and high Glc (Figs. 4 and 6A), which is unexpected considering the literature to date (Rolland et al., 2006; Eveland and Jackson, 2012). However, most of the studies showing the effect of Glc on ABA have been conducted with seedlings rather than with adult plants. Our results suggest that ABA catabolism could be enhanced in the leaves of the silenced plants; the higher Glc and Hexokinase1 transcript levels (Figs. 4 and 7E) might result in ABA glycosylation generating inactive ABA-glucosyl esters (Xu et al., 2002; Bolouri-Moghaddam et al., 2010).
GA metabolic pathway also considerably differed in these lines (Fig. 6B); ratios of a given GA and its precursor were altered (Supplemental Table S1), probably as a consequence of changes in the activities of GA 20- and GA 3-oxidases. Also, treatment of wild-type plants with GA3 increases and/or stabilizes the level of ASR1 protein (data not shown). This is in agreement with the increased transcript levels of rice (Oryza sativa) ASR5 occurring upon treatment with GA3 (Takasaki et al., 2008). There is a precedent that interactions between GAs and sugars occur in the leaves, as it has been described that GAs regulate photosynthesis and source-sink relationships via the regulation of SUC PHOSPHATE SYNTHASE and invertases (Biemelt et al., 2004; Huerta et al., 2008; Iqbal et al., 2011). As for other hormones, the regulation of the bioactivity of GAs can also be achieved by the glycosylation of its species (Bolouri-Moghaddam et al., 2010). Regulation of ASR1 by ABA, GA, and sugars thereby provides a novel link between hormonal and sugar pathways.
A remarkable aspect of the phenotype of the silenced plants is the occurrence of necrosis and chlorosis of the leaves (Fig. 2A). Along with the decrease in CO2 assimilation (Fig. 3A), the degradation of the large and the small subunits of Rubisco (Supplemental Fig. S3), the accelerated deterioration of the leaves after a period of incubation (Supplemental Fig. S2), and the increased reactive oxygen species (ROS) levels in leaves (Supplemental Fig. S1) indicate that these plants undergo a process of accelerated senescence (Yoshida et al., 2002; Rolland et al., 2006; Gregersen et al., 2008; Breeze et al., 2011). It has been proposed that developmental senescence is led by increasing levels of Glc in mesophyll cells and that the sugar signaling during senescence is dependent on HEXOKINASE1 (Dai et al., 1999; Rolland et al., 2006; Slewinski, 2011; Wingler et al., 2012). It is thus conceivable that the increments in Glc and Hexokinase1 transcript levels in silenced plants (Figs. 4 and 7E) could trigger leaf senescence. Furthermore, high Glc levels inhibit photosynthesis by down-regulating transcription of photosynthetic genes through HEXOKINASE1 (Sheen, 1990; Moore et al., 2003). In turn, photosynthesis inhibition can also lead to senescence (Dai et al., 1999). Moreover, silenced plants also show high levels of H2O2, a ROS (Supplemental Fig. S1). This molecule could also contribute to the establishment of senescence, since ROS can act as signaling molecules that activate senescence genes (Jongebloed et al., 2004; Bolouri-Moghaddam et al., 2010). Additionally, it has been proposed that a drastic overshoot of the ROS levels can serve as a trigger of senescence after the release of HEXOKINASE1 from the mitochondrion (or the chloroplast) membrane (Bolouri-Moghaddam et al., 2010). SnRK1 activity is also involved in the development of senescence. Strong evidence suggests that low SnRK1 activity accelerates senescence and the opposite occurs with high SnRK1 activity at different stages of plant development (Rolland et al., 2006; Cho et al., 2012; Wingler et al., 2012). Silenced tobacco plants displayed decreased SnRk1.1 mRNA levels (Fig. 7F), a feature that could be influencing the early onset of senescence. In brief, our results suggest that the altered levels of Glc, Hexokinase1, SnRK1.1, and ROS all contribute to the early onset of senescence observed in the silenced plants.
At the hormonal level, ABA is not necessary for the induction of senescence associated with sugars (Pourtau et al., 2004). GAs, on the other hand, delay senescence (Gan, 2010). Although the silenced plants revealed no significant differences in GA1 content (Fig. 6B), we cannot exclude that other GA species and/or other hormones may be influencing the observed phenotype. The role of ASR in senescence is also supported by the fact that levels of this protein increase as leaf age progresses (data not shown) and by the suggestion of the involvement of the maize Asr1 ortholog in stress-induced senescence (Jeanneau et al., 2002). Surprisingly, GA3 treatment provoked a remarkable deterioration in the silenced plants (Fig. 6C). This is opposite to the known effects of GAs on plant senescence (Gan, 2010). Therefore, we suppose that the observed phenotype is likely the result of a complex interaction of changes caused by the lack of ASR1 and the treatment with GA3.
In addition to accelerated senescence, silenced plants showed other phenotypic changes: dwarfism, delayed flowering, and reduced biomass (Fig. 2). These traits are complex and likely regulated at different levels. Some of the most likely causes of these changes are the lower rates of photosynthesis (Fig. 3) and the concomitant reduction in Suc export, which implies a decrease in carbon flow to sink organs (Supplemental Fig. S2), and the hormonal alterations (Fig. 6) probably caused by the increased Glc levels in the leaves. Additionally, there could be a change in the uptake of sugars by sink organs. Regarding the latter, although ASR1 is probably not directly involved in phloem unloading, as this process is symplasmic in developing tobacco leaves (Turgeon, 1987; Ding et al., 1988), it may play a role via HT1 in retrieval of the hexoses as was mentioned above. Interestingly, when sink activity decreases, carbohydrates accumulate in leaves and photosynthesis becomes inhibited by feedback control mechanisms (Paul and Foyer, 2001; Lalonde et al., 2003; Ainsworth and Bush, 2011). As such, we cannot formally exclude that both events (hexose leakage in source and sink tissues) are taking place simultaneously. To test this hypothesis, experiments with Asr1-silenced tomato plants, whose source-sink relationships are stronger, are being performed.
Dwarfism is a typical phenotype associated with a deficit of active GAs. Although there were no changes in the levels of GA1, the reduction in GA20 in mature leaves of silenced plants could potentially explain its phenotype (Fig. 6B), since it has recently been proposed that maturing leaves of tobacco shoots produce GA1 or GA20 as signal molecules necessary to stimulate the growth of stem cells (Dayan et al., 2012). On the other hand, silenced plants were able to elongate the internodes in response to GA3, confirming that GA3 sensitivity was not modified (Fig. 6D). It is noteworthy that our observations are in agreement with a previous work where manipulation of GA20 activity by means of GA 20-oxidase silencing in tobacco resulted in a dwarf phenotype and conserved sensitivity to GA3 (Biemelt et al., 2004). We also observed an increase in the biomass of wild-type plants upon GA3 treatment (Supplemental Table S2), suggesting that there is a change in the whole-plant CO2 assimilation, an effect previously described for GAs (Biemelt et al., 2004). However, silenced plants did not exhibit altered biomass (Supplemental Table S2). The observation that wild-type plants had lower levels of Suc and higher levels of Glc and Fru in the leaves (Supplemental Table S2) was probably due to the promotion of invertase activity by GA3, as was previously described (Iqbal et al., 2011). Silenced plants did not show either change in hexose or Suc levels (Supplemental Table S2), possibly owing to inhibition of invertase activity by the high Glc content.
Floral meristem development is controlled by sugar contents and their signals (Francis and Halford, 2006). There are many examples that describe that disruptions in hexose availability generate delays in flowering time (Slewinski, 2011; Eveland and Jackson, 2012). This suggests that flowering time in silenced plants may be delayed (Fig. 2) owing to impaired sugar availability. With regard to hormonal effects, GAs are known to be closely related to flowering. Particularly, in tobacco, it has previously been suggested that the active GAs in apical shoots are not imported from other organs but are locally synthesized (Gallego-Giraldo et al., 2007). Thus, it cannot be excluded that silenced plants display an altered local meristematic synthesis of GAs affecting flowering. However, we also cannot currently rule out that other hormonal changes influence the flowering phenotype of these transgenic lines.
CONCLUSION
Although a number of transcription factors have been positioned within the signaling cascade of interactions between sugars and hormones, most of these factors have been identified in Arabidopsis (Arabidopsis thaliana; for review, see Rolland et al., 2006). In this study, we provide compelling evidence that ASR1, a transcription factor not present in Arabidopsis (Carrari et al., 2004), connects sugar, ABA, and GAs. Results presented in this article, together with evidence showing that ASR proteins have transactivation activity in grape fruits (Çakir et al., 2003) and also function as a repressor of transcription of a plasma membrane hexose transporter in potato tubers (Frankel et al., 2007), lead us to propose a role for ASR1 in photosynthetic tissues. The fact that large phenotypic changes were notable in the silenced lines but not in the overexpressing lines suggests that ASR1 levels in wild-type plants are saturating with regard to their role in Glc metabolism. While further studies will clearly add more evidence about the exact mechanisms underlying these interactions, this study unequivocally associates ASR1 with the modulation of Glc levels in photosynthetic tissues through the regulation of Glc metabolism. This regulation also has an impact on Glc signaling probably through Hexokinase1 and SnRK1, which would subsequently affect photosynthesis, leaf senescence, and hormone levels.
MATERIALS AND METHODS
Generation of the Transgenic Lines
The 348-bp coding region of the tomato (Solanum lycopersicum) Asr1 gene (GenBank U86130.1) was cloned in sense or antisense orientation into the multiple cloning sites of the vector pBINAR (Liu et al., 1990) between the Cauliflower mosaic virus 35S promoter and the octopine synthase terminator. The constructs were transferred into tobacco (Nicotiana tabacum ‘Xanthi-nc’) cotyledons by an Agrobacterium tumefaciens-mediated protocol. Emerging shoots were excised and selected on Murashige and Skoog media containing kanamycin (200 mg/L). Once plants had taken root, they were transferred to soil in the greenhouse for their subsequent selection.
Selection of the Transgenic Lines
We screened 34 sense and 50 antisense T0 plants on the basis of Asr1 expression levels by northern blot as described by Frankel et al. (2007). Sense plants were further tested for ASR1 levels by western blot (for methodological details, see below). Finally, three overexpressing lines (S 22, S 14, and S 31) and three silenced lines (AS 2, AS 26, and AS 42) were selected, propagated, and transferred to the greenhouse for further characterization. Lines S 22 and AS 2 were taken to the T4 generation in order to select for homozygous seeds. Asr1 expression levels of these lines were checked by qRT-PCR. Total RNA was extracted by TRIzol (Invitrogen) according to the manufacturer’s specifications. RNA samples were treated with DNase I (Invitrogen). Complementary DNA was done using reverse transcriptase (M-MVL; Invitrogen), RNase inhibitor (RNAseOUT; Invitrogen), and random primers (Invitrogen) according to the manufacturer’s specifications. qRT-PCR was performed with a master mix (QuantiTect SYBR Green PCR kit; Qiagen) in a thermocycler (ABI 7500; Applied Biosystems). The primer sequences used for the detection of Asr1 levels were as follows: forward (FW) 5′-CCTGTTCCACCACAAGGACAA-3′ and reverse (RV) 5′-CGATTTGCTCGAGATGTTTATGG-3′. The reference gene was Elongation Factor1α (EF1α; as suggested in Schmidt and Delaney, 2010) and the primer sequences used for the detection of its levels were as follows: FW 5′-GATTGGTGGTATTGGTACTGTC-3′ and RV 5′-AGCTTCGTGGTGCATCTC -3′. Cycle threshold values and efficiencies values of PCRs were obtained with the LinRegPCR software (Ruijter et al., 2009). Ratios were obtained according to Pfaffl (2001). The statistical analysis of the data was performed by the permutation test of the Infostat software (Di Rienzo, 2009).
Growth and Measurement Conditions
Between six and eight replicates of each genotype were grown in a greenhouse under controlled conditions (80% relative humidity, 200 µmol PAR s−1 m−2, 16 h of light/8 h of darkness) at each performed experiment. All measurements (see next sections) were performed on the third or fourth expanded leaf during the vegetative phase of the plants, when they had eight to 10 expanded leaves in total.
Phenotypic Characterization
In T0 plants, fresh weight of leaves, stems, and roots was measured. In the homozygous plants, height, stem length between nodes four and eight, fresh weight of leaves, stems, and roots, and flowering time were measured. Flowering time was recorded as the number of total leaves at the time of flower bud setting.
Photosynthetic Parameters
Light-response curves of photosynthetic parameters for each homozygous line and the wild-type plants were obtained from eight replicates per genotype at increasing photon irradiances (0, 50, 100, 300, 700, 1,200, 1,500, and 1,700 μmol s−1 m−2) using a portable gas exchange system (LI-COR 6400).
Leaf Carbohydrate Levels
Leaf sugar levels of the T0 plants were measured at a light time point (13 pm) and a dark time point (5 am). Diurnal changes in leaf sugar levels were measured in homozygous plants. From each plant, we sampled 30 mg of leaf fresh weight and immediately froze the samples in liquid nitrogen. Samples were ground in liquid nitrogen, and soluble sugars were successively extracted with 250 µL of 80% (v/v) ethanol, 250 µL of 80% (v/v) ethanol, and 150 µL of 50% (v/v) ethanol. Samples were heated at 80°C during 20 min and centrifuged 5 min at 14,000 rpm after each addition of ethanol. Supernatants of each extraction were combined and the mixture was used for soluble sugar determinations. Glc, Fru, and Suc were measured by the enzymatic method (Fernie et al., 2001). The pellet obtained from the soluble sugars extraction was used for starch measurement. It was resuspended in 400 μL of 0.2 n KOH (Sigma-Aldrich), and it was incubated during 1 h at 95°C. Then, 70 μL of 1 n acetic acid (Merck) was added, and the mixture was centrifuged for 10 min at 13,900 rpm. The supernatant was separated and diluted 1:3. A starch assay kit was used for the determinations (Sigma-Aldrich).
Feeding Leaf Discs with [U-14C]Glc
Leaf discs were cut from T0 plants at the onset of the light period and washed three times with 10 mm MES-KOH (BioChemika; pH 6.5). They were incubated (18 discs in a volume of 4 mL, shaken at 90 rpm) for 1, 2, and 3.75 h in 2 mm Glc, including 1.4 kilobecquerel mmol−1 [U-14C]Glc (Amersham-Buchler). The discs were harvested, washed three times in buffer, and frozen in liquid nitrogen. Fractionation of 14C-labeled leaf discs and separation of sugars from neutral fractions on TLC plates were performed as described by Fernie et al. (2001). The value of each result for each line is the average of the label of two discs per plant from three plants. In every case, the normalization of the data was done by leaf area.
Sugar Content in Phloem Sap
Third or fourth expanded leaves were detached from the T0 plants by cutting the base of petioles under water. One leaf per plant and five plants per line were sampled. Leaves were weighed and fed through their petioles with 5 mm EDTA (pH 6.0; Sigma-Aldrich) for 1 h, then transferred to a fresh solution and incubated for further 16 h. The incubations were done in a growing chamber under controlled conditions (relative humidity 60%, temperature 23°C, 200 µmol PAR s−1 m−2). Sap was lyophilized and resuspended in 1 mL of water. Soluble sugar contents were determined enzymatically as described previously. Results of the sugar contents from the first and the 16th incubation hours are presented together. The protocol was adapted from Leggewie et al. (2003).
In Situ Localization of H2O2 in Leaves
H2O2 was detected in leaves of homozygous plants and wild-type plants using DAB dye (Sigma-Aldrich). Leaves were infiltrated under vacuum for 5 min with 0.1% (w/v) DAB in buffer Tris-HCl, pH 6.5 (Tris Base; CalBioChem). They were incubated 16 h in darkness at room temperature. Afterward, they were illuminated until brownish spots appeared. They were bleached with 100% (v/v) ethanol for better visualization of the spots. The spots were quantified by the number of pixels using Photoshop 6.0 software. The results were expressed as the percentage of spots per total leaf area.
Total Protein Profile of Leaves
Total proteins of leaves of homozygous and wild-type plants were extracted. They were quantified utilizing the Bradford method (Bio-Rad protein assay). Equal amounts of protein were run in a 15% SDS-PAGE and stained with Brilliant Blue R dye (Sigma-Aldrich). SDS-PAGE protocol was based on Sambrook et al. (1989).
ASR1 Levels in Leaves of Different Ages
Two or three leaves of different ages (ranging between leaf no. 5 and leaf no. 20) were sampled from three wild-type plants. Total proteins were extracted using the following buffer: 10% (v/v) glycerol (Merck), 25 mm Tris, pH 7.5, 1 mm EDTA (Sigma-Aldrich), 150 mm NaCl (Alcor), 10 mm dithiothreitol (Sigma-Aldrich), 2% (w/v) polyvinylpolypyrrolidone (Sigma-Aldrich), and 2 mm phenylmethanesulfonyl fluoride (Sigma-Aldrich). Quantification of total proteins was performed utilizing the Bradford method (Bio-Rad protein assay). Protein quality of the extracts was checked by SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane (Amersham Hybond ECL; GE Healthcare), and transference was checked by Ponceau S dye (Sigma-Aldrich). Membrane was incubated with anti-ASR1 serum (Maskin et al., 2007), and, after washing, it was incubated with anti-rabbit IgG serum (Sigma-Aldrich). SDS-PAGE and western-blot protocols were based on Sambrook et al. (1989).
Hormone Measurements
ABA and GAs were measured in leaves of homozygous plants. Leaves were harvested at midday, as previously described, and lyophilized. The analysis method for extraction and detection of ABA is similar to a previous report (Chen et al., 2009). For quantification, the data were normalized based on the internal standard [6-d]ABA. The analysis method for extraction and detection of GAs is similar to a previous report (Zhang et al., 2011). The analysis included the following 20 targeted GAs: 8/29/23/3/1/6/5/19/13/20/44/34/53/51/7/4/24/15/9/12. The LC-MS/MS system used is composed of a Shimadzu LC system with a LEAP CTC PAL autosampler coupled to an Applied Biosystems 4000 QTRAP mass spectrometer equipped with a TurboIon Spray electrospray ion source.
Sensitivity to Exogenous GA3
Silenced homozygous plants and wild-type plants were foliar sprayed with 10 μm GA3 (Sigma-Aldrich) twice a week, while another identical group of plants was foliar sprayed with 0.002% (v/v) ethanol (GA3 solvent) at the same time. The treatment was started at seedling emergence and was finished when the plants reached the reproductive stage in a greenhouse. A phenotypic analysis was performed as above, except for height, which was measured along time. Glc, Fru, Suc, and starch contents of the leaves were measured at 12 am (for sugar measurement details, see above). Also, ASR1 protein was detected with a western blot in the leaves of wild-type plants with or without GA3 treatment (for western-blot details, see above).
qRT-PCR Analyses of Sugar Transporters and Sensors
mRNA levels of sugar transporters and sensors in the leaves of homozygous plants were measured by qRT-PCR. RNA extractions, complementary DNA synthesis, and real-time PCRs were performed as described before. mRNA levels were measured for Ht1 (SGN-U446865 tobacco [Nicotiana tabacum], ortholog of GenBank, AY608701.1), Sut2 (SGN-U423251 tobacco, ortholog of The Arabidopsis Information Resource [TAIR], At2G02860), Tmt3 (SGN-E129731 tomato, ortholog of TAIR, At3g51490), Vgt (SGN-U430963 tobacco, ortholog of TAIR, At5g59250), Hexokinase1 (SGN-E750217 tobacco, ortholog of SGN, E1203588 Nicotiana benthamiana), and SnRK1.1 (SGN-E750838 tobacco, ortholog of TAIR, At3G01090). The primer sequences used were as follows: NtHt1 FW, 5′-CCTGGGATTTGCATCAGTTT-3′; NtHt1 RV, 5′-TGGCGGAAGCTGAAATTACT-3′; NtSut2 FW, 5′-GAGGTTGGCACAGATGGTTT-3′; NtSut2 RV, 5′-AGCGATTGGTTTGCTTGAGT-3′; SlTmt3 FW, 5′-CTGCCCCACCAGAAATAAGA-3′; SlTmt3 RV, 5′-AGCCTCCTTCATTCGACCTT-3′; NtVgt FW, 5′-GGCTCAGACTGAAGGTTTCG-3′; NtVgt RV, 5′-CCACAAGACCAAGCTGAACA-3′; NtHK1 FW, 5′-GAGGCATCAATTCCTCCAAA-3′; NtHK1 RV, 5′-GCCCTTTGTCCACCTCATAA-3′; NtSnRk1.1 FW, 5′-AGATTCGTATGCACCCTTGG-3′; NtSnRk1.1 RV, 5′-ACGCCACAGTACCCTCATTC-3′. The reference gene EF1α was used as described before.
Statistical Analyses
Statistical analyses of the data were performed by the Student’s t test, except when otherwise indicated.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Oxidative burst and hypersensitive reaction in Asr1-silenced leaves.
Supplemental Figure S2. SDS-PAGE of total proteins extracted from wild-type and transgenic lines (T4) leaves.
Supplemental Figure S3. Suc contents in leaf phloem sap.
Supplemental Table S1. Gibberellin conversion in Asr1 tobacco transgenic lines.
Supplemental Table S2. Sensitivity of Asr1-silenced plants to exogenous GA3.
Glossary
- ABA
abscisic acid
- qRT-PCR
quantitative real-time PCR
- DAB
diaminobenzidine
- H2O2
hydrogen peroxide
- PAR
photosynthetically active radiation
- TLC
thin-layer chromatography
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- ROS
reactive oxygen species
- FW
forward
- RV
reverse
- TAIR
The Arabidopsis Information Resource
References
- Ainsworth EA, Bush DR. (2011) Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol 155: 64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai-Zeigersona H, Scolnik P, Bar-Zvi D. (1995) Tomato Asrl mRNA and protein are transiently expressed following salt stress, osmotic stress and treatment with abscisic acid. Plant Sci 110: 205–213 [Google Scholar]
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P. (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14: 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Arenhart R, Lima J, Pedron M, Carvalho F, Silveira J, Rosa S, Caverzan A, Andrade C, Schünemann M, Margis R, et al. (2013) Involvement of ASR genes in aluminium tolerance mechanisms in rice. Plant Cell Environ 36: 52–67 [DOI] [PubMed] [Google Scholar]
- Baena-González E, Sheen J. (2008) Convergent energy and stress signaling. Trends Plant Sci 13: 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB. (2000) SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol 148: 6–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemelt S, Tschiersch H, Sonnewald U. (2004) Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis, and photosynthesis in transgenic tobacco plants. Plant Physiol 135: 254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri-Moghaddam MR, Le Roy K, Xiang L, Rolland F, Van den Ende W. (2010) Sugar signalling and antioxidant network connections in plant cells. FEBS J 277: 2022–2037 [DOI] [PubMed] [Google Scholar]
- Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, Kiddle S, Kim YS, Penfold CA, Jenkins D, et al. (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23: 873–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner M. (2007) The monosaccharide transporter(-like) gene family in Arabidopsis. FEBS Lett 581: 2318–2324 [DOI] [PubMed] [Google Scholar]
- Çakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R. (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15: 2165–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Fernie AR, Iusem ND. (2004) Heard it through the grapevine? ABA and sugar cross-talk: the ASR story. Trends Plant Sci 9: 57–59 [DOI] [PubMed] [Google Scholar]
- Chen JY, Liu DJ, Jiang YM, Zhao ML, Shan W, Kuang JF, Lu WJ. (2011) Molecular characterization of a strawberry FaASR gene in relation to fruit ripening. PLoS ONE 6: e24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang B, Hicks LM, Wang S, Jez JM. (2009) A liquid chromatography-tandem mass spectrometry-based assay for indole-3-acetic acid-amido synthetase. Anal Biochem 390: 149–154 [DOI] [PubMed] [Google Scholar]
- Cho YH, Hong JW, Kim EC, Yoo SD. (2012) Regulatory functions of SnRK1 in stress-responsive gene expression and in plant growth and development. Plant Physiol 158: 1955–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JR, Liu B, Feng DR, Liu HY, He YM, Qi KB, Wang HB, Wang JF. (2011) MpAsr encodes an intrinsically unstructured protein and enhances osmotic tolerance in transgenic Arabidopsis. Plant Cell Rep 30: 1219–1230 [DOI] [PubMed] [Google Scholar]
- Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Levine A, Granot D. (1999) Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11: 1253–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan J, Voronin N, Gong F, Sun TP, Hedden P, Fromm H, Aloni R. (2012) Leaf-induced gibberellin signaling is essential for internode elongation, cambial activity, and fiber differentiation in tobacco stems. Plant Cell 24: 66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers BJ, Schuurmans JA, Smeekens SC. (2008) Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol Biol 67: 151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Parthasarathy M, Niklas K, Turgeon R. (1988) A morphometric analysis of the phloem-unloading pathway in developing tobacco leaves. Planta 176: 307–318 [DOI] [PubMed] [Google Scholar]
- Di Rienzo J. (2009) fgStatistics, version 2009. Córdoba, Argentina. http://sites.google.com/site/fgstatistics
- Eveland AL, Jackson DP. (2012) Sugars, signalling, and plant development. J Exp Bot 63: 3367–3377 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ. (2001) Fructose 2,6-bisphosphate activates pyrophosphate:fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212: 250–263 [DOI] [PubMed] [Google Scholar]
- Fischer I, Camus-Kulandaivelu L, Allal F, Stephan W. (2011) Adaptation to drought in two wild tomato species: the evolution of the Asr gene family. New Phytol 190: 1032–1044 [DOI] [PubMed] [Google Scholar]
- Francis D, Halford NG. (2006) Nutrient sensing in plant meristems. Plant Mol Biol 60: 981–993 [DOI] [PubMed] [Google Scholar]
- Frankel N, Carrari F, Hasson E, Iusem ND. (2006) Evolutionary history of the Asr gene family. Gene 378: 74–83 [DOI] [PubMed] [Google Scholar]
- Frankel N, Nunes-Nesi A, Balbo I, Mazuch J, Centeno D, Iusem ND, Fernie AR, Carrari F. (2007) ci21A/Asr1 expression influences glucose accumulation in potato tubers. Plant Mol Biol 63: 719–730 [DOI] [PubMed] [Google Scholar]
- Gallego-Giraldo L, García-Martínez JL, Moritz T, López-Díaz I. (2007) Flowering in tobacco needs gibberellins but is not promoted by the levels of active GA1 and GA4 in the apical shoot. Plant Cell Physiol 48: 615–625 [DOI] [PubMed] [Google Scholar]
- Gan S. (2010) The hormonal regulation of senescence. In P Davies, ed, Plant Hormones: Biosynthesis, Signal Transduction, Action! Ed 3. Springer, New York, pp 597–617. [Google Scholar]
- Ghillebert R, Swinnen E, Wen J, Vandesteene L, Ramon M, Norga K, Rolland F, Winderickx J. (2011) The AMPK/SNF1/SnRK1 fuel gauge and energy regulator: structure, function and regulation. FEBS J 278: 3978–3990 [DOI] [PubMed] [Google Scholar]
- Gregersen PL, Holm PB, Krupinska K. (2008) Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol (Stuttg) (Suppl) 10: 37–49 [DOI] [PubMed] [Google Scholar]
- Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C. (2006) Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J 45: 180–192 [DOI] [PubMed] [Google Scholar]
- Hanson J, Smeekens S. (2009) Sugar perception and signaling: an update. Curr Opin Plant Biol 12: 562–567 [DOI] [PubMed] [Google Scholar]
- Henry IM, Carpentier SC, Pampurova S, Van Hoylandt A, Panis B, Swennen R, Remy S. (2011) Structure and regulation of the Asr gene family in banana. Planta 234: 785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YF, Yu SC, Yang CY, Wang CS. (2011) Lily ASR protein-conferred cold and freezing resistance in Arabidopsis. Plant Physiol Biochem 49: 937–945 [DOI] [PubMed] [Google Scholar]
- Huerta L, Forment J, Gadea J, Fagoaga C, Peña L, Pérez-Amador MA, García-Martínez JL. (2008) Gene expression analysis in citrus reveals the role of gibberellins on photosynthesis and stress. Plant Cell Environ 31: 1620–1633 [DOI] [PubMed] [Google Scholar]
- Iqbal N, Nazar R, Iqbal R, Khan M, Masood A, Khan N. (2011) Role of gibberellins in regulation of source-sink relations under optimal and limiting environmental conditions. Curr Sci 100: 998–1007 [Google Scholar]
- Jeanneau M, Gerentes D, Foueillassar X, Zivy M, Vidal J, Toppan A, Perez P. (2002) Improvement of drought tolerance in maize: towards the functional validation of the Zm-Asr1 gene and increase of water use efficiency by over-expressing C4-PEPC. Biochimie 84: 1127–1135 [DOI] [PubMed] [Google Scholar]
- Jha B, Lal S, Tiwari V, Yadav S, Agarwal P. (2012) The SbASR-1 gene cloned from an extreme halophyte Salicornia brachiata enhances salt tolerance in transgenic tobacco. Mar Biotechnol 14: 782–792 [DOI] [PubMed] [Google Scholar]
- Jongebloed U, Szederkényi J, Hartig K, Schobert C, Komor E. (2004) Sequence of morphological and physiological events during natural ageing and senescence of a castor bean leaf: Sieve tube occlusion and carbohydrate back-up precede chlorophyll degradation. Physiol Plant 120: 338–346 [DOI] [PubMed] [Google Scholar]
- Kalifa Y, Gilad A, Konrad Z, Zaccai M, Scolnik PA, Bar-Zvi D. (2004a) The water- and salt-stress-regulated Asr1 (abscisic acid stress ripening) gene encodes a zinc-dependent DNA-binding protein. Biochem J 381: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalifa Y, Perlson A, Gilad A, Konrad Z, Scolnik P, Bar-Zvi D. (2004b) Over-expression of the water and salt stress-regulated Asr1 gene confers an increased salt tolerance. Plant Cell Environ 27: 1459–1468 [Google Scholar]
- Konrad Z, Bar-Zvi D. (2008) Synergism between the chaperone-like activity of the stress regulated ASR1 protein and the osmolyte glycine-betaine. Planta 227: 1213–1219 [DOI] [PubMed] [Google Scholar]
- Kühn C. (2011) Sucrose transporters and plant development. In M. Geisler and K. Venema, eds, Transporters and Pumps in Plant Signaling, Signaling and Communication in Plants 7. Springer-Verlag, Berlin, pp 225–251 [Google Scholar]
- Lalonde S, Tegeder M, Throne-Holst M, Frommer W, Patrick J. (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26: 37–56 [Google Scholar]
- Leggewie G, Kolbe A, Lemoine R, Roessner U, Lytovchenko A, Zuther E, Kehr J, Frommer WB, Riesmeier JW, Willmitzer L, et al. (2003) Overexpression of the sucrose transporter SoSUT1 in potato results in alterations in leaf carbon partitioning and in tuber metabolism but has little impact on tuber morphology. Planta 217: 158–167 [DOI] [PubMed] [Google Scholar]
- Liesche J, Krügel U, He H, Chincinska I, Hackel A, Kühn C. (2011) Sucrose transporter regulation at the transcriptional, post-transcriptional and post-translational level. J Plant Physiol 168: 1426–1433 [DOI] [PubMed] [Google Scholar]
- Liu DD, Chao WM, Turgeon R. (2012) Transport of sucrose, not hexose, in the phloem. J Exp Bot 63: 4315–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Dai JR, Feng DR, Liu B, Wang HB, Wang JF. (2010) Characterization of a novel plantain Asr gene, MpAsr, that is regulated in response to infection of Fusarium oxysporum f. sp. cubense and abiotic stresses. J Integr Plant Biol 52: 315–323 [DOI] [PubMed] [Google Scholar]
- Liu XJ, Prat S, Willmitzer L, Frommer WB. (1990) cis regulatory elements directing tuber-specific and sucrose-inducible expression of a chimeric class I patatin promoter/GUS-gene fusion. Mol Gen Genet 223: 401–406 [DOI] [PubMed] [Google Scholar]
- MacMillan J. (1997) Biosynthesis of the gibberellin plant hormones. Nat Prod Rep 14: 221–243 [Google Scholar]
- Maskin L, Frankel N, Gudesblat G, Demergasso MJ, Pietrasanta LI, Iusem ND. (2007) Dimerization and DNA-binding of ASR1, a small hydrophilic protein abundant in plant tissues suffering from water loss. Biochem Biophys Res Commun 352: 831–835 [DOI] [PubMed] [Google Scholar]
- Maskin L, Maldonado S, Iusem ND. (2008) Tomato leaf spatial expression of stress-induced Asr genes. Mol Biol Rep 35: 501–505 [DOI] [PubMed] [Google Scholar]
- Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N. (2004) Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiol 134: 684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Melzer M, Truernit E, Hümmer C, Besenbeck R, Stadler R, Sauer N. (2000) AtSUC3, a gene encoding a new Arabidopsis sucrose transporter, is expressed in cells adjacent to the vascular tissue and in a carpel cell layer. Plant J 24: 869–882 [DOI] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Foyer CH. (2001) Sink regulation of photosynthesis. J Exp Bot 52: 1383–1400 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtau N, Marès M, Purdy S, Quentin N, Ruël A, Wingler A. (2004) Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 219: 765–772 [DOI] [PubMed] [Google Scholar]
- Ricardi MM, Guaimas FF, González RM, Burrieza HP, López-Fernández MP, Jares-Erijman EA, Estévez JM, Iusem ND. (2012) Nuclear import and dimerization of tomato ASR1, a water stress-inducible protein exclusive to plants. PLoS ONE 7: e41008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Rom S, Gilad A, Kalifa Y, Konrad Z, Karpasas MM, Goldgur Y, Bar-Zvi D. (2006) Mapping the DNA- and zinc-binding domains of ASR1 (abscisic acid stress ripening), an abiotic-stress regulated plant specific protein. Biochimie 88: 621–628 [DOI] [PubMed] [Google Scholar]
- Rossi M, Carrari F, Cabrera-Ponce JL, Vázquez-Rovere C, Herrera-Estrella L, Gudesblat G, Iusem ND. (1998) Analysis of an abscisic acid (ABA)-responsive gene promoter belonging to the Asr gene family from tomato in homologous and heterologous systems. Mol Gen Genet 258: 1–8 [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Saumonneau A, Agasse A, Bidoyen MT, Lallemand M, Cantereau A, Medici A, Laloi M, Atanassova R. (2008) Interaction of grape ASR proteins with a DREB transcription factor in the nucleus. FEBS Lett 582: 3281–3287 [DOI] [PubMed] [Google Scholar]
- Saumonneau A, Laloi M, Lallemand M, Rabot A, Atanassova R. (2012) Dissection of the transcriptional regulation of grape ASR and response to glucose and abscisic acid. J Exp Bot 63: 1495–1510 [DOI] [PubMed] [Google Scholar]
- Schmidt GW, Delaney SK. (2010) Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genomics 283: 233–241 [DOI] [PubMed] [Google Scholar]
- Sheen J. (1990) Metabolic repression of transcription in higher plants. Plant Cell 2: 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewinski TL. (2011) Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: a physiological perspective. Mol Plant 4: 641–662 [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Brauer M, von Schaewen A, Stitt M, Willmitzer L. (1991) Transgenic tobacco plants expressing yeast-derived invertase in either the cytosol, vacuole or apoplast: a powerful tool for studying sucrose metabolism and sink/source interactions. Plant J 1: 95–106 [DOI] [PubMed] [Google Scholar]
- Takasaki H, Mahmood T, Matsuoka M, Matsumoto H, Komatsu S. (2008) Identification and characterization of a gibberellin-regulated protein, which is ASR5, in the basal region of rice leaf sheaths. Mol Genet Genomics 279: 359–370 [DOI] [PubMed] [Google Scholar]
- Turgeon R. (1987) Phloem unloading in tobacco sink leaves: insensitivity to anoxia indicates a symplastic pathway. Planta 171: 73–81 [DOI] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. (2009) Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Urtasun N, Correa García S, Iusem ND, Bermúdez Moretti M. (2010) Predominantly cytoplasmic localization in yeast of ASR1, a non-receptor transcription factor from plants. Open Biochem J 4: 68–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignault C, Vachaud M, Cakir B, Glissant D, Dédaldéchamp F, Büttner M, Atanassova R, Fleurat-Lessard P, Lemoine R, Delrot S. (2005) VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J Exp Bot 56: 1409–1418 [DOI] [PubMed] [Google Scholar]
- Virlouvet L, Jacquemot MP, Gerentes D, Corti H, Bouton S, Gilard F, Valot B, Trouverie J, Tcherkez G, Falque M, et al. (2011) The ZmASR1 protein influences branched-chain amino acid biosynthesis and maintains kernel yield in maize under water-limited conditions. Plant Physiol 157: 917–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schaewen A, Stitt M, Schmidt R, Sonnewald U, Willmitzer L. (1990) Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J 9: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jauh G, Hsu Y, Wang C. (2003) The nuclear localization signal of a pollen-specific, desiccation-associated protein of a lily is necessary and sufficient for nuclear targeting. Bot Bull Acad Sin 44: 123–128 [Google Scholar]
- Wingler A, Delatte TL, O’Hara LE, Primavesi LF, Jhurreea D, Paul MJ, Schluepmann H. (2012) Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability. Plant Physiol 158: 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormit A, Trentmann O, Feifer I, Lohr C, Tjaden J, Meyer S, Schmidt U, Martinoia E, Neuhaus HE. (2006) Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 18: 3476–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZJ, Nakajima M, Suzuki Y, Yamaguchi I. (2002) Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiol 129: 1285–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59: 225–251 [DOI] [PubMed] [Google Scholar]
- Yang CY, Chen YC, Jauh GY, Wang CS. (2005) A Lily ASR protein involves abscisic acid signaling and confers drought and salt resistance in Arabidopsis. Plant Physiol 139: 836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Ito M, Nishida I, Watanabe A. (2002) Identification of a novel gene HYS1/CPR5 that has a repressive role in the induction of leaf senescence and pathogen-defence responses in Arabidopsis thaliana. Plant J 29: 427–437 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang B, Yan D, Dong W, Yang W, Li Q, Zeng L, Wang J, Wang L, Hicks LM, et al. (2011) Two Arabidopsis cytochrome P450 monooxygenases, CYP714A1 and CYP714A2, function redundantly in plant development through gibberellin deactivation. Plant J 67: 342–353 [DOI] [PubMed] [Google Scholar]