Overproduction of the copper/zinc superoxide dismutase and catalase in transgenic cassava dramatically improves ROS scavenging ability, abiotic stress resistance, and delayed postharvest deterioration.
Abstract
Postharvest physiological deterioration (PPD) of cassava (Manihot esculenta) storage roots is the result of a rapid oxidative burst, which leads to discoloration of the vascular tissues due to the oxidation of phenolic compounds. In this study, coexpression of the reactive oxygen species (ROS)-scavenging enzymes copper/zinc superoxide dismutase (MeCu/ZnSOD) and catalase (MeCAT1) in transgenic cassava was used to explore the intrinsic relationship between ROS scavenging and PPD occurrence. Transgenic cassava plants integrated with the expression cassette p54::MeCu/ZnSOD-35S::MeCAT1 were confirmed by Southern-blot analysis. The expression of MeCu/ZnSOD and MeCAT1 was verified by quantitative reverse transcription-polymerase chain reaction and enzymatic activity analysis both in the leaves and storage roots. Under exposure to the ROS-generating reagent methyl viologen or to hydrogen peroxide (H2O2), the transgenic plants showed higher enzymatic activities of SOD and CAT than the wild-type plants. Levels of malondialdehyde, chlorophyll degradation, lipid peroxidation, and H2O2 accumulation were dramatically reduced in the transgenic lines compared with the wild type. After harvest, the storage roots of transgenic cassava lines show a delay in their PPD response of at least 10 d, accompanied by less mitochondrial oxidation and H2O2 accumulation, compared with those of the wild type. We hypothesize that this is due to the combined ectopic expression of Cu/ZnSOD and CAT leading to an improved synergistic ROS-scavenging capacity of the roots. Our study not only sheds light on the mechanism of the PPD process but also develops an effective approach for delaying the occurrence of PPD in cassava.
Cassava (Manihot esculenta) produces an acceptable yield under the adverse climatic and nutrient-poor soil conditions occurring in some tropical and subtropical regions; therefore, it is recognized as being an important food security crop (Sayre et al., 2011). Nevertheless, the rapid postharvest physiological deterioration (PPD) of its storage roots, a unique phenomenon compared with other root crops, renders the roots unpalatable and unmarketable within 24 to 72 h of harvest, thereby adversely impacting farmers, processors, and consumers alike (Rickard, 1985; Reilly et al., 2004, 2007). While the exclusion of oxygen by storing and transporting the roots in plastic sacks, or coating individual roots with paraffin wax, has been used to minimize the problem of PPD in cassava, these approaches only provide solutions for wealthier consumers or expatriate communities and are not generally economically viable for such a low-value commodity (Reilly et al., 2004).
Early studies identified phenolic compounds whose accumulation and oxidation led to the discoloration of vascular tissues; these included scopoletin, scopolin, esculin, and proanthocyanidins (Rickard, 1985). Various other secondary metabolites including volatile compounds, which are associated with the early stages of cassava root PPD, had been detected (Buschmann et al., 2000; Iyer et al., 2010). The application of cycloheximide to inhibit protein synthesis (Beeching et al., 1998) confirmed that PPD was an active process, rather than a degenerative one, involving changes in gene expression, protein synthesis, and phenolic compound synthesis (Huang et al., 2001). Reactive oxygen species (ROS) increased very early during PPD (Iyer et al., 2010). Cellular processes, including ROS turnover, programmed cell death, defense pathways, signaling pathways, and cell wall remodeling, have been shown to be active during the deterioration response using complementary DNA microarray and isobaric tags for relative and absolute quantification-based PPD proteome analyses (Reilly et al., 2007; Owiti et al., 2011). A burst of superoxide is detected within minutes of harvesting, followed by peaks of other ROS and increased activities of ROS-scavenging enzymes (Reilly et al., 2001, 2004, 2007), suggesting that ROS plays a major role in, and may in fact initiate, the PPD response. A recent study showed that PPD is cyanide dependent, presumably resulting from cyanide-dependent inhibition of respiration (Zidenga et al., 2012). Therefore, while there is considerable evidence linking ROS accumulation to PPD, there is a need to confirm the intrinsic relationship between ROS accumulation and PPD occurrence and their regulation.
ROS, which include the superoxide anion radical, hydroxyl radical, and hydrogen peroxide (H2O2), are potentially harmful to the cell and cause oxidative damage to proteins, DNA, and lipids (Apel and Hirt, 2004). Plants have developed efficient nonenzymatic and enzymatic detoxification mechanisms to scavenge ROS. Nonenzymatic antioxidants include the major cellular redox buffers, ascorbate and glutathione, as well as tocopherol, flavonoids, alkaloids, and carotenoids. Enzymatic ROS-scavenging mechanisms in plants include superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), ascorbate peroxidase (APX; EC 1.11.1.11), and glutathione peroxidase (EC 1.11.1.7; Asada, 1999; Apel and Hirt, 2004; Mittler et al., 2004). SOD, acting as the first line of defense against ROS, converts superoxide to H2O2. There are essentially three types of SODs containing manganese, iron, or copper plus zinc as prosthetic metals (Raychaudhuri and Deng, 2000). The resultant H2O2 can be detoxified to oxygen and water by CAT or APX, which occur mainly in peroxisomes.
Several groups have addressed the overproduction of SOD (Gupta et al., 1993; Perl et al., 1993; McKersie et al., 1996, 2000) in the chloroplasts as a means to enhance tolerance to oxidative stress. In addition, transgenic plants expressing CAT had increased tolerance against various abiotic stresses (Shikanai et al., 1998; Miyagawa et al., 2000; Moriwaki et al., 2007). However, others have reported that the expression of either SOD or CAT alone led to no change in response to oxidative or environmental stress (Pitcher et al., 1991; Payton et al., 1997). These contradictory findings may be due to the complex network of plant antioxidant defenses and raise the possibility that a higher tolerance to oxidative stress might be achieved by pyramiding or stacking genes in a single genotype (Halpin, 2005). Transgenic cotton (Gossypium hirsutum) expressing both glutathione reductase and APX improved the recovery of photosynthesis following exposures to 10°C and high photon flux density (Payton et al., 2001). Transgenic Chinese cabbage (Brassica campestris ssp. pekinensis) plants expressing both SOD and CAT in chloroplasts enhanced tolerance to sulfur dioxide and salt stress (Tseng et al., 2007). Coexpression of copper/zinc SOD (Cu/ZnSOD) and APX genes in the chloroplasts or cytosol of tobacco (Nicotiana tabacum), potato (Solanum tuberosum), and sweet potato (Ipomoea batatas) enhanced tolerance to multiple abiotic stresses, including the herbicide methyl viologen (MV), chilling, high temperature, and drought stress (Kwon et al., 2002; Tang et al., 2006; Lee et al., 2007; Faize et al., 2011).
As a clonally propagated, highly heterozygous crop plant, conventional breeding for tolerance to PPD via the cross hybridization between cassava and its relatives, although possible (Chavez et al., 2000), may not be straightforward and is time consuming. Some variation in PPD tolerance has been found in various cassava landraces (Contreras Rojas et al., 2009); for example, Morante et al. (2010) reported that cassava roots with high carotenoid levels were tolerant to PPD for up to 40 d after harvest. They attributed this character to the antioxidant properties of carotenoids, but the trait proved difficult to transfer to common cultivars. Pruning the plants a few days before harvest also delays PPD, but at the expense of a reduction in dry matter content of the root (van Oirschot et al., 2000). To date, traditional breeding alternatives do not appear to offer a practical solution, and PPD has remained a difficult problem to solve. Therefore, molecular and biochemical regulation of the PPD process using transgenic technology offers alternative approaches for controlling the PPD response.
Recently, transgenic cassava overexpressing the Arabidopsis (Arabidopsis thaliana) mitochondrial alternative oxidase (AOX 1A) gene were shown to delay PPD for up to 10 d by reducing ROS accumulation (Zidenga et al., 2012). The PPD response is an enzymatically mediated oxidative process in which ROS appear to play a dual role as both a signaling molecule that induces programmed cell death as part of a more general wound response and in oxidizing phenolic compounds to produce the visible symptoms of PPD, with wound repair and antioxidant defenses being too late or inadequate to contain these effects (Reilly et al., 2004). Both SOD and CAT activities increased significantly in storage roots after harvest, although too late to contain the PPD response (Reilly et al., 2001, Owiti et al., 2011); additionally, the antioxidant effects of the two enzymes are directly linked through their converting superoxide to H2O2 and H2O2 to oxygen and water, sequentially. In this study, we used transgenic cassava overexpressing cassava SOD and CAT to study their synergized effect on the intrinsic relationship between ROS scavenging and PPD occurrence, which also leads to an appropriate approach for delaying the deterioration of cassava storage roots after harvest.
RESULTS
Molecular Characterization of Transgenic Plants and Increased Expression of SOD and CAT1 Showed No Visible Phenotypic Changes
In order to test how ROS regulates the PPD response, MeCu/ZnSOD and MeCAT1 stacked in the binary vector pC-P54::MeCu/ZnSOD-35S::MeCAT1 (Supplemental Fig. S1A) were used to produce transgenic cassava. Since ROS were mainly generated from the cells of vascular tissues, the expression of the MeCu/ZnSOD gene were driven by the vascular-specific promoter p54/1.0 from cassava (Zhang et al., 2003), while the MeCAT1 gene was placed under the control of the cauliflower mosaic virus (CaMV) 35S promoter in order to avoid transgene silencing caused by using the same promoter. Nine independent transgenic plant lines (named SC for short) were regenerated from transformed embryogenic suspension on hygromycin-containing medium. In vitro plants were tested by PCR for the presence of the MeCu/ZnSOD and MeCAT1 transgenes (data not shown). The copy number of the transgenes in the transgenic plants was assessed by Southern-blot analysis using the XbaI-digested genomic DNA hybridized with digoxigenin-labeled hygromycin phosphotransferase (HPT) and CAT1 probes (Supplemental Fig. S1B). The transgenic cassava plants were truly independent lines and have a pattern of transfer DNA integration ranging from one to two copies.
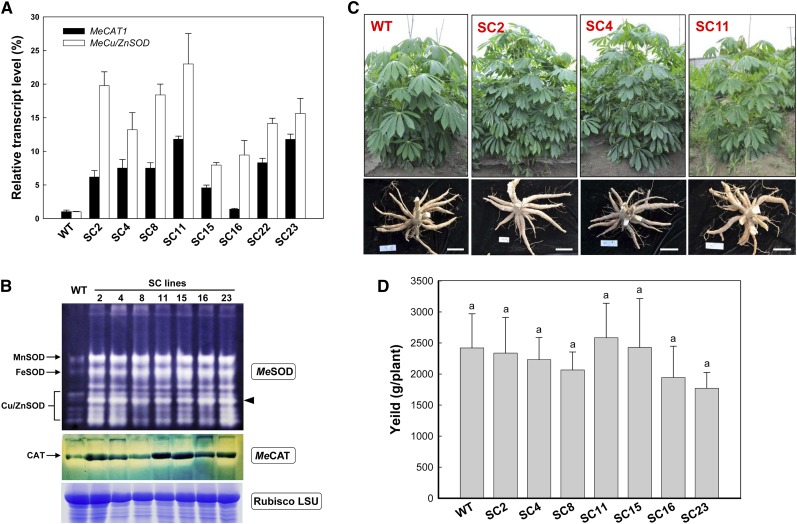
Different transcriptional levels of MeCu/ZnSOD and MeCAT1 in the eight single-copy lines compared with the nontransformed control were detected by quantitative reverse transcription (qRT)-PCR (Fig. 1A). The expression of MeCu/ZnSOD and MeCAT1 increased to 20- and 10-fold, respectively. The increased enzymatic abundances of the different SOD and CAT isoforms were detected, and the results are shown in Figure 1B. A typical SOD isoenzyme banding pattern (Chen and Pan, 1996), which consisted of manganese SOD (MnSOD), iron SOD (FeSOD), and Cu/ZnSOD, was found. In all transgenic lines, MnSOD, FeSOD, and Cu/ZnSOD bands were stronger than those of the wild type. Comparison of the intensities of the Cu/ZnSOD bands showed that one band was greatly enhanced compared with the wild type (Fig. 1B, arrowhead), due to the overproduction of the cytoplasmic MeCu/ZnSOD protein in the transgenic lines. The transgenic lines also showed a stronger CAT isoenzyme band than the wild type. These results demonstrated the elevated levels of SOD and CAT enzymes in transgenic plants.
Figure 1.
Analyses of cassava SOD and CAT transcript and abundance and phenotypic evaluation of transgenic plants harvested from the field. A, qRT-PCR analysis of MeCu/ZnSOD and MeCAT1 expression levels both in wild-type (WT) and transgenic cassava. Total RNA was extracted from leaves, and the data are shown relative to the wild type, using β-actin as an internal control. Data are presented as means ± sd of three independent RNA samples. B, SOD and CAT isoforms in leaves of wild-type and transgenic plants detected by activity staining of a nondenaturing polyacrylamide gel. Three SOD isoforms, MnSOD, FeSOD, and Cu/ZnSOD, are indicated. A substantial increase of the cytoplasmic Cu/ZnSOD is highlighted by the black arrowhead. The Rubisco LSU protein was used as a loading control. C and D, Normal growth of transgenic plants with fully developed storage roots (C) and unchanged yield (D) in comparison with the wild type in the field. Bars = 15 cm. No significant difference was found by Duncan’s multiple comparison tests at P < 0.05. [See online article for color version of this figure.]
None of the transgenic plants, grown in either the greenhouse or the field, showed any visible phenotypic and yield differences compared with the wild type under the same growth conditions (Fig. 1, C and D), indicating that the overexpression of the stacked MeCu/ZnSOD and MeCAT1 genes occurs without phenotypic defects in the transgenic cassava.
The Transgenic Plants Have Enhanced Tolerance to Oxidative Stresses
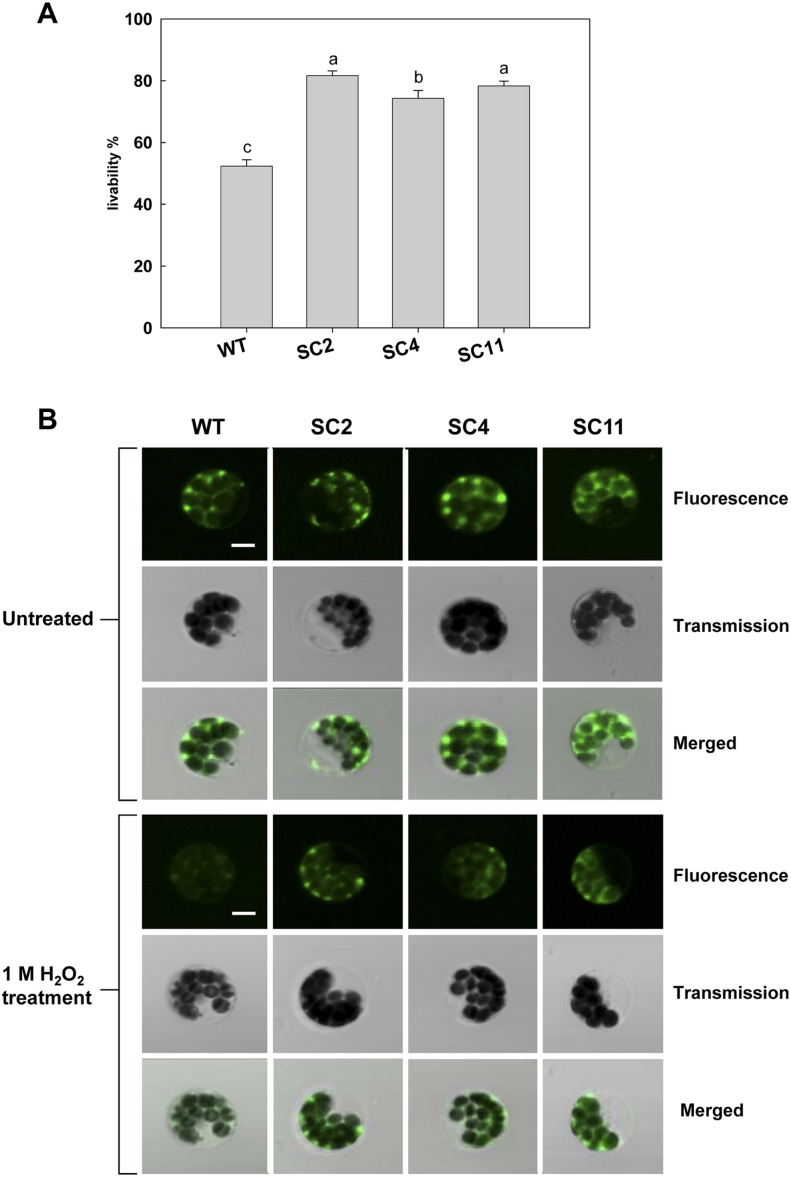
To assess ROS-scavenging capacity in the transgenic cassava, tolerance to exogenous H2O2 stress using protoplasts of transgenic plants was determined. Under 1 m H2O2 treatment, the viability of transgenic protoplasts from the leaves of SC2, SC4, and SC11 remained 80%, 75%, and 79%, respectively, which was significantly higher than that of the wild type, which only showed 50% viability (Fig. 2A). The protoplasts isolated from all cassava leaves showed intense green fluorescence under the untreated condition (Fig. 2B). When treated with 1 m H2O2, only a diffuse and weak fluorescent signal was observed, after the rhodamine 123 reaction, in the wild-type protoplasts, in contrast to the transgenic protoplasts, which still exhibited a strong green fluorescence (Fig. 2B). These results imply that the transgenic cells have enhanced oxidative stress tolerance compared with wild-type cells.
Figure 2.
Changes in protoplast viability and mitochondrial membrane integrity in the presence of 1 m H2O2. A, The viability of cassava mesophyll protoplasts after H2O2 treatment was estimated by fluorescein diacetate staining. Data are represented as means of six replicates ± sd (more than 300 cells were counted for each experiment per genotype). Values labeled with different letters (a, b, and c) are significantly different by Duncan’s multiple comparison tests at P < 0.05. B, Mitochondrial membrane integrity under 1 m H2O2 stress. Cassava protoplasts were stained with rhodamine 123, and the fluorescent signal was observed with a confocal microscope. Bars = 5 μm. [See online article for color version of this figure.]
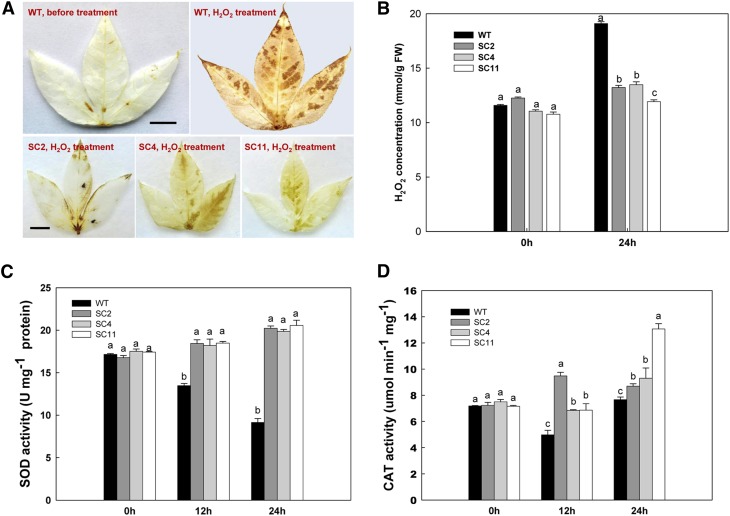
To confirm the enhanced ROS-scavenging capacity of the transgenic plants at the plant level, these were treated with 0.5 m H2O2 oxidative stress. Before treatment, wild-type leaves showed only a basal level of H2O2 production, as indicated both by 3,3′-diaminobenzidine (DAB) staining and colorimetric detection (Fig. 3, A and B). At this time point, no significant difference was found between wild-type and transgenic plants (Fig. 3B). After 0.5 mm H2O2 treatment for 24 h, wild-type leaves accumulated H2O2 significantly, mostly in epidermal cells, and reached a concentration of 19.1 mmol g−1, a 1.65-fold change compared with untreated ones (Fig. 3B). In contrast to the wild type, transgenic leaves showed much less H2O2 accumulation, as evidenced by slight DAB staining in leaves (Fig. 3A) and a low level of H2O2 concentration (Fig. 3B). For example, the H2O2 concentration in the leaves of SC2 was 13.2 mmol g−1, only a 7.8% increase. The accumulation of H2O2 in these transgenic lines was only about 70% of the wild type.
Figure 3.
Enhanced tolerance to H2O2-mediated oxidative stress in transgenic leaves. A, H2O2 accumulation in leaves detected by DAB staining. Bars = 0.5 cm. B, Changes in the levels of H2O2 concentration between wild-type (WT) and transgenic cassava during 0.5 m H2O2 treatment. C and D, Changes in SOD (C) and CAT (D) activities between wild-type and transgenic cassava during H2O2 treatment. Values represent means of three independent experiments ± sd. Values labeled with different letters (a, b, and c) are significantly different by Duncan’s multiple comparison tests at P < 0.05. [See online article for color version of this figure.]
Without H2O2 treatment, both wild-type and transgenic leaves showed only basal SOD and CAT activities, with no significant difference between them (Fig. 3, C and D). After treatment, the SOD activity of the wild type decreased over the time course. At 24 h, SOD activity was reduced to 52.5% of that at 0 h. However, a consistent increase of SOD activity was detected in the leaves of all three transgenic lines. For example, in SC2, the activity increased from 16.8 units mg−1 at 0 h to 20.2 units mg−1 protein by 24 h, about a 20% increase (Fig. 3C). CAT activity was also affected by H2O2 treatment (Fig. 3D). Unlike SOD, CAT decreased its activity at 12 h (about 32.2% compared with 0 h) but recovered by 24 h in the wild type. The CAT of transgenic leaves showed increased activity after H2O2 treatment, especially in SC11: the activity level at 24 h increased 2-fold as compared with 0 h (Fig. 3D), reaching 13.1 μmol min−1 mg−1. At 24 h, all transgenic leaves showed significantly higher activity in comparison with the wild type. These results confirm that the improved performance of transgenic cassava leaves against oxidative stress is due to elevated SOD and CAT activities.
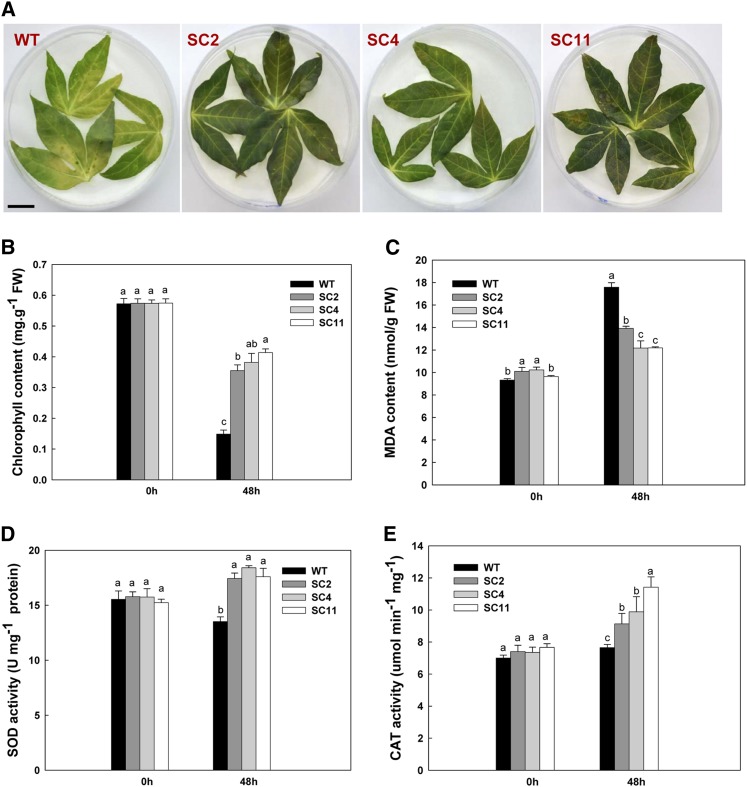
The efficacy of endogenous ROS scavenging in transgenic leaves was tested using the ROS-generating reagent MV. When subjected to 100 µm MV treatment for 2 d, cassava leaves were greatly affected, showing discoloration (Fig. 4A) and reduced chlorophyll content (Fig. 4B). The reduction of chlorophyll content in transgenic leaves was 38%, 33%, and 45% in SC2, SC4, and SC11, respectively, which was significantly less than the wild type (74%; Fig. 4B).
Figure 4.
Enhanced tolerance to MV-mediated oxidative stress in transgenic leaves. A, Leaves treated with 100 µm MV showing the senescence phenotype of wild type (WT) plants and the stay-green phenotype of transgenic plants. Bar = 0.5 cm. B and C, Chlorophyll and MDA contents in the first leaf of MV-treated and untreated plants. FW, Fresh weight. D and E, Changes in SOD and CAT activities between wild-type and transgenic cassava during MV treatment. Data are presented as means ± sd from triplicate independent measurements. Values labeled with different letters (a, b, and c) at the same time point are significantly different by Duncan’s multiple comparison tests at P < 0.05. [See online article for color version of this figure.]
After the MV treatment, increased malondialdehyde (MDA) content, an indicator of lipid peroxidation, was observed in both wild-type and transgenic leaves (Fig. 4C). However, transgenic leaves only showed about a 13% increase in comparison with 50% in the wild type, indicating less cellular membrane damage in the transgenic leaves (Fig. 4C). Additionally, both SOD and CAT activities were significantly enhanced (Fig. 4, D and E), with average increases of 30% for SOD and 20% for CAT in transgenic leaves, in contrast to their reduced activities in the wild type, results that were similar to those obtained with H2O2 treatment. These data confirm that the coexpression of MeCu/ZnSOD and MeCAT1 renders transgenic cassava leaves more resistant to both exogenous and endogenous oxidative stresses.
Storage Roots of Transgenic Cassava Show Delayed PPD Occurrence
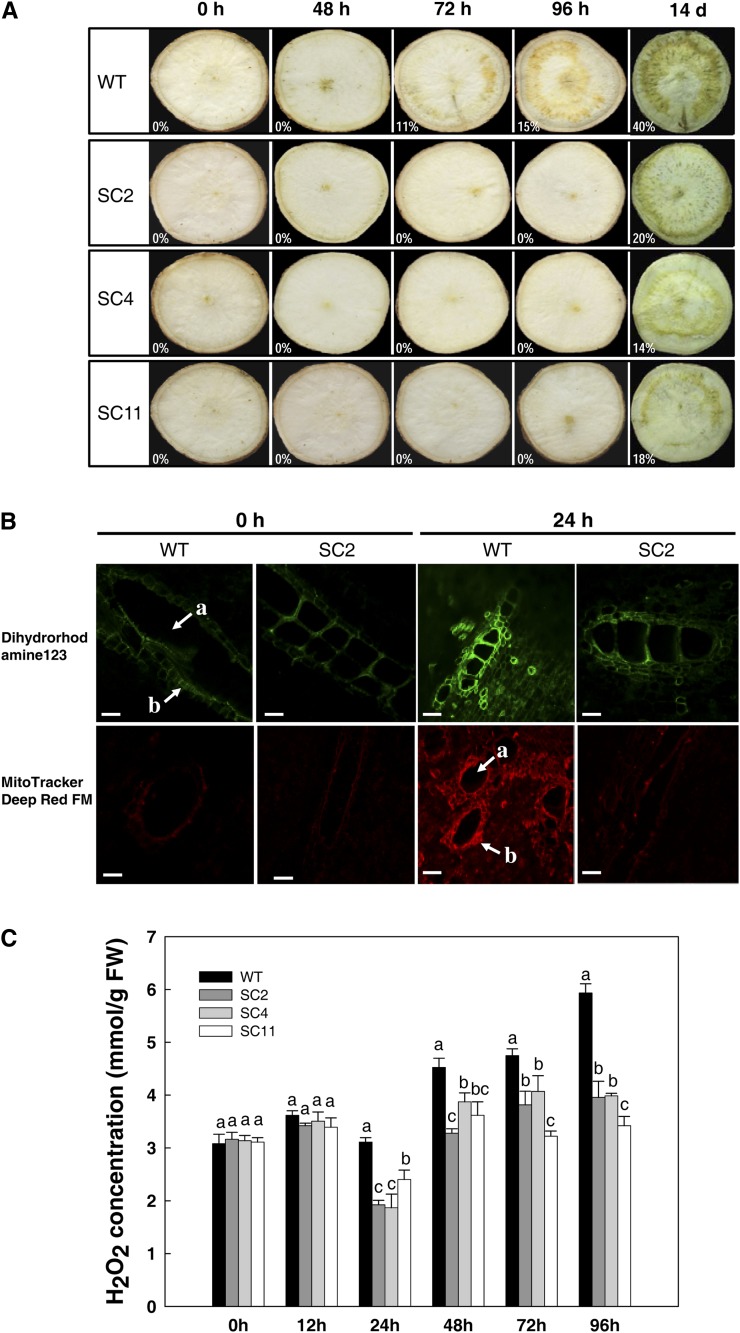
Analysis of field-grown plants showed that the transgenic plants had no obvious difference in terms of root production and yield (Fig. 1, C and D). However, it was also pertinent to determine whether the enhanced capacity of ROS scavenging influenced the storage root PPD response. At 48 h post harvest, PPD symptoms could be seen in the storage roots of wild-type cassava; these roots showed the typical symptom of brown vascular streaking. More visible dark-brown coloration was observed in the starch-rich parenchyma section from 72 h, with 11% vascular discoloration level (Fig. 5A). Compared with the wild type, storage roots of the three transgenic lines did not show typical PPD symptoms even after 96 h (Fig. 5A), indicating a tolerance to PPD development. No visible PPD occurrence was noticed in 10 d. However, by 14 d after harvest, clear PPD symptoms could also be observed in their storage root sections (the vascular discoloration levels of the wild type, SC2, SC4, and SC11 were 40%, 20%, 14%, and 18%, respectively), although different lines responded differentially (Fig. 5A).
Figure 5.
Delayed PPD occurrence of storage roots from transgenic cassava by visual and fluorescence determination. A, Visual detection of PPD occurrence using the International Center for Tropical Agriculture method (Wheatley et al., 1985; Morante et al., 2010). The levels of vascular discoloration are indicated by percentages using ImageJ processing software. B, Inhibition of mitochondrial ROS generation during the PPD process in transgenic storage roots detected by fluorescent probes. The fluorescence intensity of oxidized rhodamine was observed with a fluorescence microscope (Zeiss LSM 510 META) with excitation/emission of 488/515 nm and 635/680 nm for DHR and MitoTracker-Deep Red FM, respectively. a, Xylem vessel; b, bundle sheath. Bars = 20 μm. C, Changes of H2O2 concentrations during PPD between wild-type (WT) and transgenic cassava. Values labeled with different letters (a, b, and c) at the same time point are significantly different by Duncan’s multiple comparison tests at P < 0.05. FW, Fresh weight. [See online article for color version of this figure.]
To test whether delayed PPD occurrence was related to a reduced oxidative burst, ROS generation and mitochondrial localization were assayed by the use of the oxidation-sensitive fluorescent probe dihydrorhodamine 123 (DHR) and MitoTracker-Deep Red FM, two mitochondrion-selective probes, on the storage roots of the wild type and SC2 (Fig. 5B). Immediately after harvest, the fresh storage roots from the wild type and SC2 showed only a very weak fluorescent signal along the xylem vessels (Fig. 5B), no difference being observed between the wild type and the transgenic line. After 24 h, bright fluorescence was detected, not only in the xylem vessels but also in the parenchyma cells of the wild-type storage roots, thereby showing an increased production and accumulation of ROS during the PPD process (Fig. 5B); the fluorescent signal in the SC2 storage roots was much weaker, indicating either a relatively lower mitochondrial ROS generation or an improved capacity of ROS scavenging (Fig. 5B). Overall, ROS, especially H2O2, were mainly located in the cortical and internal storage parenchyma associated with xylem vessels at the onset of PPD.
To determine whether the additional overexpression of MeCu/ZnSOD and MeCAT1 was involved in scavenging excess ROS in storage roots during the postharvest period, H2O2 content at different PPD time points was measured. Generally, the trend of H2O2 content in wild-type storage roots increased during the time course (Fig. 5C), although a small reduction at 24 h was observed, indicating a possible enhanced turnover of H2O2 at that stage. After 12 h post harvest, all transgenic lines accumulated significantly less H2O2 in their storage roots than the wild type (Fig. 5C). For example, the change of H2O2 content in the SC2 sample at 12 and 96 h was from 3.42 to 3.96 mmol g−1 fresh weight, only a 15.7% increase; for the wild type, a 64% increase of H2O2 content was detected over the same period. Thus, there was a reduction in ROS accumulation in the transgenics compared with the wild type. We deduce that this is due to increased ROS turnover, but it could also be due to reduced ROS production.
Expression and Activity Patterns of SOD and CAT Enzymes during the PPD Process
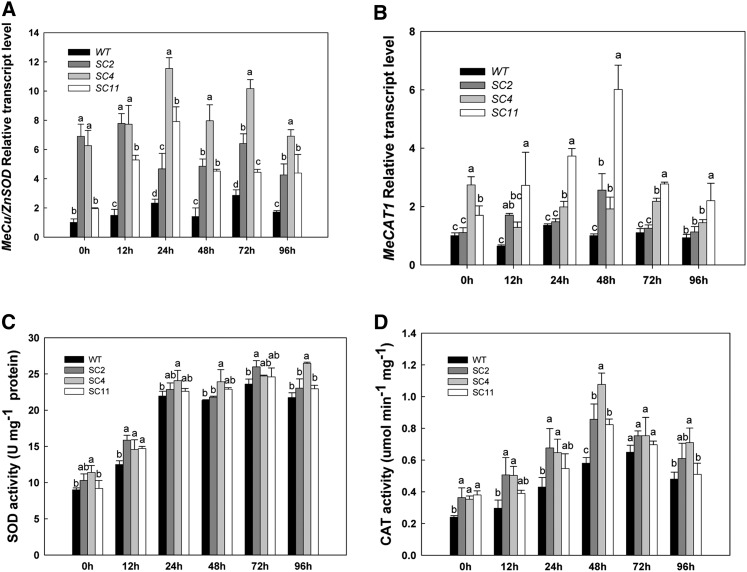
During the PPD process, relative higher levels of MeCu/ZnSOD and MeCAT1 transcripts were detected at all time points in transgenic samples than in the wild type by qRT-PCR analysis (Fig. 6, A and B). In the three transgenic lines, the expression levels of MeCu/ZnSOD were higher than those of MeCAT1, which indicated that the p54/1.0 promoter from cassava was stronger than the CaMV 35S promoter in the storage roots, consistent with the report by Zhang et al. (2003). Furthermore, the cassava promoter was not affected or inducible by the PPD process (data not shown). The three independent lines also showed some variation in transcript patterns of MeCu/ZnSOD and MeCAT1 between each line, although all showed significantly increased expression levels for both gene constructs compared with the wild type.
Figure 6.
Expression and activity patterns of SOD and CAT enzymes during the PPD process. A and B, Overall up-regulated expression of MeCu/ZnSOD and MeCAT1 during PPD in transgenic plants compared with the wild type (WT). C and D, Higher levels of SOD and CAT activities during PPD in transgenic cassava compared with the wild type. Values labeled with different letters (a, b, and c) at the same time point are significantly different by Duncan’s multiple comparison tests at P < 0.05.
Total cellular SOD activities in wild-type and transgenic plants dramatically increased from 0 to 24 h during the postharvest period and remained at a high level from 24 to 96 h (Fig. 6, C and D). For the wild type, the SOD activity was 9 units mg−1 protein at 0 h and 23.6 units mg−1 protein at 72 h. For transgenic line SC2, its activity was 10.3 units mg−1 protein at 0 h and 26 units mg−1 protein at 72 h. The pattern of CAT activity was different from that of SOD, showing a gradual increase followed by a decline after 72 h for the wild type and after 48 h for transgenic lines (Fig. 6D). The SOD and CAT activities in storage roots after harvest reflect their central role in ROS turnover and scavenging, which is key to the modulation of the PPD response.
DISCUSSION
Cassava PPD is a complex physiological and biochemical process involving changes of gene expression, protein synthesis, and the accumulation of secondary metabolites (Huang et al., 2001; Reilly et al., 2007). Studies on the mechanism of the PPD response are of importance, not only in increasing our molecular understanding of this unique biological phenomenon in an important root crop but also for their potential in developing an effective approach to control postharvest losses. Several studies have shown that PPD is caused by an oxidative burst in the vascular bundle cells and starch-rich parenchyma cells of cassava storage roots and that ROS appear to play a central role both in triggering the onset of PPD and in symptom causation through the oxidation of phenolic compounds, as evidenced by the up-regulation of gene expression and protein biosynthesis related to ROS turnover (Reilly et al., 2007; Owiti et al., 2011). A recent study showed that cyanide released by mechanical damage of cassava roots caused during harvesting results in the buildup of ROS and that the overexpression of Arabidopsis AtAOX (a cyanide-resistant terminal oxidase in plants) in cassava reduced the accumulation of ROS and delayed PPD (Zidenga et al., 2012). However, while PPD was delayed, field-grown roots had reduced biomass compared with controls, which might be due to the heterologous expression of AtAOX affecting normal mitochondrial activity during root development (Zidenga et al., 2012).
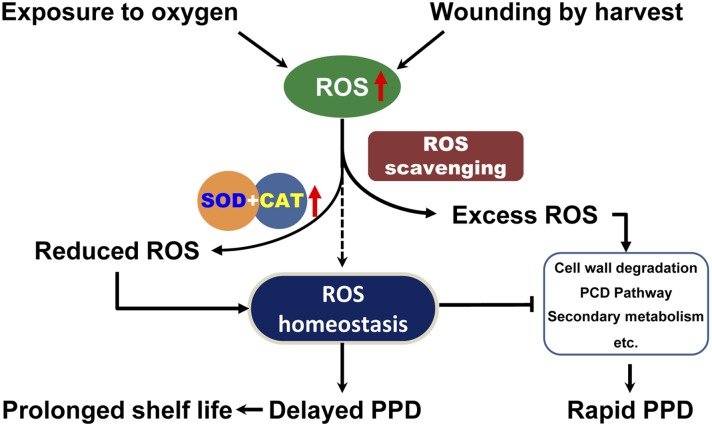
In plants, ROS homeostasis, a delicate balance between cell defense and signaling, plays important roles in a diverse range of biological processes and responses to environmental stimuli (Alscher et al., 2002; Miller et al., 2010). In cassava, increases of ROS have been observed from immediately after root harvesting into the early stages of PPD, via ROS detection or by using radical-specific fluorescent imaging (Reilly et al., 2000, 2001, 2004; Iyer et al., 2010; Zidenga et al., 2012). In this study, we confirmed that the transcripts of MeCu/ZnSOD and MeCAT1 were up-regulated during PPD in the wild type (Fig. 6), which is consistent with previous reports (Reilly et al., 2007, Owiti et al., 2011). However, their profiles, together with the results of SOD and CAT enzymatic activity, were shown to be different, indicating different behaviors of the two genes in response to the oxidative burst in storage roots. We found that H2O2 content increased slightly between 0 and 12 h and then suddenly dropped to a low amount by 24 h over the PPD time course. These data suggest that in the early stages, the superoxide radical is immediately converted to H2O2 and that the available CAT was sufficient to catalyze the conversion of H2O2 to water and oxygen, thereby leading to the reduction of H2O2 concentration at 24 h (Fig. 5C). However, with the continued accumulation of more H2O2, the total capacity of CAT was not sufficient to scavenge the excess H2O2, despite its activity gradually increasing, a possible reason for the initiation of PPD symptoms, as highlighted by our model (Fig. 7). The additional expression of MeCu/ZnSOD and MeCAT1 in the transgenic plants resulted in enhanced ROS scavenging, as observed through fluorescent detection (Fig. 5B), leading to the delay of PPD occurrence (Fig. 5A). This confirms the intrinsic relationship between ROS scavenging and PPD occurrence (Fig. 7).
Figure 7.
Schematic illustration of the intrinsic relationship between ROS production, ROS scavenging, and ROS homeostasis for regulating PPD in cassava storage roots. Exposure to oxygen or mechanical wounding during harvest leads to increased ROS production in the storage root. Inefficient endogenous ROS scavenging results in excess ROS, which triggers rapid PPD responses, and stable ROS homeostasis in harvested cassava roots is never achieved. Ectopic expression of SOD and CAT leads to timely scavenging of excess ROS, thereby keeping the ROS homeostasis balanced and delaying PPD occurrence. [See online article for color version of this figure.]
Generally, more ROS will be produced in stressed cells, which leads to cellular damage (An et al., 2012). In transgenic cassava, leaves under MV and H2O2 treatment showed stronger tolerance against these stresses and improved viability compared with the wild type (Figs. 2–4), demonstrating that the overexpression of MeCu/ZnSOD and MeCAT1 reduced ROS accumulation in transgenic plants. The overexpression of SODs can lead to protection against specific stresses, implying that SOD may be the first line of defense against ROS (Gupta et al., 1993; Perl et al., 1993; McKersie et al., 1996, 2000). But Tepperman and Dunsmuir (1990) were unable to detect high resistance to MV in tobacco plants that expressed a petunia (Petunia hybrida) chloroplast Cu/ZnSOD 50-fold compared with control plants. However, when the overexpression resulted in a moderate increase in SOD activity, the transgenic plants were tolerant to oxidative stress, as the equilibrium between oxygen radicals and H2O2 was maintained (Perl et al., 1993). The authors suggested that elevating SOD without coelevating enzymes that remove H2O2 could not provide protection against ROS toxicity. Importantly, CAT, a key H2O2-scavenging enzyme that is mainly located in peroxisomes, had been shown to be important in protecting plant cells against stresses; overexpression of CAT in tobacco or rice (Oryza sativa) improved paraquat, drought, or salt tolerance (Shikanai et al., 1998; Miyagawa et al., 2000; Moriwaki et al., 2007). CAT activity was higher in the less susceptible cultivars during the postharvest period (Reilly et al., 2001), while our data show that the ectopic overexpression of CAT led to delayed PPD, suggesting that high CAT activity may play a pivotal role in modulating PPD in cassava.
Several studies have demonstrated that gene stacking (e.g. of SOD and APX) could synergistically increase ROS turnover, thereby enhancing stress tolerance in a range of plant species (Kwon et al., 2002; Tang et al., 2006; Lee et al., 2007; Faize et al., 2011). In this study, the in vivo imaging of ROS using the fluorescent probe rhodamine-123, a stain readily sequestered by active mitochondria, in cassava mesophyll protoplasts or DAB staining of leaves showed the improved tolerance of transgenic cassava cells to oxidative stress caused by H2O2 and MV (Figs. 3 and 4). MV treatment in the presence of light leads to the generation of superoxide radicals and H2O2 in chloroplasts. The increased SOD and CAT enzyme activities were able to rapidly scavenge ROS at the site of generation as well as prevent the formation of hydroxyl radicals. Therefore, this study convincingly demonstrates that coexpression of the antioxidant enzymes SOD and CAT in transgenic cassava leads to a synergistic effect that not only reduces ROS levels but also delays cassava PPD (Fig. 7).
In plant cells, most of the ROS produced originate from chloroplasts or peroxisomes, but in nongreen tissues, or in the dark, mitochondrial ROS production predominates. Therefore, the fluorescent probe DHR and MitoTracker-Deep Red FM were used to monitor ROS in cassava storage root organelles during PPD. The DHR probe targets mitochondria and, when oxidized by H2O2 or superoxide anion radical, yields fluorescent cationic and lipophilic probes, while MitoTracker-Deep Red FM just targets and labels mitochondria (Gomes et al., 2005; Swanson et al., 2011). Root parenchyma tissue stained with the two probes revealed a strong fluorescence (Fig. 5), which initially localized to the vicinity of the xylem vessels where vascular streaking symptoms occur. Thus, we showed that the early accumulation of H2O2 as well as superoxide anion radicals in mitochondria in cassava roots leads to postharvest deterioration. These data imply the centrality of ROS and their modulation in the PPD response, confirming the important role of ROS in the development of PPD (Reilly et al., 2007; Iyer et al., 2010; Zidenga et al., 2012).
In conclusion, coexpression of MeCu/ZnSOD and MeCAT1 in cassava could dramatically improve ROS-scavenging ability, leading to reduced H2O2 accumulation, improved abiotic stress resistance, and delayed PPD occurrence. It also confirms the current model of oxidative burst as a key player in initiating ROS and that enhanced ROS-scavenging capacity represses PPD occurrence.
MATERIALS AND METHODS
Plasmid Construction and Transformation
Cassava (Manihot esculenta) Cu/ZnSOD (GenBank accession no. AY642137) and CAT1 (GenBank accession no. AY170272) were controlled by the vascular-specific promoter p54/1.0 (GenBank accession no. AY217353.1; Zhang et al., 2003) and the CaMV 35S promoter, respectively. The two expression cassettes were inserted into the binary vector pCAMBIA1301 containing the hygromycin phosphotransferase gene (hpt) under the control of the CaMV 35S promoter to generate pC-P54::MeCu/ZnSOD-35S::MeCAT1 (Supplemental Fig. S1A). The construct was introduced into Agrobacterium tumefaciens strain LBA4404, which was then used for genetic transformation. Embryogenic callus induction of cassava TMS60444 and A. tumefaciens-mediated genetic transformation were performed as described by Zhang et al. (2000).
Plant Materials
Four-week-old in vitro plantlets of transgenic and control plants were transplanted into pots (25 cm in diameter × 20 cm in height) containing 5 kg of well-mixed soil (soil:peat:perlite, 1:1:1) and grown in the greenhouse (16 h/8 h of light/dark, 30°C/22°C day/night). One-month-old plants were taken for subsequent physiological analysis. For field evaluation, 10 2-month-old plantlets per transgenic line and the wild type were planted on May 20, 2011, in the Wushe Plantation for Transgenic Crops, Shanghai (31°13′48.00″N, 121°28′12.00″E), and harvested on November 1, 2011. The entire trial plot was surrounded by a border row of nontransgenic plants to reduce edge effects. The performance of field plants was recorded regularly until harvest.
Molecular Analysis of Transgenic Plants
Genomic DNA was isolated from in vitro-cultured cassava leaf tissue according to the procedure of Soni and Murray (1994). To screen positive transgenic plants, one primer was designed to bind the promoter region (5′-ATGGCCCTCCATTATTTACACT-3′ for p54/1.0 and 5′-TGTGAAGATAGTGGAAAAGGAAGG-3′ for CaMV 35S) and another one bound to the gene region (5′-AACAACGACTGCCCTTCCTACAAT-3′ for MeCu/ZnSOD and 5′-CTCAGGATGGTGTGATAAGAAGTC-3′ for MeCAT1). Nontransformed plants were used as a control. PCR was performed using the following conditions: 95°C for 5 min, 30 cycles of 95°C for 1 min, 57°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 10 min. Finally, PCR products (759 bp for MeCu/ZnSOD and 823 bp for MeCAT1) were separated on a 1.0% agarose gel and visualized by ethidium bromide staining.
For Southern-blot analysis, 20 µg of genomic DNA was digested with XbaI, separated by electrophoresis on a 0.8% (w/v) agarose gel, and transferred onto a positively charged nylon membrane (Roche). HPT (1 kb) and MeCAT1 (1.5 kb) were labeled with digoxigenin using the PCR DIG Probe Synthesis Kit (Roche). Hybridization and detection were performed according to the manufacturer’s instructions using the DIG-High Prime DNA Labeling and Detection Starter Kit II (Roche).
The expression levels of MeCu/ZnSOD and MeCAT1 transgenes were determined by real-time qRT-PCR. β-Actin was used as a reference for normalization. Total RNA was extracted from cassava leaves and/or storage roots using the RNA Plant Plus Reagent (Tiangen). The RNA samples were digested with DNase I, and first-strand complementary DNA was synthesized from 5 µg of total RNA from each sample using Moloney murine leukemia virus reverse transcriptase (Toyobo). qRT-PCR was performed using the Bio-Rad CFX96 thermocycler SYBR Green I Master mix (Toyobo) according to the supplier’s protocols. PCR amplification was conducted by a 1-min preincubation step at 95°C followed by 40 cycles of 95°C for 15 s, 60°C for 15 s, and 72°C for 20 s. The primers were as follows: β-Actin (forward, 5′-TGATGAGTCTGGTCCATCCA-3′; reverse, 5′-CCTCCTACGACCCAATCTCA-3′), MeCu/ZnSOD (forward, 5′-ATGTTCATGCCCTTGGAGAC-3′; reverse, 5′-GATCACCAGCATGACGAATG-3′), and MeCAT1 (forward, 5′-TGGGAAACAACTTCCCTGTC-3′; reverse, 5′-ACATCATCGAAGAACCAGGC-3′).
Isolation and Viability Assay of Mesophyll Protoplasts
In vitro-cultured cassava seedlings were grown at 25°C with light at 200 µmol m−2 s−2 and a photoperiod of 16 h/8 h of light/dark. The second and third fully expanded leaves (from the apex) were used as a source of protoplasts. Protoplasts were isolated as described by Anthony et al. (1995). Protoplast viability was determined by the uptake of fluorescein diacetate and observed using a fluorescence microscope (Nikon TE2000-S; Larkin, 1976). The purified protoplasts were treated with CPW9M solution (27.2 mg L−1 KH2PO4, 101 mg L−1 KNO3, 1,480 mg L−1 CaCl2∙2H2O, 246 mg L−1 MgSO4∙7H2O, and 9% mannitol, pH 7.0) supplemented with 1 m H2O2 for 5 min and then stained with 0.01% fluorescein diacetate. Each sample was counted for about 300 cells. The viable percentage = (number of protoplasts with green fluorescence)/(number of total mesophyll protoplasts) × 100 (Duan et al., 2009).
Analysis of Mitochondrial Integrity
Cassava mesophyll protoplasts were treated with 1 m H2O2 for 5 min after staining with 10 µg mL−1 rhodamine123 (Molecular Probes-Invitrogen). Fluorescence was detected using a confocal laser scanning microscope (Zeiss LSM 510 META) with excitation/emission of 488/515 nm. Twenty to 25 cells were measured for each sample.
Treatment with MV and H2O2
Fully expanded healthy leaves were excised from plants and floated on different solutions of chemicals in 10-cm-diameter petri dishes. For the MV treatment, leaves were floated on 50 mL of 100 µm MV, while for the H2O2 treatment, leaves were floated on 50 mL of 0.5 m H2O2. Leaves were incubated at 25°C for 48 h under continuous light.
Determination of Chlorophyll Content
Chlorophyll was isolated from leaves according to the procedure of Hu et al. (2005). One gram of leaf tissues was ground and extracted with 10 mL of absolute ethyl alcohol. The absorbance of the supernatant was measured spectrophotometrically at 663, 646, and 470 nm, and chlorophyll content was calculated as described by Porra et al. (1989).
Determination of Lipid Peroxidation
Lipid peroxidation in leaf tissues was measured in terms of MDA in the samples according to Dhindsa and Matowe (1981). Extraction was performed by homogenization of 1 g of leaf tissue with 10 mL of 10% (w/v) TCA, after which the homogenate was centrifuged at 10,000g for 10 min. Then, 2 mL of 10% TCA containing 0.67% (w/v) thiobarbituric acid was added to 2 mL of the supernatant. The mixture was incubated at 100°C for 15 min and centrifuged at 10,000g at 4°C for 10 min. The absorbance of the supernatant was recorded at 532 nm and corrected for nonspecific turbidity by subtracting the A600.
Determination of H2O2 Content and DAB Staining
The H2O2 content was measured colorimetrically as described by Velikova et al. (2000). One gram of tissues was homogenized in an ice bath with 10 mL of 0.1% TCA. The homogenate was centrifuged at 10,000g for 15 min. One milliliter of the supernatant was added to 1 mL of 10 mm potassium phosphate buffer (pH 7.0) and 2 mL of 1 m potassium iodide. The absorbance of the supernatant was read at 390 nm. The content of H2O2 was given on a standard curve.
H2O2 was visualized by staining with DAB according to Thordal-Christensen et al. (1997). Detached leaves were infiltrated with 10 mL of DAB solution (1 mg mL−1 DAB, pH 3.8) for 8 h. Leaves were immersed in 95% (w/v) boiling ethanol for 10 min to decolorize the chloroplast.
Enzyme Assays
For the analysis of SOD isozymes, the protein extracts were separated on a 10% native polyacrylamide gel with a 4% stacking gel in standard Tris-Gly buffer (pH 8.3). Samples were electrophoresed at 100 V through the stacking gel for 20 min and at 120 V through the separating gel for 60 min. After electrophoresis, the gel was soaked in 0.1% (w/v) nitroblue tetrazolium (NBT) solution for 15 min, rinsed with distilled water, and transferred to 100 mm potassium phosphate buffer (pH 7.0) containing 0.028 mm riboflavin and 28 mm N,N,N′,N′-tetramethyl-ethylenediamine for another 15 min. After being washed with distilled water, the gel was illuminated on a light box, with a light intensity of 30 mE m−2 s−1 for 15 min, to initiate the photochemical reaction (Chen and Pan, 1996).
SOD activity was determined based on the method of Beauchamp and Fridovich (1971), which measured inhibition of the photochemical reduction of NBT at 560 nm. Each 3-mL reaction mixture contained 50 mm potassium phosphate buffer (pH 7.8), 13 mm Met, 75 mm NBT, 2 mm riboflavin, 0.1 mm EDTA, and 100 mL of enzyme extract. The reaction was carried out in test tubes at 25°C under a light intensity of 5,000 lux. One unit of SOD was defined as the amount of enzyme required to inhibit the reduction of NBT by 50%.
For the analyses of CAT isozymes, the protein extracts were separated on a 7.5% native polyacrylamide gel with a 3.5% stacking gel in standard Tris-Gly buffer (pH 8.3). Samples were electrophoresed in the same conditions as for the SOD isozymes. After electrophoresis, the gel was soaked in 0.01% H2O2 solution for 5 min, washed twice in water, and incubated for 5 min in 1% FeCl3 and 1% K3[Fe(CN)6] (Zimmermann et al., 2006).
CAT activity was determined by following the consumption of H2O2 at 240 nm for 4 min (Aebi, 1983). The reaction mixture contained 50 mm potassium phosphate buffer (pH 7.0), 10 mm H2O2, and 200 mL of enzyme extract in a final volume of 2 mL at 25°C.
Visual PPD Evaluation
Based on the method of PPD evaluation from the International Center for Tropical Agriculture (Wheatley et al., 1985; Reilly et al., 2004; Morante et al., 2010; Zidenga et al., 2012), the proximal and distal ends of cassava storage roots were removed immediately after harvest. Proximal ends were exposed in the air, and distal ends of the root were covered with Parafilm. Roots were stored at 21°C to 28°C and 70% to 80% relative humidity. Evaluation of root sections was conducted at 0, 12, 24, 48, 72, and 96 h. Quantitation of vascular discoloration was done on captured images using ImageJ image-processing and analysis software (http://rsb.info.nih.gov/ij/).
Fluorescence Measurement of PPD in Storage Root
The formation of ROS was determined by DHR and MitoTracker-Deep Red FM, which detect mitochondria oxidation-sensitive specific localization (Joo et al., 2001; Miller et al., 2009).
Fresh cassava storage roots from wild-type and transgenic lines were cut into smaller segments about 5 mm in length and width, then immediately soaked in sodium phosphate buffer (0.1 m). DHR (50 µm; Molecular Probes-Invitrogen) and MitoTracker-Deep Red FM (250 nm; Molecular Probes-Invitrogen) were added to each solution for 10 and 20 min, respectively. Fluorescence was detected using a confocal laser scanning microscope (Zeiss LSM 510 META) with excitation/emission of 488/515 nm and 635/680 nm for DHR and MitoTracker-Deep Red FM, respectively.
Statistical Analyses
All data are represented as means ± sd from at least three independent experiments with three replicates. Statistical analysis was conducted using ANOVA, which was performed by using SPSS Statistics 17.0 to Duncan’s multiple comparison tests. A value of P < 0.05 was considered a statistically significant difference.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AY642137, AY170272, and AY217353.1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Schematic presentation of the transfer DNA region of pC-P54::MeCu/ZnSOD-35S::MeCAT1 and Southern-blot analysis of transgenic cassava lines.
Acknowledgments
We thank Mr. Xiaoshu Gao (Core Facility, Shanghai Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences) for helping with the confocal laser scanning microscope and Prof. John Fellman (Washington State University) for technical advice on ROS imaging in cassava root.
Glossary
- PPD
postharvest physiological deterioration
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- SOD
superoxide dismutase
- APX
ascorbate peroxidase
- CAT
catalase
- MV
methyl viologen
- CaMV
cauliflower mosaic virus
- qRT
quantitative reverse transcription
- DAB
3,3′-diaminobenzidine
- MDA
malondialdehyde
- DHR
dihydrorhodamine 123
- NBT
nitroblue tetrazolium
References
- Aebi HE (1983) Catalase. In HU Bergmeyer, ed, Methods of Enzymatic Analyses, Vol 3. Verlag Chemie, Weinheim, Germany, pp 273–286 [Google Scholar]
- Alscher RG, Erturk N, Heath LS. (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53: 1331–1341 [PubMed] [Google Scholar]
- An D, Yang J, Zhang P. (2012) Transcriptome profiling of low temperature-treated cassava apical shoots showed dynamic responses of tropical plant to cold stress. BMC Genomics 13: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony P, Davey MR, Power JB, Lowe KC. (1995) An improved protocol for the culture of cassava leaf protoplasts. Plant Cell Tissue Organ Cult 42: 229–302 [Google Scholar]
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Asada K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44: 276–287 [DOI] [PubMed] [Google Scholar]
- Beeching JR, Han Y, Gómez-Vásquez R, Day RC, Cooper RM. (1998) Wound and defense responses in cassava as related to post-harvest physiological deterioration. Recent Adv Phytochem 32: 231–248 [Google Scholar]
- Buschmann H, Rodriguez MX, Tohme J, Beeching JR. (2000) Accumulation of hydroxycoumarins during postharvest deterioration of tuberous roots of cassava (Manihot esculenta Crantz). Ann Bot (Lond) 86: 1153–1160 [Google Scholar]
- Chavez AL, Bedoya JM, Sánchez T, Iglesias C, Ceballos H, Roca W. (2000) Iron, carotene, and ascorbic acid in cassava roots and leaves. Food Nutr Bull 21: 410–413 [Google Scholar]
- Chen CN, Pan SM. (1996) Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Bot Bull Acad Sin 37: 107–111 [Google Scholar]
- Contreras Rojas M, Pérez JC, Ceballos H, Baena D, Morante N, Calle F. (2009) Introduction of inbreeding and analysis of inbreeding depression in eight S1 cassava families. Crop Sci 49: 543–548 [Google Scholar]
- Dhindsa RS, Matowe W. (1981) Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot 32: 79–91 [Google Scholar]
- Duan XG, Song YJ, Yang AF, Zhang JR. (2009) The transgene pyramiding tobacco with betaine synthesis and heterologous expression of AtNHX1 is more tolerant to salt stress than either of the tobacco lines with betaine synthesis or AtNHX1. Physiol Plant 135: 281–295 [DOI] [PubMed] [Google Scholar]
- Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G, Clemente-Moreno MJ, Alcobendas R, Artlip T, Hernandez JA. (2011) Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot 62: 2599–2613 [DOI] [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima JL. (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65: 45–80 [DOI] [PubMed] [Google Scholar]
- Gupta AS, Heinen JL, Holaday AS, Burke JJ, Allen RD. (1993) Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 90: 1629–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin C. (2005) Gene stacking in transgenic plants: the challenge for 21st century plant biotechnology. Plant Biotechnol J 3: 141–155 [DOI] [PubMed] [Google Scholar]
- Hu Y, Jia W, Wang J, Zhang Y, Yang L, Lin Z. (2005) Transgenic tall fescue containing the Agrobacterium tumefaciens ipt gene shows enhanced cold tolerance. Plant Cell Rep 23: 705–709 [DOI] [PubMed] [Google Scholar]
- Huang J, Bachem C, Jacobsen E, Visser RGF. (2001) Molecular analysis of differentially expressed genes during postharvest deterioration in cassava (Manihot esculenta Crantz) tuberous roots. Euphytica 120: 85–93 [Google Scholar]
- Iyer S, Mattinson DS, Fellman JK. (2010) Study of the early events leading to cassava root postharvest deterioration. Tropical Plant Biol 3: 151–165 [Google Scholar]
- Joo JH, Bae YS, Lee JS. (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126: 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SY, Joeng YJ, Lee HS, Kim JS, Cho KY, Allen RD, Kwak SS. (2002) Enhanced tolerance of transgenic tobacco plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against methyl viologen mediated oxidative stress. Plant Cell Environ 25: 873–882 [DOI] [PubMed] [Google Scholar]
- Larkin PJ. (1976) Purification and viability determinations of plant protoplasts. Planta 128: 213–216 [DOI] [PubMed] [Google Scholar]
- Lee SH, Ahsan N, Lee KW, Kim DH, Lee DG, Kwak SS, Kwon SY, Kim TH, Lee BH. (2007) Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol 164: 1626–1638 [DOI] [PubMed] [Google Scholar]
- McKersie BD, Bowley SR, Harjanto E, Leprince O. (1996) Water-deficit tolerance and field performance of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol 111: 1177–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Murnaghan J, Jones KS, Bowley SR. (2000) Iron-superoxide dismutase expression in transgenic alfalfa increases winter survival without a detectable increase in photosynthetic oxidative stress tolerance. Plant Physiol 122: 1427–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Honig A, Stein H, Suzuki N, Mittler R, Zilberstein A. (2009) Unraveling delta1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J Biol Chem 284: 26482–26492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33: 453–467 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Miyagawa Y, Tamoi M, Shigeoka S. (2000) Evaluation of the defense system in chloroplasts to photooxidative stress caused by paraquat using transgenic tobacco plants expressing catalase from Escherichia coli. Plant Cell Physiol 41: 311–320 [DOI] [PubMed] [Google Scholar]
- Morante N, Sánchez T, Ceballos H, Calle F, Pérez JC, Egesi C, Cuambe CE, Escobar AF, Ortiz D, Chávez AL, et al (2010) Tolerance to postharvest physiological deterioration in cassava roots. Crop Sci 50: 1333–1338 [Google Scholar]
- Moriwaki T, Yamamoto Y, Aida T, Funashi T, Shishido T, Asada M, Prodhan SH, Komanine A, Motohashi T. (2007) Overexpression of the Escherichia coli catalase katE, enhances tolerance to salinity stress in the transgenic indica rice cultivar, BR5. Plant Biotechnol Rep 2: 41–46 [Google Scholar]
- Owiti J, Grossmann J, Gehrig P, Dessimoz C, Laloi C, Hansen MB, Gruissem W, Vanderschuren H. (2011) iTRAQ-based analysis of changes in the cassava root proteome reveals pathways associated with post-harvest physiological deterioration. Plant J 67: 145–156 [DOI] [PubMed] [Google Scholar]
- Payton P, Allen RD, Trolinder N, Holaday AS. (1997) Overexpression of chloroplast-targeted Mn superoxide dismutase in cotton (Gossypium hirsutum L., cv. Coker 312) does not alter the reduction of photosynthesis after short exposures to low temperature and high light intensity. Photosynth Res 52: 233–244 [Google Scholar]
- Payton P, Webb R, Kornyeyev D, Allen R, Holaday AS. (2001) Protecting cotton photosynthesis during moderate chilling at high light intensity by increasing chloroplastic antioxidant enzyme activity. J Exp Bot 52: 2345–2354 [DOI] [PubMed] [Google Scholar]
- Perl A, Perl-Treves R, Galili S, Aviv D, Shalgi E, Malkin S, Galun E. (1993) Enhanced oxidative stress defence in transgenic potato expressing tomato Cu,Zn superoxide dismutases. Theor Appl Genet 85: 568–576 [DOI] [PubMed] [Google Scholar]
- Pitcher LH, Brennan E, Hurley A, Dunsmuir P, Tepperman JM, Zilinskas BA. (1991) Overproduction of petunia chloroplastic copper/zinc superoxide dismutase does not confer ozone tolerance in transgenic tobacco. Plant Physiol 97: 452–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedmann PE. (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Raychaudhuri SS, Deng XW. (2000) The role of superoxide dismutase in combating oxidative stress in higher plants. Bot Rev 66: 89–98 [Google Scholar]
- Reilly K, Bernal D, Cortés DF, Gómez-Vásquez R, Tohme J, Beeching JR. (2007) Towards identifying the full set of genes expressed during cassava post-harvest physiological deterioration. Plant Mol Biol 64: 187–203 [DOI] [PubMed] [Google Scholar]
- Reilly K, Gómez-Vásquez R, Buschmann H, Tohme J, Beeching JR. (2004) Oxidative stress responses during cassava post-harvest physiological deterioration. Plant Mol Biol 56: 625–641 [DOI] [PubMed] [Google Scholar]
- Reilly K, Han Y, Tohme J, Beeching JR (2000) Oxidative stress related genes in cassava post-harvest physiological deterioration. In LJCB Carvalho, AM Thro, AD Vilarinhos, eds, IV International Scientific Meeting Cassava Biotechnology Network, Embrapa, Brasilia. pp 560–571 [Google Scholar]
- Reilly K, Han Y, Tohme J, Beeching JR. (2001) Isolation and characterisation of a cassava catalase expressed during post-harvest physiological deterioration. Biochim Biophys Acta 1518: 317–323 [DOI] [PubMed] [Google Scholar]
- Rickard JE. (1985) Physiological deterioration in cassava roots. J Sci Food Agric 36: 167–176 [Google Scholar]
- Sayre R, Beeching JR, Cahoon EB, Egesi C, Fauquet C, Fellman J, Fregene M, Gruissem W, Mallowa S, Manary M, et al. (2011) The BioCassava plus program: biofortification of cassava for sub-Saharan Africa. Annu Rev Plant Biol 62: 251–272 [DOI] [PubMed] [Google Scholar]
- Shikanai T, Takeda T, Yamauchi H, Sano S, Tomizawa KI, Yokota A, Shigeoka S. (1998) Inhibition of ascorbate peroxidase under oxidative stress in tobacco having bacterial catalase in chloroplasts. FEBS Lett 428: 47–51 [DOI] [PubMed] [Google Scholar]
- Soni R, Murray JAH. (1994) Isolation of intact DNA and RNA from plant tissues. Anal Biochem 218: 474–476 [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Choi WG, Chanoca A, Gilroy S. (2011) In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu Rev Plant Biol 62: 273–297 [DOI] [PubMed] [Google Scholar]
- Tang L, Kwon SY, Kim SH, Kim JS, Choi JS, Cho KY, Sung CK, Kwak SS, Lee HS. (2006) Enhanced tolerance of transgenic potato plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against oxidative stress and high temperature. Plant Cell Rep 25: 1380–1386 [DOI] [PubMed] [Google Scholar]
- Tepperman JM, Dunsmuir P. (1990) Transformed plants with elevated levels of chloroplastic SOD are not more resistant to superoxide toxicity. Plant Mol Biol 14: 501–511 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Tseng MJ, Liu CW, Yiu JC. (2007) Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol Biochem 45: 822–833 [DOI] [PubMed] [Google Scholar]
- van Oirschot QEA, O’Brien GM, Dufour D, El-Sharkawy MA, Mesa E. (2000) The effect of pre-harvest pruning of cassava upon root deterioration and quality characteristics. J Sci Food Agric 80: 1866–1873 [Google Scholar]
- Velikova V, Yordanov I, Edreva A. (2000) Oxidative stress and some antioxidant system in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151: 59–66 [Google Scholar]
- Wheatley C, Lozano C, Gomez G (1985) Post-harvest deterioration of cassava roots. In JH Cock, JA Reyes, eds, Cassava Research, Production and Utilization. United Nations Development Programme-International Center for Tropical Agriculture, Cali, Colombia, pp 655–671 [Google Scholar]
- Zhang P, Bohl-Zenger S, Puonti-Kaerlas J, Potrykus I, Gruissem W. (2003) Two cassava promoters related to vascular expression and storage root formation. Planta 218: 192–203 [DOI] [PubMed] [Google Scholar]
- Zhang P, Potrykus I, Puonti-Kaerlas J. (2000) Efficient production of transgenic cassava using negative and positive selection. Transgenic Res 9: 405–415 [DOI] [PubMed] [Google Scholar]
- Zidenga T, Leyva-Guerrero E, Moon H, Siritunga D, Sayre R. (2012) Extending cassava root shelf life via reduction of reactive oxygen species production. Plant Physiol 159: 1396–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Heinlein C, Orendi G, Zentgraf U. (2006) Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ 29: 1049–1060 [DOI] [PubMed] [Google Scholar]