Abstract
Purpose
The aim of this prospective study is the analysis of the clinical and radiological outcomes of active thoraco-lumbar spinal tuberculosis treated with isolated posterior instrumentation without any posterior bone grafting or anterior inter-body bone grafting or anterior instrumentation.
Methods
The study was a prospective follow-up of 25 patients with active thoraco-lumbar spinal tuberculosis who underwent posterior spinal instrumentation with pedicle screws and rods. These patients had posterior stabilization of the involved segment of the spine without anterior or posterior bone grafting. The mean duration of follow-up was 3.3 years and the minimum duration of follow-up was 2 years.
Results
The mean kyphotic angle improved from 32.4° pre-operatively to 7.2° in the early follow-up period. Following a minor loss of correction during follow-up, the mean kyphotic angle settled at 11.5° at the time of final follow-up. Inter-body bony fusion was noticed at the final follow-up in all patients despite the absence of anterior bone grafting or cages.
Conclusion
Posterior instrumented stabilization followed by chemotherapy seems to be adequate for obtaining satisfactory healing of the lesions. Anterior inter-body bony arthrodesis occurs despite the absence of anterior bone grafts or cages. Careful patient selection is critical for successful outcome with this technique.
Keywords: Spinal tuberculosis, Posterior spinal fusion, Posterior instrumentation, Tubercular spondylitis
Introduction
Surgical management of tuberculosis of the spine has evolved considerably, since 1895 when Ménard decompressed tubercular abscess around spinal cord with gratifying results [1]. Posterior spinal fusion with bone grafting alone was introduced in 1911 by Hibbs and Albee [2, 3]. It was however, demonstrated independently by McKee and Seddon [4, 5] around 1937 that posterior bone grafting alone had either no palpable benefit and was even harmful in the presence of active tuberculosis. Anterior radical debridement and non-instrumented fusion was described by Ito and Asami in 1934 followed by Hodgson and Stock in 1956 [6, 7]. It became apparent in the later years that even anterior debridement and bone grafting was often unsuccessful in correction or prevention of progression of kyphosis. Higher degree of progression of kyphosis has been observed following non-instrumented anterior fusion compared to non-operative treatment [8]. With the advent of modern segmental spinal instrumentation systems, isolated anterior decompression and fusion and combined anterior and posterior fusion have been described by numerous authors. The aim of the study was to assess the functional and radiological outcomes of isolated posterior instrumented fusion in skeletally mature adults having tuberculous spondylitis with active disease that was associated with paraplegia of early onset in many patients. We suggest that posterior instrumented fusion alone is sufficient in adults with tuberculous spondylitis due to the following reasons: (1) effective chemotherapy is available at present to sterilize the vertebral body lesions without the need for aggressive anterior debridement or fusion, (2) complications peculiar to anterior approaches to the dorso-lumbar spine can be avoided, (3) posterior instrumentation serves to minimize the progress of kyphosis, while the anterior vertebral body lesion is healing, (4) para-spinal abscess, if present can be drained through the posterior approach itself, and (5) since the lesion heals with anterior inter-body bony fusion, there is no need for posterior bone grafting also.
Patients and methods
This was a prospective study of 25 patients diagnosed with tubercular spondylitis involving the dorsal and lumbar spine. Patients underwent single-stage posterior decompression laminectomy, drainage of abscess and posterior pedicle screw instrumentation. The procedures were done between October 2007 and April 2010. Patients with active tubercular spondylitis and patients with early-onset neurological involvement (para-paresis associated with active tubercular disease) were included in the study. Pre-operative kyphosis angle varied from 5° to 53°. Patients with severe and very severe kyphosis (more than 60°–70°), patients with late-onset paraplegia of healed disease with bony internal gibbus and multi-segmental disease with destruction of 3–4 vertebral bodies are not obviously suited for this type of intervention. Patients with disease characteristics were excluded from the study. Permission of the hospital ethics committee and written informed consent of the patients were obtained prior to commencing the study. There were 11 males and 14 females. The mean age of the patients was 44.9 years (range 17–80 years). The mean duration of follow-up was 3.3 years (range 2–4.5 years). Minimum follow-up period was 2 years. 13 patients had involvement of the dorsal spine, four patients had involvement of dorso-lumbar junctional area, and eight patients had involvement of the lumbar spine. 21 patients had single level disease and four patients had disease involving two contiguous disks. The percentage of involvement of the vertebral bodies varied from 25 % to near total collapse of the vertebral body. Duration of symptoms prior to initial presentation ranged from 1 to 8 months (mean of 4 months). 11 patients among the total of 25 patients had neurological involvement of the lower limbs at the time of initial presentation. Neurological assessment was performed using the Frankel grading. Three patients were in Frankel grade A, two patients were in Frankel grade B, three patients were in grade C, and three patients were in Frankel grade D. 14 patients without neurological deficits were included in Frankel grade E. All patients had pre-operative antero-posterior and standing radiographs of the spine, CT scan, MRI, and hematological investigations including complete blood counts, ESR, CRP, and Monteux tuberculin test. MRI was used to document the presence of vertebral body and cord edema, cord compression, para vertebral abscess, and skip lesions.
Surgery was performed under the cover of chemotherapy. Chemotherapy was instituted soon after the diagnosis was suspected, together with bracing of the spine and measures to improve general health and nutrition. Selection of patients for surgery was done using the “middle path regime” of Tuli as a guide [9]. Surgery was considered in the presence of the following indications: (1) persistent marked pain despite chemotherapy for 2 months, (2) significant vertebral body destruction or kyphosis at the time of initial presentation, and (3) progression of neurological deficit or appearance of fresh deficit during treatment with chemotherapy. Patients with kyphosis due to healed tubercular lesions, neurological deficit associated with old, healed lesion (paraplegia of late onset), bony internal gibbus, and marked intrinsic cord lesions were excluded from the study. These patients were excluded, because anterior and posterior decompression and spinal osteotomies are necessary in these patients for deformity correction and cord decompression. Four-drug regimen was started soon after the diagnosis was suspected (INH, Rifampicin, Ethambutol, and Pyrazinamide). Following the operation, four-drug chemotherapy was continued for 4 months followed by two-drug regimen (INH and Rifampicin) for a further period of 8 months.
Under general anesthesia, patients were positioned prone on the spinal table using the Wilson’s frame. The position of the affected vertebrae was confirmed using fluoroscopy. The involved segment was exposed via mid-line posterior approach. The screws were temporarily connected by a rod on one side to avoid instability during decompression. Hemi-laminectomy or total laminectomy was done at the involved segment. Granulation tissue was debrided and abscess, if present, was drained through the inter-transverse space. Pedicle screws were connected by rods and the compression was applied to correct kyphosis. No posterolateral or anterior inter-body bone grafting or cage application was done. Intra-operative biopsy was obtained and sent for histopathological examination and PCR test. Instrumentation extended from one to two vertebral levels above the involvement to one to two levels below the level of involvement. The span of the fixation depended on the bone quality of the involved vertebrae and the vertebrae above and below the lesion and the degree of kyphosis. When adequate bone was left in the involved vertebrae (50 % or more of vertebral body was intact), pedicle screws were inserted into the involved vertebrae and one vertebra above and below the involved vertebrae. In the involved vertebrae, the screws were directed away from the involved end plates. When screws were not inserted into the involved vertebrae, two healthy vertebrae above and two vertebrae below the involved segments were instrumented to ensure rigid fixation. Kyphosis was corrected as much as possible by applying compression between the screws before tightening them to the rods.
Mobilization was started in ambulant patients on the second post-operative day and physiotherapy and wheel-chair mobilization was started in non-ambulant patients. Post-operative bracing was continued for a minimum period of 3 months following surgery in all patients. Knight-Taylor brace (TLSO) was used in patients with vertebral involvement below D6 vertebral level and a modified Milwaukee brace (CTLSO) was used in patients with disease above the D5–D6 level. The disease was considered as healed when clinical evaluation showed no spinal tenderness or spasm, hematological parameters returned to near normal levels and sound fusion was evident on follow-up radiographs. Radiographically, fusion was considered as sound when there was bony bridge with trabeculation of the density equal to the adjacent vertebrae, radiolucent zones were absent, and no abnormal mobility (less than 5° of movement) was seen at the fused segment on post-operative flexion–extension radiographs. These criteria were described earlier by Schofferman et al. [10] and Kim et al. [11].
Results
The mean duration of operation was 156 min (range 128–191 min). The mean intra-operative blood loss was 667 ml (range 532–910 ml). The mean hospital stay was 6 days (range 4–8 days). All patients with neurological deficits showed improvement in Frankel grading post-operatively (Table 1). The mean pre-operative kyphosis angle was 32.4° (range 5°–54°). The angle of kyphosis reduced to a mean of 7.2° in the immediate post-operative period (range 0°–12°) and the difference was statistically significant (P < 0.005, student’s t test). At the time of final follow-up, the mean kyphotic angle was 11.5° (range 0°–17°). There was a mean 4.3° loss of kyphosis correction between immediate post-operative radiographs and final follow-up radiographs. Complications were minor in nature and included delayed wound healing in two patients, sinus formation in one patient. There were no major complications. The anterior vertebral lesion healed through sound bony fusion between 6 and 8 months post-operatively (Figs. 1, 2, 3). There was no significant loss of kyphosis correction once bony inter-body fusion was apparent on the radiographs. Among the 11 patients with neurological deficits, those patients in Frankel grades B and C improved to Frankel grades D/E following surgery. Three patients in grade D improved to grade E post-operatively. Among the three patients in grade A, one patient improved to grade D, and one patient improved to grade C. The remaining one patient, who was 80 years in age, improved to grade B and was able to sit with support post-operatively. He died 3 years later as a result of co-morbidities. Patients in Frankel grade E pre-operatively, continued to remain so following surgery without any deterioration in neurological function.
Table 1.
Pre- and post-operative Frankel grades
| Frankel grading (pre-operative) | Frankel grading (post-operative) | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| A | 1 | 1 | 1 | ||
| B | 2 | ||||
| C | 3 | ||||
| D | 3 | ||||
| E | 14 | ||||
Numerals refer to number of patients
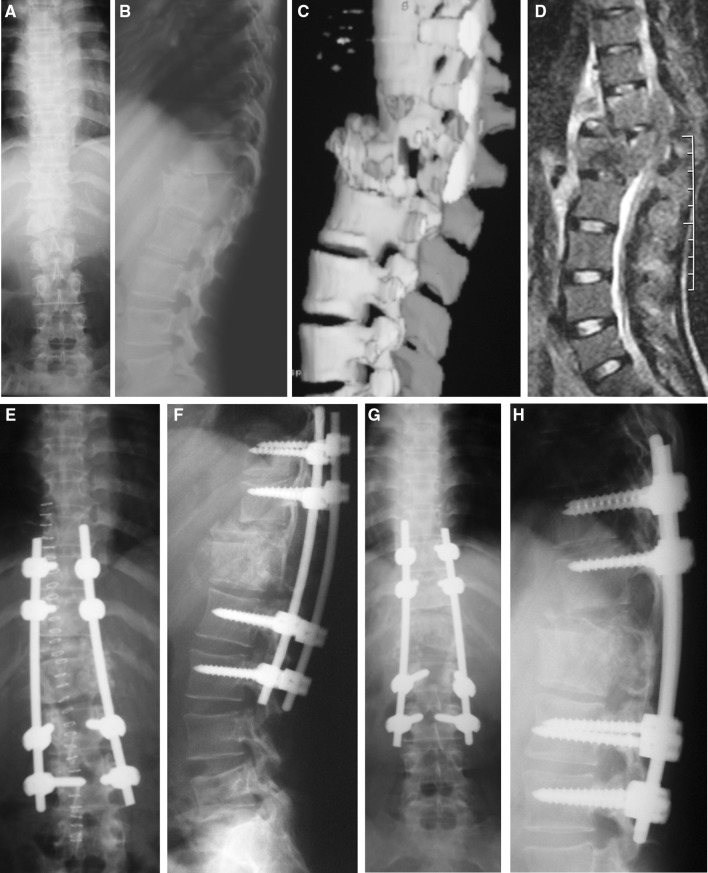
Fig. 1.
a Plain radiograph (A-P view) showing tubercular spondylitis involving the dorso-lumbar junction, b plain radiograph (lateral view) showing complete collapse of D12 body and posterior translation of D11 vertebra, c sagittal 3D CT image showing the amount of destruction of the D12 and L1 vertebrae, d sagittal T2 weighted MR image showing the degree of spinal canal compromise, e plain radiograph (A-P view) showing pedicle screw instrumentation of D10 and 11 proximally and L2 and 3 vertebrae distally (early post-operative phase), f plain radiograph (lateral view) showing restoration of sagittal profile at the dorso-lumbar junction (early post-operative phase), g plain radiograph (A-P view) showing maintenance of correction at 2 years post-operatively, and h plain radiograph (lateral view) showing bony fusion at D11–L1 junction with acceptable sagittal profile at 2 years
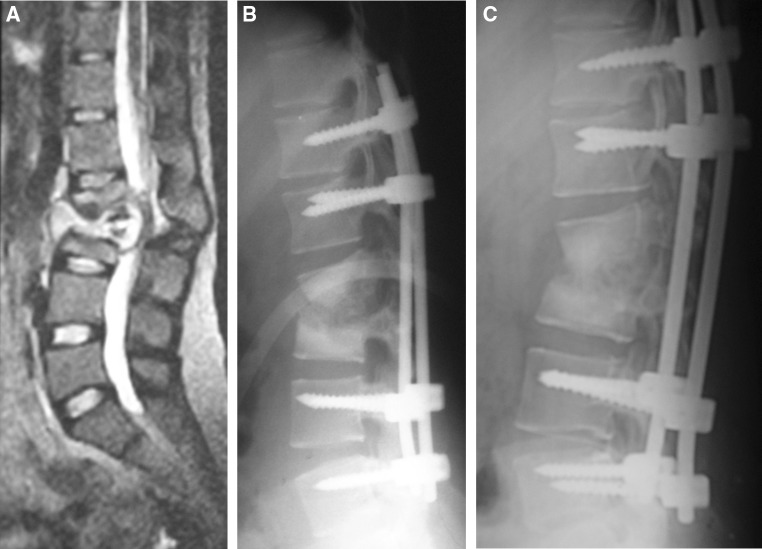
Fig. 2.
a Sagittal T2 weighted MR image showing tuberculous spondylitis at the L2–L3 disk space with canal compromise, b plain radiograph (lateral) showing pedicle screw instrumentation of D12 and L1 proximally and L4 and L5 distally (early post-operative phase), and c plain radiograph (lateral) showing maintenance of sagittal profile and bony fusion at L2–L3 disk space
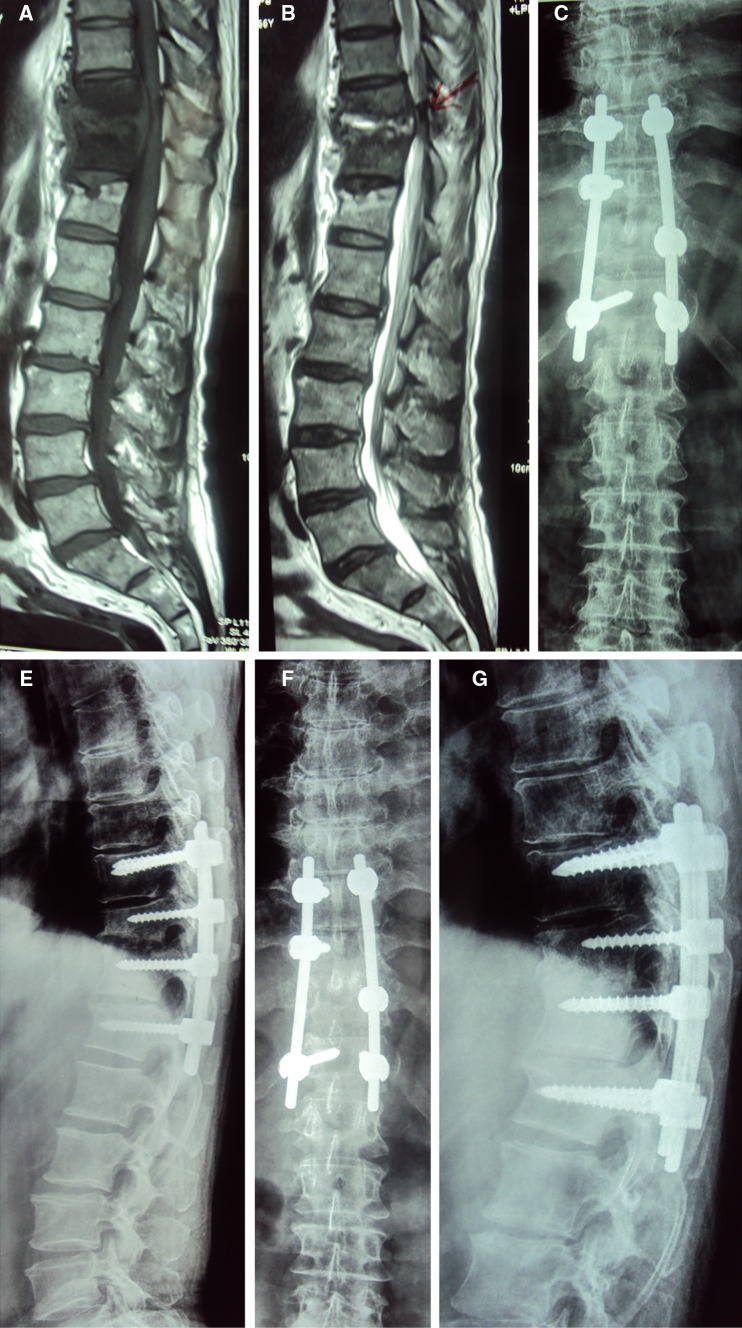
Fig. 3.
a T1 weighted MR image showing tuberculous spondylitis at the D10–D11 junction, b T2 weighted MR image of the same, c plain radiograph (A-P view) showing instrumentation from D9 to D12 vertebrae, d plain radiograph (lateral view) showing the gap at the D10–D11 disk space after instrumentation, e plain radiograph (A-P view) showing maintenance of correction at final follow-up, and f plain radiograph (lateral view) showing maintenance of sagittal profile and bony fusion at the D10–D11 disk space at final follow-up
Discussion
Following the advent of modern spinal instrumentation systems, several authors have reported combined anterior and posterior fusion either as a single-stage or as a two-stage procedure following the initial description of the procedure by Yau et al. [12–23] in 1974. Earlier apprehensions regarding the use of metal implants in the presence of active tubercular infection were overcome following the realization that tubercle bacilli had slow rates of division, lower bacillary counts than pyogenic infections, and do not produce adhesion molecule and bio-film [24, 25]. Two reports of isolated anterior instrumented fusion have been published where the authors have claimed good results [26, 27]. However, other authors have reported good outcomes with posterior fusion. Guven et al. [28] reported good results with isolated posterior instrumentation and fusion even without anterior debridement. Garst [29] reported that anterior debridement alone was insufficient and best results were achieved by combining posterior fusion with anterior debridement.
Anterior spinal surgery is not without complications. Some authors have considered anterior approach to be too invasive and often unnecessary in the context of spinal tuberculosis. Anterior approach may involve division of the diaphragm and segmental spinal vessels [26, 27]. Campbell et al. [30] have reported higher rates of complications with isolated anterior fusion and combined anterior and posterior spinal fusion compared to isolated posterior fusions. McDonnell et al. [31] have reported higher complication rates for single event anterior and posterior fusions compared with staged anterior and posterior fusions. They found 11 % incidence of major and 24 % incidence of minor complications. Chiriano et al. [32] found the incidence of minor vascular injuries (requiring repair) to be 24 %, while the incidence of serious vascular injury was 3 %. Jarrett et al. [33] reported that even the use of an expert “access surgeon” for anterior trans-lumbar approach did not reduce the incidence of complications. Vascular complications and retrograde ejaculation were notable among the complications reported by them. Extra-cavitary approaches to the anterior elements of the vertebrae have been in use for many decades and these include antero-lateral decompression and costo-transversectomy (for debridement and anterior bone grafting). As these approaches remain extra-pleural, post-operative intra thoracic complications can be minimized. However, bleeding from segmental vessels and occasionally from major vessels is still possible even with these approaches. In addition, morbidity of rib resection remains with these approaches also.
Anterior approach and antero-lateral approach have been conventionally preferred, because they provide direct access to involved vertebrae and allow direct decompression of the cord by removal of the tubercular debris and granulation tissue which cause predominantly anterior compression on the cord. They enable removal of internal gibbus (that may impinge upon the cord when the kyphosis is corrected) and increase space for the cord anteriorly [34, 35]. While these arguments are valid in the management of kyphosis of healed disease and late-onset paraplegia, they are not necessarily true in the management of kyphosis of active disease and early-onset paraplegia. In early-onset deformity or paraplegia there is no internal gibbus formation and there is no significant shortening of anterior neural structures in the spinal cord. The pathology of early tubercular spondylitis involves the presence of granulation tissue, edema, and abscess formation which have been shown to regress following chemotherapy. With chemotherapy, spontaneous inter-body bony healing has been observed to occur without anterior surgery [36, 37]. Natural history studies have also shown that spinal tuberculosis can lead to bony inter-body fusion once the intervertebral disk has been destroyed by infection [9].
Another reason for the popularity of anterior approach is the traditionally held concern that posterior decompression in spinal tuberculosis entails removal of the healthy posterior vertebral elements. In the presence of diseased anterior column, laminectomy is said to aggravate the spinal instability [32, 33]. Stability of the vertebral column following posterior decompression is no longer a major concern following the advent of contemporary posterior segmental spinal instrumentation systems. Moon et al. [38] reported the use of isolated posterior spinal fusion using Harrington rods in 1985. Subsequently, many authors have reported that posterior instrumentation alone is adequate to achieve significant deformity correction and stable fixation in patients with spinal tuberculosis. These authors have used segmental spinal instrumentation with pedicle screws and rods. In most of these reports, additional procedures such as posterior closing wedge osteotomy, and posterior inter-body fusion using bone grafts with or without cages have been performed [37, 39–44].
To the best of our knowledge, no other study has been reported previously documenting isolated posterior instrumented fusion using the contemporary segmental spinal instrumentation, without any additional procedure such as posterior wedge osteotomy and without anterior or posterior fusion. We avoided posterior bone grafting also, because the post-operative healing is through inter-body bony fusion following chemotherapy. This approach has the advantages of avoiding donor site morbidity related to posterior bone grafting and also, preserves both iliac crests for harvesting of bone grafts, if the procedure is required at a later date. Our observations are particularly valid in the present day management of tubercular spondylitis, where early diagnosis has been enabled due to the advent of MR imaging, and effective ambulatory chemotherapy is available to control the disease in early stages and limit bony destruction. Careful selection of patients is essential for this procedure. Prompt bony healing anteriorly is essential for success with this technique as a strong anterior bony column would satisfy the principles of “load-sharing.” According to the classification of Kumar (1998), tuberculous spondylitis with early active disease and belonging to the stages of early destruction, mild kyphosis (10°–30°), and moderate kyphosis (30°–60°) are best suited for this procedure [45]. Patients with severe and very severe kyphosis, patients with late-onset paraplegia of healed disease with bony internal gibbus and multi-segmental disease with destruction of 3–4 vertebral bodies are not obviously suited for this type of intervention. These categories of patients require combined anterior and posterior decompression and instrumented fusion for optimal outcomes. Patients with higher probability of having multi-drug resistant tuberculosis should not be selected for this method of treatment, since bone healing is likely to be delayed and unpredictable in these patients. This category of patients includes those with immunodeficiency states such as HIV associated tuberculosis and patients with previous history of non compliance with chemotherapy and those with relapsed tuberculosis and infection with known drug resistant strains. It is better to add fusion along with instrumentation in these categories of patients to minimize the risk of pseudarthrosis. Management techniques in severe post tubercular kyphosis have been discussed in detail by other authors [46–48].
There was a minor loss of kyphosis correction in the first post-operative year in our series of patients. After the first year, there was no further loss of kyphosis correction and our results were comparable to the results of posterior instrumentation and anterior inter-body fusion reported by Dai et al. [49]. Kyphosis did not increase in the late post-operative phase in our series, probably due to the fact that inter-body bony fusion occurred in all patients within a few months post-operatively and further vertebral collapse did not occur after this point. Chances of continued progression of kyphosis are increased in the presence of longer segment disease involvement, healing by fibrous ankylosis than with bony ankylosis and in children [34, 50]. Since our series did not include skeletally immature children, we cannot comment on the suitability of the procedure in this age group.
Conclusion
In this study, posterior segmental spinal instrumentation using pedicle screws and rods has been found to be adequate in reducing the kyphotic deformity and achieving anterior and posterior debridement of the involved segment in early, active tubercular spondylitis involving one or two disk spaces. Despite the absence of posterolateral or anterior inter-body bone grafts and the absence of anterior instrumentation, healing occurred reliably in all patients through spontaneous inter-corporeal bony fusion. There was a small degree of loss of kyphosis correction in the early post-operative period, but the correction remained stable after a few months and did not alter once bone healing had occurred. Careful patient selection is essential and the procedure is probably not suitable for rigid deformity associated with healed tuberculosis, late-onset para-paresis, patients who are non-compliant with chemotherapy, and patients with multi-drug resistant tuberculosis (healing of the tubercular lesions can be delayed in these patients). Its role in children needs to be examined in the future. Bracing is advisable for a minimum period of 3 months post-operatively and compliance with chemotherapy is mandatory to ensure healing of the lesions. Further studies are essential in this regard as isolated posterior instrumentation carries the potential benefits of less-invasive surgery without the morbidity of anterior exposures and bone graft donor sites.
Conflict of interest
None.
References
- 1.Moon M-S. Development in the management of tuberculosis of the spine. Curr Orthop. 2006;20:132–140. doi: 10.1016/j.cuor.2005.12.002. [DOI] [Google Scholar]
- 2.Hibbs RA. An operation for Pott’s disease of the spine. J Am Med Assoc. 1911;59:433–436. [PMC free article] [PubMed] [Google Scholar]
- 3.Albee FH. Transplantation of a portion of the tibia into the spine for Pott’s disease. A preliminary report. J Am Med Assoc. 1911;57:885–886. doi: 10.1001/jama.1911.04260090107012. [DOI] [PubMed] [Google Scholar]
- 4.McKee GK. A comparison of the results of spinal fixation operation and non-operative treatment in Pott’s disease in adults. Br J Surg. 1937;24:456–468. doi: 10.1002/bjs.1800249507. [DOI] [Google Scholar]
- 5.Seddon HJ. Pott’s paraplegia, prognosis and treatment. Br J Surg. 1938;22:769. doi: 10.1002/bjs.1800228813. [DOI] [Google Scholar]
- 6.Ito H, Asami T. A new radical operation for Pott’s disease. Report of ten cases. J Bone Jt Surg. 1934;15:499. [Google Scholar]
- 7.Hodgson AR, Stock FE. Anterior spinal fusion. A preliminary communication on radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44:266–275. doi: 10.1002/bjs.18004418508. [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran S, Soundarapandian S. Progression of kyphosis in tuberculosis of the spine treated by anterior arthrodesis. J Bone Jt Surg Am. 1989;71:1314–1323. [PubMed] [Google Scholar]
- 9.Tuli SM (2010) Tuberculosis of the skeletal system, 4th edn. Jaypee Publishers, Delhi, p 215
- 10.Schofferman J, Slosar P, Reynolds J, Goldthwaite N, Koestler M. A prospective randomized comparison of 270 degree fusions to 360 degree fusions (circumferential fusions) Spine. 2001;26(10):E207–E212. doi: 10.1097/00007632-200105150-00019. [DOI] [PubMed] [Google Scholar]
- 11.Kim KT, Lee SH, Lee YH, Bae SC, Suk KS. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine. 2006;31(12):1351–1357. doi: 10.1097/01.brs.0000218635.14571.55. [DOI] [PubMed] [Google Scholar]
- 12.Yau AC, Hsu LC, O’Brien JP, Hodgson AR. Tuberculous kyphosis correction with spinal osteotomy, halo pelvis distraction and anterior and posterior fusion. J Bone Jt Surg Am. 1974;56:1419–1434. [PubMed] [Google Scholar]
- 13.Moon MS. Combined posterior instrumentation and anterior inter-body fusion for active tuberculosis kyphosis of the thoraco-lumbar spine. Curr Orthop. 1991;5:177. doi: 10.1016/0268-0890(91)90039-3. [DOI] [Google Scholar]
- 14.Louw JA. Spinal tuberculosis with neurological deficit: treatment with anterior vascularized rib grafts, posterior osteotomies and fusion. J Bone Jt Surg Br. 1990;72:686–693. doi: 10.1302/0301-620X.72B4.2380228. [DOI] [PubMed] [Google Scholar]
- 15.Laheri VJ, Badhe NP, Dewnany GT. Single stage decompression, anterior inter-body fusion and posterior instrumentation for tuberculous kyphosis of the dorso-lumbar spine. Spinal Cord. 2001;39:429–436. doi: 10.1038/sj.sc.3101185. [DOI] [PubMed] [Google Scholar]
- 16.Kim DJ, Yun YH, Moon SH, Riew KD. Posterior instrumentation using compressive laminar hooks and anterior inter-body arthrodesis for the treatment of tuberculosis of the lower lumbar spine. Spine. 2004;29(13):275–279. doi: 10.1097/01.BRS.0000129027.68574.06. [DOI] [PubMed] [Google Scholar]
- 17.Saraph VJ, Bach CM, Krismer M, Wimmer C. Evaluation of spinal fusion using autologous anterior strut grafts and posterior instrumentation for thoracic/thoraco-lumbar kyphosis. Spine. 2005;30(14):1594–1601. doi: 10.1097/01.brs.0000170299.48246.28. [DOI] [PubMed] [Google Scholar]
- 18.Talu U, Gogus A, Ozturk C, Hamzaoglu A, Domanic U. The role of posterior instrumentation and fusion after anterior radical debridement and fusion in the surgical treatment of spinal tuberculosis: experience of 127 cases. J Spinal Disord Tech. 2006;19(8):554–559. doi: 10.1097/01.bsd.0000211202.93125.c7. [DOI] [PubMed] [Google Scholar]
- 19.Suh KT, Seong YJ, Lee JS. Simultaneous anterior and posterior surgery in the management of tuberculous spondylitis with psoas abscess in patients with neurological deficits. Asian Spine J. 2008;2(2):94–101. doi: 10.4184/asj.2008.2.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chunguang Z, Limin L, Rigao C, Yueming S, Hao L, et al. Surgical treatment of kyphosis in children in healed stages of spinal tuberculosis. J Pediatr Orthop. 2010;30(3):271–276. doi: 10.1097/BPO.0b013e3181d39899. [DOI] [PubMed] [Google Scholar]
- 21.El-Sharkawi MM, Said GZ. Instrumented circumferential fusion for tuberculosis of the dorso-lumbar spine. A single or double stage procedure? Int Orthop. 2012;36(2):315–324. doi: 10.1007/s00264-011-1401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Yuan H, Geng G, Shi J, Jin W. Posterior mono-segmental fixation, combined with anterior debridement and strut graft, for treatment of the mono-segmental lumbar spine tuberculosis. Int Orthop. 2012;36:325–329. doi: 10.1007/s00264-011-1475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao-bin Wang, Li J, Guo-hua Lü, Wang Bin LuC, Yi-Jun Kang. Single-stage posterior instrumentation and anterior debridement for active tuberculosis of the thoracic and lumbar spine with kyphotic deformity. Int Orthop. 2012;36:373–380. doi: 10.1007/s00264-011-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oga M, Arizono T, Takasita M, et al. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis. Clin Biol Study Spine. 1993;18:1890–1894. doi: 10.1097/00007632-199310000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Ha KY, Chung YG, Ryoo SJ. Adherence and bio-film formation of Staphylococcus epidermis and Mycobacterium tuberculosis on various spinal implants. Spine. 2004;29(24):1–6. doi: 10.1097/01.brs.0000147801.63304.8a. [DOI] [PubMed] [Google Scholar]
- 26.Benli IT, Acaroğlu E, Akalin S, Kis M, Duman E, Un A. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J. 2003;12(2):224–234. doi: 10.1007/s00586-002-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin D, Qu D, Chen J, Zhang H. One-stage anterior inter-body auto grafting and instrumentation in primary surgical management of thoraco-lumbar spinal tuberculosis. Eur Spine J. 2004;13(2):114–121. doi: 10.1007/s00586-003-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guven O, Kumano K, Yalcin S, Karahan M, Tsuji S. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine. 1976;19(9):1039–1043. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Garst RJ. Tuberculosis of the spine: a review of 236 operated cases in an underdeveloped region from 1954 to 1964. J Spinal Disord. 1992;5(3):286–300. doi: 10.1097/00002517-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Campbell PG, Malone J, Yadla S, Maltenfort MG, Harrop JS, et al. Early complications related to approach in thoracic and lumbar spine surgery: a single center prospective study. World Neurosurg. 2010;73(4):395–401. doi: 10.1016/j.wneu.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 31.McDonnell MF, Glassman SD, Dimar JR, 2nd, Puno RM, Johnson JR. Perioperative complications of anterior procedures on the spine. J Bone Jt Surg. 1996;78(6):839–847. doi: 10.2106/00004623-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Chiriano J, Abou-Zamzam AM, Jr, Urayeneza O, Zhang WW, Cheng W. The role of the vascular surgeon in anterior retroperitoneal spine exposure: preservation of open surgical training. J Vasc Surg. 2009;50(1):148–151. doi: 10.1016/j.jvs.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Jarrett CD, Heller JG, Tsai L. Anterior exposure of the lumbar spine with and without an “access surgeon”: morbidity analysis of 265 consecutive cases. J Spinal Disord Tech. 2009;22(8):559–564. doi: 10.1097/BSD.0b013e318192e326. [DOI] [PubMed] [Google Scholar]
- 34.Jain AK, Dhammi IK, Jain S, Mishra P. Kyphosis in spinal tuberculosis–prevention and correction. IJO. 2010;44(2):127–136. doi: 10.4103/0019-5413.61893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuli SM. Tuberculosis of the spine: a historical review. Clin Orthop Relat Res. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- 36.Jain S. Comment on Huang et al. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop. 2010;34(5):769–770. doi: 10.1007/s00264-010-0975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pu X, Zhou Q, He Q, Dai F, Xu J, Zhang Z, Branko KA. Posterior versus anterior surgical approach in combination with debridement, inter-body auto grafting and instrumentation for thoracic and lumbar tuberculosis. Int Orthop. 2012;36:307–313. doi: 10.1007/s00264-011-1329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon M-S, et al. Harrington rods in treatment of active spinal tuberculosis with kyphosis. J West Pac Orthop Assoc. 1986;23:53. [Google Scholar]
- 39.Guzey FK, Emel E, Bas NS, Hacisalihoglu S, Seyithanoglu MH, Karacor SE, Ozkan N, Alatas I, Sel B. Thoracic and lumbar tuberculous spondylitis treated by posterior debridement, graft placement, and instrumentation: a retrospective analysis in 19 cases. J Neurosurg Spine. 2005;3(6):450–458. doi: 10.3171/spi.2005.3.6.0450. [DOI] [PubMed] [Google Scholar]
- 40.Lee JS, Moon KP, Kim SJ, Suh KT. Posterior lumbar inter-body fusion and posterior instrumentation in the surgical management of lumbar tuberculous spondylitis. J Bone Jt Surg Br. 2007;89(2):210–214. doi: 10.2106/JBJS.F.00437. [DOI] [PubMed] [Google Scholar]
- 41.Lee SH, Sung JK, Park YM. Single stage transpedicular decompression and posterior instrumentation in the treatment of thoracic and thoraco-lumbar spinal tuberculosis: a retrospective case series. J Spinal Disord Tech. 2006;19:595–602. doi: 10.1097/01.bsd.0000211241.06588.7b. [DOI] [PubMed] [Google Scholar]
- 42.Gokce A, Ozturkmen Y, Mutlu S, Caniklioğlu M. Spinal osteotomy: correcting sagittal balance in tuberculous spondylitis. J Spinal Disord Tech. 2008;21(7):484–488. doi: 10.1097/BSD.0b013e3181586023. [DOI] [PubMed] [Google Scholar]
- 43.Bezer M, Kucukdurmaz F, Guven O. Transpedicular decancellous osteotomy in the treatment of post-tuberculous kyphosis. J Spinal Disord Tech. 2007;20:209–215. doi: 10.1097/01.bsd.0000211271.89485.f1. [DOI] [PubMed] [Google Scholar]
- 44.Kalra KP, Dhar SB, Shetty P, Dhariwal Q. Pedicle subtraction for rigid post tuberculous kyphosis. J Bone Jt Surg Br. 2006;88:925–927. doi: 10.1302/0301-620X.88B7.17366. [DOI] [PubMed] [Google Scholar]
- 45.Kumar K. Tuberculosis of the spine—natural history of the disease and its judicious management. J West Pac Orthop Assoc. 1998;25:1–18. [Google Scholar]
- 46.Erturer E, Tezer M, Aydogan M, Mirzanlı C, Ozturk I. The results of simultaneous posterior-anterior-posterior surgery in multilevel tuberculosis spondylitis associated with severe kyphosis. Eur Spine J. 2010;19(12):2209–2215. doi: 10.1007/s00586-010-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajasekaran S, Vijay K, Shetty AP. Single-stage closing-opening wedge osteotomy of spine to correct severe post-tubercular kyphotic deformities of the spine: a 3-year follow-up of 17 patients. Eur Spine J. 2010;19(4):583–592. doi: 10.1007/s00586-009-1234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Zhang Y, Zhang X, Wang Z, Mao K, Chen C, Zheng G, Li G, Wood KB. Posterior-only multilevel modified vertebral column resection for extremely severe Pott’s kyphotic deformity. Eur Spine J. 2009;18(10):1436–1441. doi: 10.1007/s00586-009-1067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai LY, Chen WH, Jiang LS. Anterior instrumentation for the treatment of pyogenic vertebral osteomyelitis of thoracic and lumbar spine. Eur Spine J. 2008;17(8):1027–1034. doi: 10.1007/s00586-008-0661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Upadhyay SS, Saji MJ, Sell P, Sell B, Hsu LC. Spinal deformity after childhood surgery for tuberculosis of the spine: a comparison of radical surgery and debridement. J Bone Jt Surg Br. 1994;76:91–98. [PubMed] [Google Scholar]