Abstract
Purpose
Our aim is to define the role of embolization in the treatment of aneurysmal bone cyst of the spine in order to include this option in the decision making process.
Methods
From April 2004 to November 2009, seven patients with primary aneurysmal bone cyst of the mobile spine treated by embolization have been prospectively followed-up. All clinical presentations and imagings were recorded. There are many options of embolic agent and techniques used, but all aim to devascularize the tumor. The therapeutic protocol includes: embolization repeated every 8 weeks until the appearance of radiographic signs of healing. Complications, rate of healing and clinical outcome were analyzed.
Results
The number of embolizations varied from one to a maximum of seven without related intra- or post-operative complications. One patient, after four selective arterial embolizations, underwent direct percutaneous injection of embolic agents into the cyst. A clinical and radiographical response was achieved in all patients who were found alive and completely free of disease at mean follow-up of 46 months after last treatment and nobody crossed to surgical option.
Conclusion
Embolization seems to be the first option for spinal aneurysmal bone cyst treatment because of the best cost-to-benefit ratio. It is indicated in intact aneurysmal bone cyst, when diagnosis is certain, when technically feasible and safe and when no pathologic fracture or neurologic involvements are found. If embolization fails, other options for treatment would still be available.
Keywords: Aneurysmal bone cyst, Primary bone tumor, Mobile spine, Embolization
Introduction
Aneurysmal bone cyst (ABC) is a benign cystic lesion of bone, composed of blood-filled spaces separated by connective tissue septa containing fibroblasts, osteoclast-type giant cells and reactive woven bone [1]. Its origin is unknown. It was first described by Jaffe and Lichtenstein [2] in 1942, when it was differentiated from hemangiomas and other tumors with giant cells. It can occur as primary bone lesion (which consists of 70 % of the cases), or secondary (in about 30 % of the cases) [1], when ABC-like areas are found inside other bone conditions (giant cell tumors, chondroblastoma, osteoblastoma, telangiectatic osteosarcoma) [3].
Primary ABC is a rare entity and is included among benign tumors (representing 1.0–1.4 % of all primary bone tumors) even if its origin could not be neoplastic [1]. The metaphyseal region of long bones are most likely to be affected, especially femur and tibia [1]. 10 to 30 % is located in the mobile spine and corresponds to 15 % of all primary spine bone tumors [4].
As a benign lesion, ABC can be classified according to Enneking [5] in stage 1 (benign latent), stage 2 (benign active) and stage 3 (benign but locally aggressive). Some lesions can present with a very rapid growth and local aggressiveness. The most common symptom is local pain and swelling; however, when it is localized in the spine, it can cause cord or root compression associated with neurological deficit, deformity and instability.
Most cases are identified in the first two decades of life with predominance between 10 and 20 years of life. It rarely occurs after 30 years of age, even though it has been described in patients older than 50 years old [1]. Diagnosis is achieved by biopsy on histologic typical pattern, but imaging is sometimes pathognomonic when “fluid–fluid” levels are found, in computerized tomography scan (CT) and conventional radiography (X-ray), confirmed in magnetic resonance images (MRI).
The treatment of ABC varies widely. Options for treating ABCs have included simple curettage with or without bone grafting, complete excision, embolization, radiation therapy, or a combination of these methods [3, 4]. Progression to malignancy has been described in the literature after treated with radiation therapy [6–8]. Although there are good results regarding the type of treatment, local recurrence has been described within all protocols of therapy and cannot be predicted. The cases treated by “en bloc” resection were never followed by recurrence, but this treatment possibly exposes the patient to high surgical morbidity [9] and theoretically could be considered as overtreatment.
Because ABC is a relatively rare lesion, most of the reports found in the literature are small series of cases or retrospective series analyses. There are six major retrospective series reports [3, 6, 8, 10–12]. In the previous article published by the senior author [3], a retrospective report on a series of 41 patients that were treated by all sorts of therapeutic modalities, showed that good results can be obtained independently by the type of treatment performed. The authors also concluded that selective arterial embolization (SAE) should be the first choice of treatment for most of the patients with ABC in the spine, because of the low cost-to-benefit ratio.
Based on this conclusion, a prospective study was designed and started in 2004. The aim of the study was to answer the research question: can SAE heal ABC? All the eligible patients with a diagnosis of ABC were treated with embolization, possibly repeated.
Methods
The inclusion criteria were primary ABC lesions of the mobile spine, no previous treatment, no pathological fracture, neither instability (defined according to SINS, Spine Instability Neoplastic Score [13]: score inferior to 12) nor symptomatic cord compression.
All the eligible patients referred starting April 2004 were submitted to the same diagnostic protocol including: standard radiograms, CT and MRI. Histological diagnosis was confirmed by the same pathologist on a specimen achieved by CT scan guided biopsy.
The therapeutic protocol includes: embolization by the same neuro-radiologist according to the technique later described, repeated each 8 weeks until the appearance of radiographic signs of healing.
In the period from April 2004 to November 2009, seven consecutive patients (Table 1) with primary and intact ABC lesions in the spine entered the study. In the considered time no case occurred with exclusion criteria, except two cases occurring in the sacrum. ABC of the sacrum is associated with different clinical and therapeutic problems.
Table 1.
Summary of presentation, treatments and follow-up
| ID | Age (years) | Gender | Pain duration (weeks) | Other symptoms | Frankel initial | Site | Staging (WBB) sectors/layers | Years of first SAE | Number of SAE | SAE with particles > feeding arteries originated from: | SAE with Acrylic glue > feeding arteries originated from: | SAE with Coils | Microwire dissection particles > feeding arteries originated from: | Direct injection | Follow-up (months) after last treatment | Frankel final | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.M. | 16 | M | 54 | E | L1 | 7–2/A–D | 2004 | Ia | T12 right and L2 right | L1 right | CDF (49) | E | |||||
| IIa | T12 right and L1 right L1 left | ||||||||||||||||
| IIIa | L1 right | T12 right | |||||||||||||||
| M.G. | 6 | F | 50 | E | C4 | 2–4/B–C | 2005 | Ia | Thyrocervical trunk and ascending cervical left | CDF (64) | E | ||||||
| D.B.M.L | 12 | F | 24 | E | C7 | 8–1/A–D | 2005 | Ia | Ascending cervical artery right | Ascending cervical artery right | CDF (62) | E | |||||
| IIa | Thyrocervical trunk right | ||||||||||||||||
| IIIa | Ascending cervical artery right | ||||||||||||||||
| IVa | Ascending cervical artery right | Ascending cervical artery right | |||||||||||||||
| Va | Thyrocervical trunk right | ||||||||||||||||
| VIa | Thyrocervical trunk right | ||||||||||||||||
| VIIa | Thyrocervical trunk right | ||||||||||||||||
| C.G. | 12 | F | 45 | Root pain and deltoid weakness (right) | E | C5 | 8–10/A–F | 2006 | Ia | Right vertebral artery | CDF (52) | E | |||||
| IIa | Thyrocervical trunk right | ||||||||||||||||
| C.G. | 21 | M | 12 | Root pain and quadriceps weakness (left) | E | L3 | 2–6/A–D | 2007 | Ia | L3 right + left | CDF (42) | E | |||||
| IIa | L2 left | ||||||||||||||||
| IIIa | L3 left | L2 left | |||||||||||||||
| IVa | L3 left | L3 left | |||||||||||||||
| Ia | |||||||||||||||||
| IIa | |||||||||||||||||
| IIIa | |||||||||||||||||
| IVa | |||||||||||||||||
| Va | |||||||||||||||||
| VIa | |||||||||||||||||
| S.S. | 12 | F | 78 | E | T11 | 4–9/B–D | 2007 | Ia | T11 left | CDF (31) | E | ||||||
| D.G. | 41 | M | 12 | Root pain (right) and subjective lower limbs weakness | E | T5 | 5–1/B–D | 2009 | Ia | T6 right | CDF (24) | E | |||||
| T6 | 9–11/B–D | ||||||||||||||||
| IIa | T5 e T6 right | ||||||||||||||||
| IIIa | T5 e T6 right | ||||||||||||||||
| IVa | T5 right + left | ||||||||||||||||
| Va | T4 right and T5 right + left | ||||||||||||||||
There were four women, and three men, with the mean age of 17 years ranging from 6 to 41. Three cases occurred in the cervical spine, two in the thoracic, two in the lumbar spine.
A gradual onset of pain (mean 39 weeks, range 12–78) was the constant symptom.
The extent of the lesion was described in CT scans axial view according to the Weinstein-Boriani-Biagini system: in five patients the epidural space was involved (layer D) [14].
Nerve root compressions were observed in three cases.
A case of CT scan detected cord compression (Fig. 5) was not considered a contraindication to inclusion in the protocol as he just complained of some lower legs subjective feeling of weakness without any objective clinical sign or reflex change.
Fig. 5.
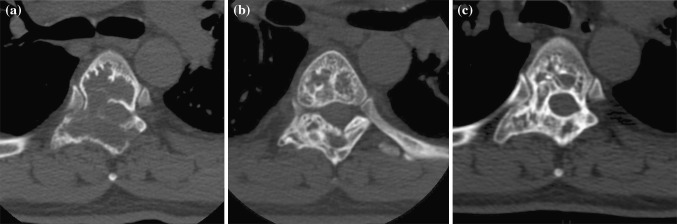
D.G., T5-T6 aneurysmal bone cyst in a 41-year-old man. a CT scan at presentation detects cord compression. b CT scan 8 weeks from the last SAE. c Follow-up CT scan, 24 months after the last treatment session: the cyst ossified
Treatment protocol
After the first embolization, the patients had to be reviewed after 8 weeks, CT scan and/or MRI carefully observed to detect any sign of peripheral ossification or progression of the disease and angiogram to evaluate the ABC vascularization. If progression is seen with volume increasing, instability or neurological deterioration, cross-over to surgery had to be considered. Conversely, a second embolization is performed. This process had to be repeated as many times as necessary as long as consistent signs of healing are observed. Based on the previous experience, signs of healing are defined as: peripheral sclerotic bone rim formation, shrink of the ABC mass, disappearance of the double content image, and bone formation inside the ABC mass.
Once these images achieved, the patient had to be followed for 2 further years each 6 months with MRI.
Angiography and embolization technique
All patients underwent angiography and embolization under local anesthesia and with intravenous analgesia when necessary.
The angiographic protocol included selective angiography of the vertebral arteries, thyro-costocervical trunks, ascending cervical arteries and external carotid arteries on both sides for cervical lesions; vertebral arteries, and supreme intercostal arteries for upper thoracic lesions; the segmental arteries within at least two levels above and below the tumor site for thoracic and L1 lesions; upper and the lower lumbar arteries for lesions from L2 to L4. In our series, the arteries supplying tumors in C4 and C7 originated from the ascending cervical artery and thyrocervical trunk, while in C5 from the vertebral artery and the thyrocervical trunk. All feeding arteries in tumors from thoracolumbar spine arose from the intercostal or lumbar arteries.
Special attention was paid to identification of the anterior spinal artery (ASA), because inadvertent embolization may injure the pyramidal tracts and cause a severe motor deficit. The presence of an ASA at the same pedicle is an obvious contraindication for embolization. In addition, particular caution must be taken when attempting embolization of the feeders in the adjacent levels because of the numerous collateral branches surrounding the vertebral column and to avoid accidental diffusion of embolic material to the nearest metameric artery from which the ASA arises (especially when located below).
Selective arterial embolization
A total number of 23 embolizations were performed. The technique includes microcatheterization and infusion of embolic agents only into the feeding arteries via the microcatheter thus sparing normal uninvolved arteries (Fig. 1).
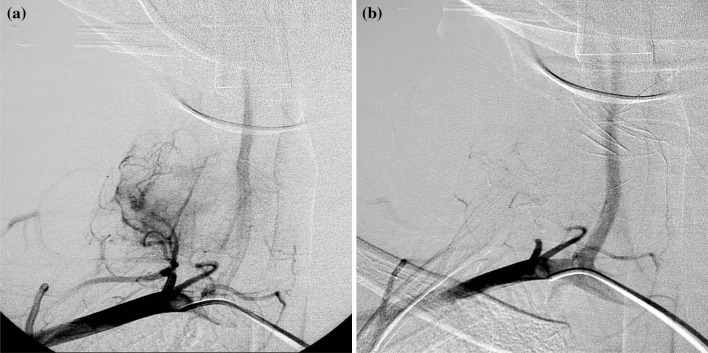
Fig. 1.
D.B.M.L., aneurysmal bone cyst of C7 in a 12-year-old woman. Angiography shows: a a feeding arteries originated from the thyrocervical trunk; b devascularization of pathological circulation by SAE with acrylic glue
Selective arterial embolization procedures were performed using particles (14 cases) and acrylic glue (11 cases); in 5 cases the embolic materials were used simultaneously in the same session.
The particles were nonabsorbable tris-acryl gelatine microspheres (TAGs), ranging in size from 100 to 300 μm (Embosphere®).
Particles have several advantages like good penetration within the lesion, easy to handle and available in different sizes depending on the type of pathological circulation. Yet, they do entail some disadvantages.
Make the embolization “unstable”: recanalization of the thrombosed vessel is readily triggered by a proteolytic enzyme cascade at different times after embolization depending on the type of particles injected.
Small particles have the advantage of good intralesional penetration, but they can lead to hazardous embolization of unplanned locations through invisible shunts in intra and extracranial locations or in the spinal cord; on the contrary larger particles (250–350 micron or more) are safer, but tend to agglutinate allowing a more proximal embolization.
In view of these drawbacks, when the characteristics of tumor vascularization and flow are amenable, we prefer to use acrylic glue like Glubran 2® (GEM Srl, Viareggio, Italy), suitable for permanent SAE of the pathological circulation, good tumor penetration even into minute comb-shaped vessels, resembles the angiographic image of superselective catheterization and avoids the too distal embolization typical of particles.
Intravascular penetration closely depends on dilution with Lipiodol® (Guerbet, Roissy CdG Cedex, France) that was mixed at different concentrations (1:1, 1:2, 1:3). A high dilution of the glue (Glubran 2®:Lipiodol® mixture of 1:3) proved especially useful for easier, more effective penetration to reach more distal branches and was the mixture rate most often used by us.
Injection
One patient, after four SAE, underwent direct percutaneous injection into the cyst via a 18 G needle, with a mixture of Glubran 2 and iodized oil, under CT guidance.
Coil
In one patient with C5 tumor (Fig. 2), the vertebral artery was occluded with detachable coils (GDC®, Boston Scientific, Natick, Massachusetts).
Fig. 2.
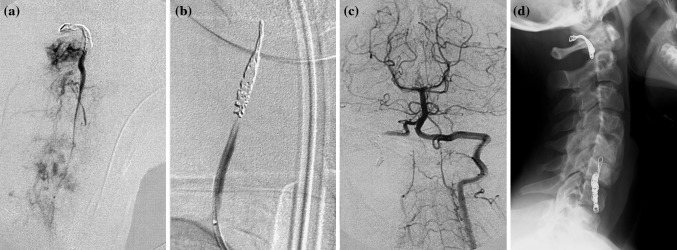
C.G., aneurysmal bone cyst of C5 in a 12-year-old woman. a Proximal spiral coil to the level of C1/C2 (V2–V3 angle) below the PICA origin. b Distal coil below the lesion (C6). c Control angiography shows lack of blood flow from C2 to C6. d Xray in sagittal view shows the coil
In the diagnostic phase the caliber of the contralateral vertebral artery was verified: it was larger than the vertebral artery to be occluded, a temporary vertebral artery occlusion test was not considered necessary. A 5F guide-catheter was placed at the origin of vertebral artery, then the microcatheter for coil detachment was pulled in close proximity to the level of C1/C2 (V2–V3 angle), below the posterior inferior cerebellar artery (PICA) origin, to form a stable anchor in the artery at this level. For this purpose, a spiral coil of a slightly oversized dimension was chosen and built a stable basket by pull-and-push movements of the microcatheter. Before coil detachment, the stability of the basket was verified and the clinical status of the patient as well for at least 10 min. Creation of the distal coil basket was followed by placement of a series of microcoils until occlusion was deemed to have been obtained. No neurological symptoms occurred after coil embolization.
Mechanical dissection
Embolization was contraindicated in three cases by the presence of the ASA arising from the same pedicle feeding the lesion. The small feeders were occluded by mechanical dissection due to the microwire. The occlusion was stable and effective for the purposes of the procedure.
Control angiography
Irrespective of the chosen SAE technique, a repeat angiogram was obtained immediately after embolization.
Results
The hospital discharge protocol included standard radiograms and CT scan for all patients every time that they were submitted to embolization. The mean duration of hospitalization was 2.2 days, varying from 2 to 5 for each time that the patient was submitted to the procedure.
All the patients were followed prospectively, with the follow-up time ranging from 24 to 64 months (average 46 months) after the occurrence of healing images.
CT evidence of regression and recalcification of the lesions was noted in all patients at last follow-up. No complications related to the embolization were observed.
The number of embolizations varied from one to a maximum of seven in one female patient with C7 lesion (D.B.M.L.).
Three patients with a SINS between 8 and 12 were included in the protocol and gained full stability after the treatment.
The cervical spine of one of these patients (D.B.M.L.) was immobilized with a Halo-vest the first 45 days, then with orthosis until healing of the lesion occurred at 11 months after the first SAE. She is continuing disease free at 62 months of follow-up (Fig. 3).
Fig. 3.
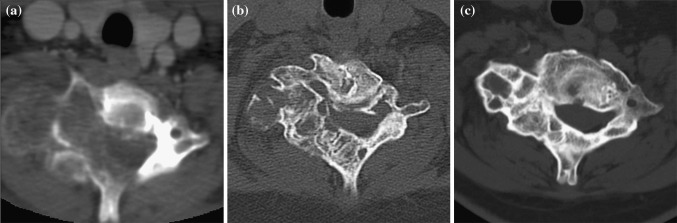
D.B.M.L., aneurysmal bone cyst of C7 in a 12-year-old woman. a CT scan at presentation shows the right vertebral artery completely embedded in the lesion. b CT scan 8 weeks from the last SAE. c Follow-up CT scan, 62 months after the last treatment session: the lesion was completely ossified
In two patients with nerve root motor weakness, embolization was performed with the improvement of neurological symptoms and healing.
One patient with a L3 lesion (C.G., Fig. 4) had been referred with a huge mass compressing the L3 nerve root and provoking pain and quadriceps weakness (2/5). These symptoms improved since the first SAE. Despite this he had recurrence of pain after three embolizations. He was submitted to another SAE, which was followed by relief of the symptoms. Afterward he underwent six direct injections of acrylic glue, until his lesion was considered clearly regressed and he was completely symptom-free. He was further followed for 42 months after last SAE without any detection of evidence of disease.
Fig. 4.

C.G., 21-year-old man affected by an aneurismal bone cyst of L3. a CT scan at presentation shows the extension of the cyst in the soft tissues. b CT scan 8 weeks from the last injection. c Follow-up CT scan, 42 months after the last treatment session: the mass appears completely ossified
The second case (C.G.) presented with a moderate C5 root pain and deltoid weakness (3/5). After three embolizations, she was completely pain-free with full motor recovery. She was still free of symptoms at 52 months of follow-up.
One case (D.G., Fig. 5), presented with root pain and subjective lower limbs weakness at the admission (motor function 5/5), was submitted to five embolizations of an ABC located in T5-T6.
After the first embolization the cord compression signs are gone, while was reported an incomplete pain improvement after the last procedure even though that there was a considerable improvement in the radiological images. This patient was complaining back pain for 6 more months, fully disappearing at the last follow-up (24 months).
All of the patients presented satisfying evolution, and none crossed to surgical option. There were no recurrences or progression of the disease in any of the cases until the last follow-up.
Discussion
ABC of the spine can be a very challenging surgical due to the expected profuse intraoperative bleeding from the cyst [15]. The current methods of treatment include curettage (intralesional excision) with or without stabilization [4, 15–18], resection [4, 17], intracystic injections [19], and SAE [20]. Several complications were associated to more aggressive modalities of treatment, including massive bleeding, limitation of growth and range of motion in cases of arthrodesis, and even fatal event after resection [8]. The only treatment free of recurrence is “en bloc” resection, however, due to the benign histology of the tumor and the good results achieved with other less aggressive treatments, “en bloc” resection should be considered an overtreatment and performed only in very selected cases, as in posteriorly located lesions where the amount of bleeding would be less than curettage. Good outcomes have been reported after intralesional curettage and fusion in children [21], even such aggressive approach should be avoided in growing spine.
Radiation therapy has also been used, with good results, but due the risk of radio induced sarcoma and cord myelopathy this procedure should be considered only when all other treatments fail [1, 22]. In one of the cases treated in our Institution before 2004, acute leukemia followed RT performed on a C2 recurrent ABC. Radiation therapy [23] was decided as three intralesional excisions could not control the disease. Leukemia regressed after medical oncology treatment and the ABC did not show any sign of evolution 8 years after RT. Since that experience we decided to approach this lesion (which is mostly considered not even a tumor but rather a pseudo-tumoral hyperplastic condition) by a less aggressive strategy. In our critical analysis we re-considered the historical experience, published in a previous article [3]: 41 patients treated by all sorts of modalities (including SAE alone) had good evolution regardless of the used technique. So we decided to adopt the less invasive strategy.
In the literature, SAE was first used to reduce the intraoperative bleeding, and for palliative or curative in very selected cases [12, 20, 24]. The first case fully treated with SAE was reported in 1990 by DeRosa et al. [25]. Later on, several papers reported good results in patients treated only with SAE [3, 7, 12, 20, 24, 25]. Marushima et al. [26] recently reported a case of 12 years old boy, with a T10 lesion treated only with one SAE that showed regression of the lesion and decompression of the cord. The patient was still disease free after 3 years of follow-up, and had returned to his normal daily activities. The limit of the procedure is the risk of embolization of the Adamkiewicz artery [26]. In case of detecting the feeding artery as branch of the artery to be embolized, the procedure must be aborted and the treatment crossing over to surgery.
The rationale for this prospective study is to assess the validity of SAE in the treatment of ABC to the purpose of finding a strategy allowing to heal the ABC avoiding the higher morbidity of the other treatment modalities.
The endpoint of the study was to observe the radiographic evolution of ABC submitted to repeated embolization till full healing. This could demonstrate the validity of SAE in the treatment of ABC.
All the cases here reported healed without need of surgery. No cross-over was required.
The limitation of the study can be represented by the different technical modalities of SAE, as described above, but this is related to the typical variability of this condition. The techniques must always be adapted to the disease and not viceversa.
Conclusion
Embolization is a safe and effective method of treatment for ABC. It should be the first option for the patients presenting without severe instability and or neurologic compromise. The protocol must include repeated procedures unless tumor progression, instability and neurological compromise occur. The morbidity of this protocol is far less than surgical and or radiation treatments. The limits are the long time of the treatment protocol (till 7 and more embolization) and the need of repeated imaging and angiographic controls. The procedure is precluded by the detection of arteries feeding the Adamkiewicz artery. The risk of cord ischemia and para/tetraplegia is by far higher by embolization of the AKA than by the surgical ligation of a radiculo-medullary artery feeding the AKA. Surgery can always be performed in case of unsuccessful evolution.
Acknowledgments
The Authors are indebted to Mr. Carlo Piovani for design, archive research, image and editorial assistance.
Conflict of interest
The authors declare that they have no conflict of interest related to the publication of this manuscript and no funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Campanacci M (1990) Aneurysmal bone cyst. In: Bone and soft tissue tumors. Springer, New York, p 725–751
- 2.Jaffe HL, Lichtenstein L. Solitary unicameral bone cysts: with emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch Surg. 1942;44:1004–1025. doi: 10.1001/archsurg.1942.01210240043003. [DOI] [Google Scholar]
- 3.Boriani S, De lure F, Campanacci L, et al. Aneurysmal bone cyst of the mobile spine. Report of 41 cases. Spine. 2001;26:27–35. doi: 10.1097/00007632-200101010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Vergel De Dios AM, Bond JR, Shives TC, et al. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69:2920–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::AID-CNCR2820691210>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9–24. [PubMed] [Google Scholar]
- 6.Capanna R, Albisinni U, Picci P, et al. Aneurysmal bone cyst of the spine. J Bone Joint Surg. 1985;67(4):527–531. [PubMed] [Google Scholar]
- 7.Kyriakos M, Hardy D. Malignant transformation of aneurysmal bone cyst, with an analysis of the literature. Cancer. 1991;68:1770–1780. doi: 10.1002/1097-0142(19911015)68:8<1770::AID-CNCR2820680821>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Papagelopoulos PJ, Currier BL, Shaughnessy WJ, et al. Aneurysmal bone cyst of the spine. Management and outcome. Spine. 1998;23:621–628. doi: 10.1097/00007632-199803010-00018. [DOI] [PubMed] [Google Scholar]
- 9.Boriani S, Bandiera S, Donthineni R, et al. Morbidity of en bloc resections in the spine. Eur Spine J. 2010;19(2):231–241. doi: 10.1007/s00586-009-1137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay MC, Paterson D, Taylor TK (1978) Aneurysmal bone cysts of the spine. J Bone Joint Surg 60-B(3):406–411 [DOI] [PubMed]
- 11.Ameli NO, Abbassioun K, Saleh H, et al. Aneurysmal bone cysts of the spine. Report of 17 cases. J Neurosurg. 1985;63:685–690. doi: 10.3171/jns.1985.63.5.0685. [DOI] [PubMed] [Google Scholar]
- 12.deKleuver M, van der Heul RO, Veraart BE. Aneurysmal bone cyst of the spine: 31 cases and the importance of the surgical approach. J Pediatr Orthop B. 1998;7:286–292. doi: 10.1097/01202412-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine. 2010;35(22):1221–1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 14.Boriani S, Weinstein JN, Biagini R. Spine update. Primary bone tumors of the spine: terminology and surgical staging. Spine. 1997;22:1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho MF, Letko LJ, Jensen RG, Harms J. Surgical treatment of aneurysmal bone cysts of the spine. Columna. 2007;6(1):1–6. [Google Scholar]
- 16.Cottalorda J, Bourelle S. Current treatments of primary aneurysmal bone cysts. J Pediatr Orthop. 2006;15:155–167. doi: 10.1097/01.bpb.0000210588.50899.29. [DOI] [PubMed] [Google Scholar]
- 17.Szendroi M, Cser I, Konya A, et al. Aneurysmal bone cyst: a review of 52 primary and 16 secondary cases. Arch Orthop Trauma Surg. 1992;111:318–322. doi: 10.1007/BF00420058. [DOI] [PubMed] [Google Scholar]
- 18.Mankin HJ, Hornicek FJ, Ortiz-Cruz E, et al. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23:6756–6762. doi: 10.1200/JCO.2005.15.255. [DOI] [PubMed] [Google Scholar]
- 19.Adamsbaum C, Mascard E, Guinebretiere JM, et al. Intralesional Ethibloc injection in primary aneurysmal bone cysts: an efficient and safe treatment. Skeletal Radiol. 2003;32:559–566. doi: 10.1007/s00256-003-0653-x. [DOI] [PubMed] [Google Scholar]
- 20.Rossi G, Rimondi E, Bartalena T, et al. Selective arterial embolization of 36 aneurysmal bone cysts of the skeleton with N-2-butyl cyanoacrylate. Skeletal Radiol. 2010;39:161–167. doi: 10.1007/s00256-009-0757-z. [DOI] [PubMed] [Google Scholar]
- 21.Garg S, Metha S, Dormans JP. Modern Surgical Treatment of Primary Aneurysmal Bone Cyst of the Spine in Children and Adolescents. J Pediatr Orthop. 2005;25:387–392. doi: 10.1097/01.bpo.0000152910.16045.ee. [DOI] [PubMed] [Google Scholar]
- 22.Maeda M, Tateishi H, Takaiwa H, et al. High-energy, low dose radiation therapy for aneurysmal bone cyst. Clin Orthop. 1989;243:200–203. [PubMed] [Google Scholar]
- 23.Boriani S, Biagini R, De Iure F, et al. Primary bone tumors of the spine: a survey of the evaluation and treatment at the Istituto Ortopedico Rizzoli. Orthopedics. 1995;18(10):993–1000. doi: 10.3928/0147-7447-19951001-09. [DOI] [PubMed] [Google Scholar]
- 24.Koci TM, Mehringer CM, Yamagata N, et al. Aneurysmal bone cyst of the thoracic spine: evolution after particulate embolization. Am J Neuroradiol. 1995;16:857–860. [PMC free article] [PubMed] [Google Scholar]
- 25.DeRosa GP, Graziano GP, Scott J. Arterial embolization of aneurysmal bone cyst of the lumbar spine. J Bone Joint Surg. 1990;72(5):777–780. [PubMed] [Google Scholar]
- 26.Marushima A, Matsumaru Y, Suzuki K, et al. Selective arterial embolization with n-butyl cyanoacrylate in the treatment of aneursymal bone cyst of the thoracic vertebra-a case report. Spine. 2009;34:230–234. doi: 10.1097/BRS.0b013e31818f8f7c. [DOI] [PubMed] [Google Scholar]