Abstract
Purpose
Radiotherapy has been the mainstay treatment for nasopharyngeal carcinoma (NPC) and has achieved good disease control. However, irradiation is associated with potential complications such as osteoradionecrosis (ORN) and infection. There is sparse description in the literature of such complications and how they are best managed. The objectives of the study are: (1) to describe the complications at the cervical spine after surgical and radiotherapy treatment for NPC (2) to identify key principles in the diagnosis and treatment of these complications.
Methods
A retrospective review of all patients with cervical spine complications after radiation treatment and surgery for NPC treated in a tertiary referral center, since 1990.
Results
Fourteen patients with cervical spine ORN and infections were found with an average duration to diagnosis of 8.6 years. All 14 patients had mucosal and deep biopsies and none had tumor recurrence. Four patients had ORN, eight had osteomyelitis and two patients had both ORN and osteomyelitis.
Conclusions
Radiotherapy complications usually have delayed and subtle presentations. ORN progresses slowly and can often be treated conservatively. Infections should be treated aggressively with surgical debridement and the results are generally good. Patients should be regularly followed-up with transoral examination to assess the integrity of the posterior pharyngeal wall and imaging to assess for ORN. Pharyngeal defects raise concern for cervical spine infections. Coverage of pharyngeal defects in these patients is important to prevent recurrent infection.
Keywords: Nasopharyngeal carcinoma, Radiotherapy, Cervical spine
Introduction
Radiotherapy is the primary treatment modality for nasopharyngeal carcinoma (NPC) and has had good efficacy in eradicating the disease. Orthopedic surgeons usually do not treat this group of patients unless they present with bone metastases. However, we may encounter cervical spine complications such as osteoradionecrosis (ORN) and infections that are related to the treatment of NPC.
Complications of radiotherapy for NPC involving the central nervous system such as focal necrosis, diffuse white matter injury, optic neuritis, and vasculopathy has been well-documented in the literature [1]. Other complications include fatty replacement of bone marrow, ORN, and soft-tissue edema. Post-irradiation neoplasms are also of concern as radiation causes abnormal cellular repair and abnormal expression of oncogenes [1].
Radiotherapy can lead to development of ORN but can also cause adjacent soft-tissue complications such as atlantoaxial instability due to the destruction of soft-tissue constraints [2]. The close proximity of nasal and oral secretions may also result in contamination and subsequent infection which can further impede wound healing. Sequelae of this infection can include osteomyelitis, systemic infections, pathological fractures, and fistula formation [3].
Radiotherapy effects include inhibition of osteoblasts and osteoclasts, vascular damage and loss of cellular and metabolic balance leading to osteolysis and increased susceptibility to infection [3]. ORN is a process of ischemic bone necrosis associated with soft-tissue necrosis in the absence of malignancy. The mandible is notorious for ORN but spine involvement has also been reported [2–9]. One study found that ORN occurs in 10–15 % of patients who underwent radiotherapy [4]. Cervical spine infections can be related to ORN or manifest on its own. Ulceration of the pharyngeal mucosa or oral cavity can arise secondary to radiotherapy [5]. This results in tissue breakdown and formation of a chronic non-healing wound, which can lead to fistula formation. Microorganisms could gain direct access to the cervical spine from these defects leading to cellulitis, osteomyelitis of the vertebra and/or pathological fracture [3]. Radiotherapy and radical nasopharyngectomy can also destroy the lymphoid tissue surrounding the nasopharynx, predisposing to bony infection [5]. In addition, radiotherapy can increase the permeability of the blood brain barrier leading to the spread of infection to the central nervous system [10]. Gram-negative bacilli are the most common pathogen responsible for meningitis [11].
The aim of this study is to (1) describe the complications at the cervical spine after surgical and radiotherapy treatment for NPC and (2) identify key principles in the diagnosis and treatment of these complications.
Materials and methods
This study was approved by the institutional review board. This was a case series of all patients with NPC who had undergone radiotherapy or salvage surgery and presented to our department for management of cervical spine complications. Records of all patients with NPC treated at our tertiary referral center since 1990 were traced. A total of 70 patients (presented to our department with history of NPC) were found and their electronic data was screened. We included all patients with history of NPC with surgical or irradiation treatment who had documented cervical spine complications. Complications caused by bone metastasis or NPC progression were excluded. Data on the patients’ treatment of NPC, time between treatment and presentation of cervical spine complication, the type of complication, presentation, method of diagnosis, treatment of the complication, and outcome were recorded.
Results
A total of 14 patients fulfilled our inclusion criteria (see Table 1 for details). There was a predominance of male patients (ratio 10:4) and the average age of all patients, when they presented to our department was 51 years (range 42–60). Thirteen patients had irradiation therapy only while the remaining patient had primary irradiation therapy and salvage nasopharyngectomy for tumor recurrence. Seven patients had second courses of radiotherapy for tumor recurrence and six of them developed infection. In our series, only 4 of our 14 patients had radiotherapy after the year 2000 whereas 7 patients had radiotherapy in the 1980s and 3 had radiotherapy in the 1990s.
Table 1.
Patients with cervical spine complications after NPC treatment
| No. | Patient | NPC treatment | Elapsed time (years) | Complication | Symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | M/55 years | Irradiation × 2 courses (60 Grays each) | 1 | C4/5 infective spondylitis | Incidental finding on X-ray | Antibiotics | Recovered |
| 2 | M/42 years | Irradiation × 2 courses | 2 | Pyogenic meningitis and odontoid ORN, C1 epidural abscess | Headache and fever | C1 anterior arch removal and debridement, antibiotics (ciprofloxacin and clindamycin) | C1/2 subluxation requiring PSF 1 year later |
| 3 | M/45 years | Irradiation × 2 courses (40 Grays each) | 3 | C1/2 infective spondylitis | Neck pain, stiffness, palm numbness | Augmentin | Stiff neck |
| 4 | F/56 years | Irradiation | 11 | C1/2 abscess with cord compression | Neck pain and fever | Anterior transoral decompression, clindamycin and metronidazole | Died of meningitis and pneumonia during same hospitalization |
| 5 | M/49 years | Irradiation × 2 courses (60 Grays each) | 2 | C2/3 infective spondylitis | Neck pain, no neurology | Clindamycin | Recovered |
| 6 | F/58 years | Irradiation | 19 | C2 erosion | Neck pain, no neurology | Conservative | Died 2 years later of pneumonia |
| 7 | M/55 years | Irradiation × 2 courses (68 Grays each) | 1 | C7 osteonecrosis | Neck pain, no neurology | Conservative | Recovered |
| 8 | M/51 years | Irradiation | 22 | Odontoid process erosion, C1/2 subluxation | Neck pain, no neurology | Halo vest | Stiff neck |
| 9 | F/51 years | Irradiation | 17 | C2–3 osteomyelitis and C2–4 ORN Perforated nasopharyngeal wall |
Incidental finding on FU MRI | ASF C2–C4 and PSF (occipital to C6), antibiotics (imipenem/cilastatin, vancomycin, fluconazole) SCM myocutaneous flap |
Recovered |
| 10 | M/42 years | Irradiation | 11 | C5–6 ORN with instability | Left C5–6 weakness | ASF C4–6 | Mild neck pain but stiff |
| 11 | M/52 years | Irradiation (76 Grays) | 8 | Retropharyngeal abscess, C1/2 spondylitis and pathological fracture | Neck pain | Halo vest and augmentin for 3 months | Recovered and removed halo at 3 months |
| 12 | M/53 years | Irradiation × 2 courses and nasopharyngectomy | 1 | C1–6 epidural abscess Persistent infection and posterior pharyngeal defect |
Neck pain, RUL weakness, fever | Right C2–6 hemilaminectomy and antibiotics (augmentin and levofloxacin for 3 months) Augmentin |
Life-long antibiotics |
| 13 | F/60 years | Irradiation × 2 | 11 | C1/2 osteomyelitis and posterior pharyngeal defect | Fever | Augmentin | Life-long antibiotics |
| 14 | M/46 years | Irradiation | 11 | C1/2 subluxation and C2 osteomyelitis | Neck pain | Ertapenem for 6 weeks | Resolved neck pain |
Elapsed time: time between last NPC treatment (irradiation or surgery) and onset of complication
ORN osteoradionecrosis
The average duration to diagnosis of the cervical complication after NPC treatment was 8.6 years (range 1–22). Two patients were diagnosed incidentally on imaging and six patients presented with neck pain alone. Three patients presented with neurological deficit and four patients presented with fever. Three patients with infection also had posterior pharyngeal wall defects. Some patients had multiple presentations (see Table 1).
Diagnostic investigations mainly involve MRI and biopsies, but only biopsies can confirm the diagnosis. MRI findings suggestive of ORN include T1 hypointensity due to the loss of marrow signal which was present in eight patients. MRI findings of osteomyelitis include T2 hyperintensity (edema or inflammatory changes in acute infections) which was present in ten patients. All ten of these patients were diagnosed with osteomyelitis by biopsy. Four patients with osteomyelitis also had T1 hyperintensity. Soft-tissue inflammatory masses were present in seven patients. All 14 patients had mucosal and deep biopsies performed and none of them had tumor recurrence. Four patients had ORN, eight patients had osteomyelitis, and two patients had both ORN and osteomyelitis diagnosed by biopsy.
ORN was treated conservatively in most cases except for one patient (patient #10) who required anterior spinal fusion for cervical instability caused by vertebral body destruction of C5–6 with neurological complications. For the ten patients with infection diagnosed by biopsy, five patients required surgery, two patients were treated with halo vest immobilization and three patients were treated with antibiotics only. Only five of these ten patients had positive cultures.
Out of the 14 patients, 7 patients had other complications of radiotherapy including multiple cranial nerve palsies (vestibulocochlear nerve, glossopharyngeal nerve, vagus nerve, accessory nerve, and hypoglossal nerve) and temporal lobe necrosis.
Case examples (see Table 1 for details)
The following two cases illustrate two different approaches to patients with infection and posterior pharyngeal wall defects.
Posterior pharyngeal wall defect with flap coverage (patient #9)
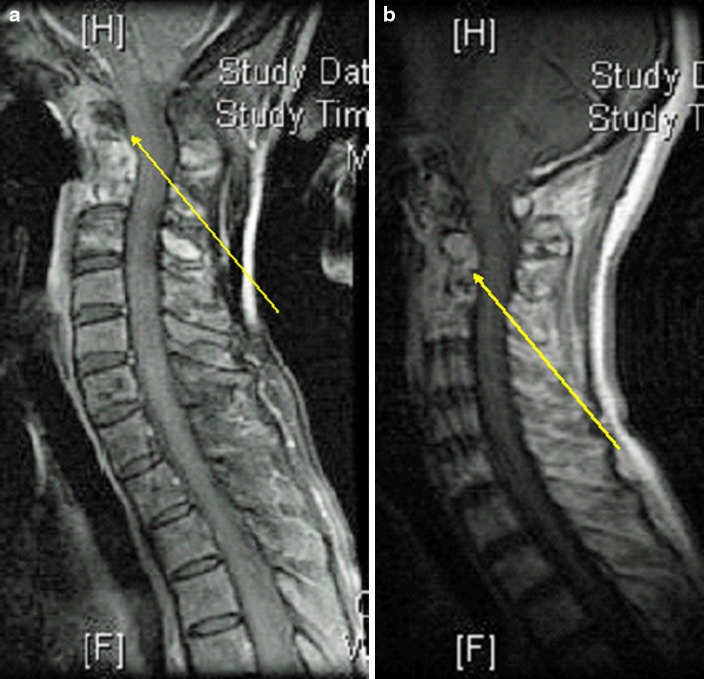
This was a 51-year-old woman, who had C2–3 osteomyelitis and C2–4 ORN diagnosed incidentally on the follow-up MRI scan (Fig. 1a, b). MRI showed T1 and T2 hyperintensity from C1–3 with soft-tissue lesion around the odontoid process. She presented 17 years after radiotherapy. There was no neurological deficit but definite C2/3 rotatory subluxation was present with minimal deformity. She underwent anterior spinal fusion from C2–4, but developed anterior nonunion of C2–3 likely due to a posterior pharyngeal wall defect. This defect was a barrier to bone union as the bone graft site was persistently exposed to oropharyngeal microorganisms. This patient subsequently required posterior spinal fusion from occiput to C6 and myocutaneous sternocleidomastoid flap for coverage (Fig. 2). There was growth of Serratia marcescens, extended-spectrum beta-lactamase (ESBL)-producing E. coli, methicillin-resistant Staphylococcus aureus, enterococcus, and Candida albicans and was treated with 6 months of imipenem, vancomycin, and fluconazole. The patient recovered and had no recurrence of infection up to 10 years of follow-up.
Fig. 1.
a Osteomyelitis (T2 hyperintense indicating inflammatory change; patient 9). b ORN (T1 hypointense indicating loss of fat signal in bone marrow; patient 9)
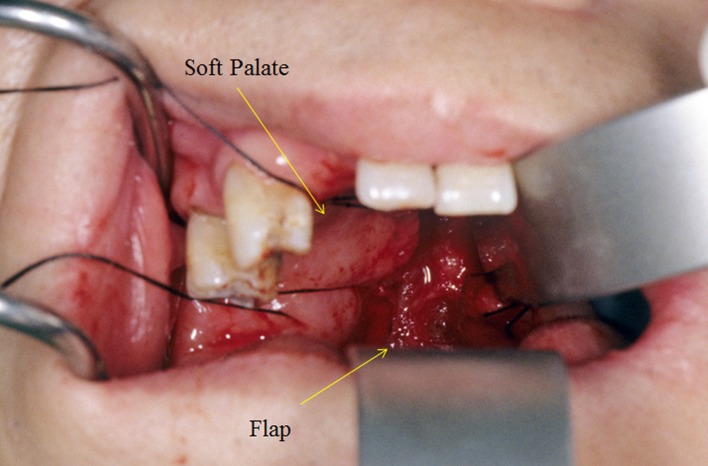
Fig. 2.
Flap coverage of the posterior pharynx
No flap surgery for a posterior pharyngeal wall defect (patient #12)
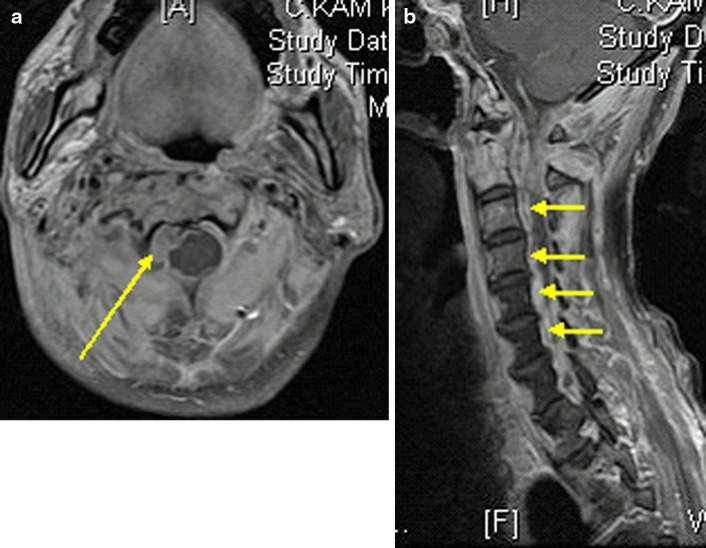
This was a 53 year old man who had recurrent NPC with two doses of radiotherapy done 1 year apart and nasopharyngectomy performed 2 years after the last dose of radiotherapy due to tumor recurrence. He developed sudden onset of right upper limb weakness and fever with MRI showing an epidural abscess at C2–5 mainly on the right side (Fig. 3a, b) 1 year after his last dose of radiotherapy. MRI also showed T1 hypointensity and T2 hyperintensity at C1/2 with soft-tissue lesion around the dens. Emergency decompression with right hemilaminectomy performed for drainage of pus. The patient had full neurological recovery and was given augmentin (intravenous for 2 weeks) for 3 months with normalized inflammatory markers. He was readmitted 2 months later for increasing neck pain. His inflammatory markers were raised again and X-ray showed erosion of C2 dens and C1/2 subluxation (Fig. 4). Nasoendoscopy showed a 2 cm defect in the posterior pharyngeal wall with exposed anterior C1 arch and dens. Repeat MRI showed C1/2 osteomyelitis but no obvious abscess collection. This patient was put on intravenous augmentin (amoxicillin and clavulanic acid) and preoperative halo traction. He was subsequently managed with posterior occipital-C3 instrumented fusion with C1 sublaminar wiring for improved stability (Fig. 5). Augmentin was switched to oral form once fever was settled. Unfortunately, this patient refused flap surgery for the pharyngeal defect and is currently put on life-long antibiotics (augmentin). Up to current 2 years follow-up, this patient does not have any recurrence of infection on repeated MRI and serology screening.
Fig. 3.
a Axial MRI scan showing right sided epidural abscess (patient 12). b Sagittal MRI scan showing epidural abscess from C2–5 (patient 12)
Fig. 4.
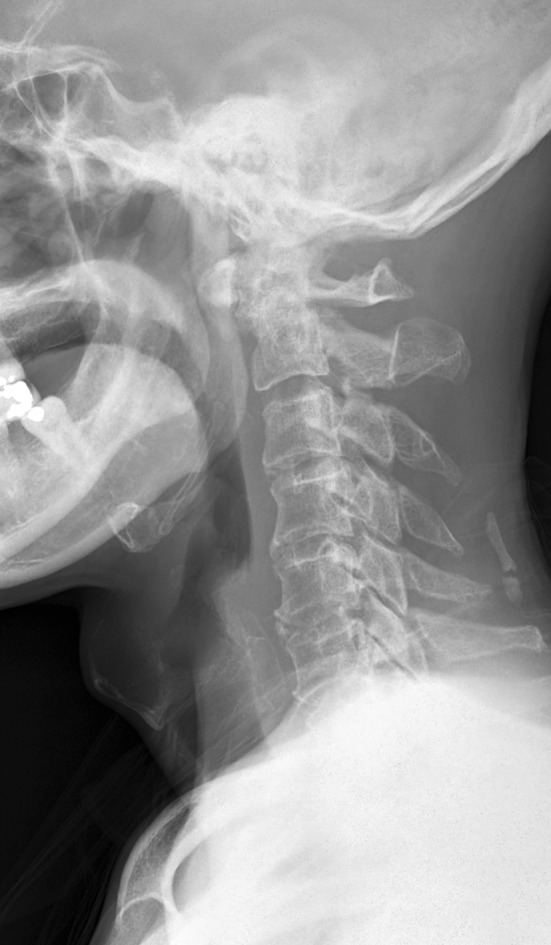
Lateral cervical spine radiograph C2 erosion and C1/2 subluxation with minimal soft-tissue swelling (patient 12)
Fig. 5.
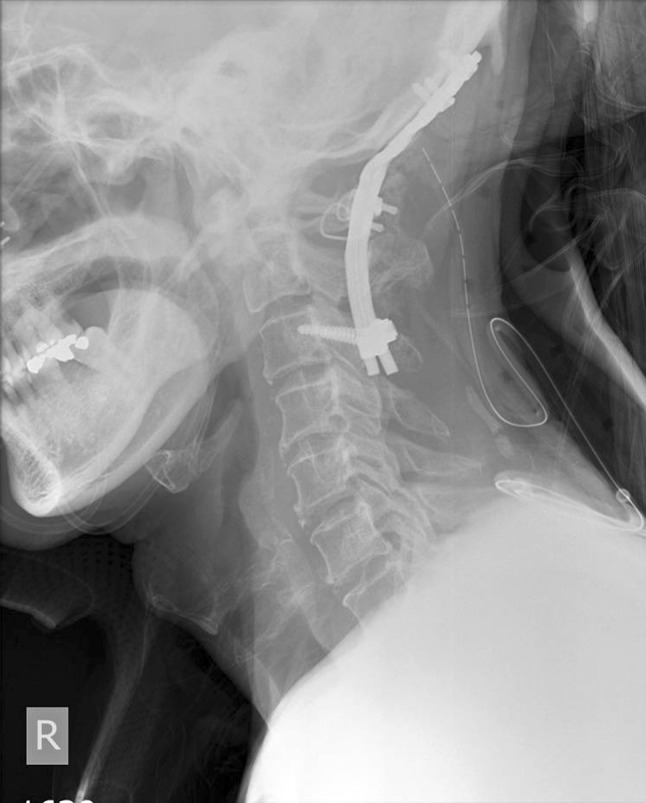
Postoperative lateral cervical spine radiograph; Occipital-C3 fusion with C1 sublaminar wiring to stabilize the C1/2 instability (patient 12)
Discussion
There have been rapid advances in the development of radiotherapy in recent decades from conventional two dimensional radiotherapy to three dimensional conformal radiotherapy to the latest stereotactic guided irradiation that causes less side effects with accurate targeting and delivery of higher dose radiotherapy in different directions [12]. It is important during follow-up of patients who have undergone radiotherapy in the past to actively look for spine complications as these patients are at most risk. In our series, most patients had radiotherapy in the 1980s, 20–30 years before the presentation of symptoms which mirrors that of other centers [9, 13]. In our experience, we have come across two main groups of complications, namely ORN and osteomyelitis.
ORN
The time elapsed between radiotherapy and presentation of ORN could take decades as evidenced by our series. Nevertheless, most patients with ORN do not have any significant sequelae and can be treated conservatively most of the time as it mainly presents as pain and analgesics are adequate [3]. This is the main reason why most patients of ORN are not identified until complications occur. Surgical treatment may only be required in cases of vertebral collapse due to pathological fracture. Yet, this only occurs in the early months after the insult when the bone is weak during resorption and remodeling via creeping substitution. In our series only one of the patients with ORN required surgery due to vertebral body destruction of C5–6 leading to cervical instability and neurological complications. Otherwise, ORN rarely causes instability in the later years unless accompanied by a low-grade infection that disrupts ligamentous complexes (usually atlanto-axial). Surgery for instability is indicated when it is progressive and leads to spinal deformity and cord compression [4].
The presence of ORN, however, has prognostic indications because it is a predisposing factor for infection. Necrotic bone is a good medium for microorganisms and these patients may be prone to infections in the future. This is an important finding as most ORN patients are not identified before complications such as instability or infection. It may be prudent to follow-up on patients with previous radiotherapy done.
Infection
Infections on the other hand require a more aggressive approach. As compared to ORN, these patients usually have more florid presentations including fever (4 patients) and symptoms of nerve compression (2 patients). However, high index of suspicion is still required as many patients present with only neck pain (3 patients) or no symptoms at all (incidental finding in 2 patients). Eight of our patients had infection without ORN diagnosed as by biopsy. Radiotherapy and radical nasopharyngectomy can also destroy the lymphoid tissue surrounding the nasopharynx, predisposing to bony infection [5]. Thus, this patient group is more susceptible to seeding by infectious organisms despite the absence of ORN.
In our series, only five of ten patients with infection had positive culture growth probably due to antibiotics given before tissue culture sampling. These infections were usually caused by the common oropharyngeal flora and were typically polymicrobial. Regular communication with the microbiologist and monitoring of the clinical progress can guide the proper antibiotic regimen and duration. Treatment of infection is usually more aggressive as half of our patients with infection required surgery (5 of 10 patients) and two patients required halo vest immobilization due to complications of cervical instability. Those with epidural abscess compression on the spinal cord would likely require surgical decompression.
Another important aspect in the workup for these patients with infection is the presence of posterior pharyngeal wall defects. In theory, patients with posterior pharyngeal wall defects should be treated with flap coverage of the defect regardless of the investigation results. Without adequate soft-tissue coverage, the risk of recurrent infection is high and any operative procedure would likely fail due to bacterial contamination. Flap surgery (Fig. 2) to cover exposed bone should be performed with debridement of the osteomyelitis to improve the rates of healing by providing a new vascular bed. The repair requires a reliable, high quality pedicle flap of sufficient size. Three muscular and musculocutaneous pedicle flaps commonly used are the latissimus dorsi, paraspinal muscles, and trapezius by rotation-transposition, reverse-flap or free-flap coverage,respectively [7]. The more reliable and frequently used flap is the ascending pedicle flap of the lower trapezius. This pedicle flap is supplied by the descending branch of the transverse cervical artery [7].
Two of our patients (patients #12 and #13) refused flap surgery despite the presence of a defect. The only alternative we can offer them in this scenario is life-long antibiotics. At 2 years of follow-up, both of these patients have not had recurrence of infection evidenced by MRI and serology tests. Although our series does not identify a definite role of flap surgery for reconstruction of posterior pharyngeal wall defects, we believe that life-long antibiotics are not advised as prolonged antibiotic use could lead to other complications such as liver and kidney damage and development of antibiotic-resistant strains of microorganisms in the future. There are visible benefits to flap surgery nonetheless. In our single case (patient #9) with successful flap coverage, the outcome at 10 years after the flap surgery has been very satisfactory without any recurrence of infection. One other report of reconstruction of a posterior pharyngeal wall defect with a radial forearm free flap was performed with no recurrence of infection at 22 months of follow-up [14].
Management pearls
As discussed before, both ORN and infection may have subtle presentations. In addition, inflammatory markers and white cell count may not be significantly elevated. Thus, without knowledge of the connection between these complications and NPC, these diagnoses are easily missed. A simple and helpful procedure to perform during routine examination of these patients is the transoral examination (Fig. 6) using a tongue depressor and torch to visualize the posterior pharynx. This should be done in all patients with treated NPC as part of a complete examination. In the presence of a posterior pharyngeal wall defect, infection will have to be excluded until proven otherwise. Occasionally, the trismus in post-irradiation patients may be too severe to allow adequate exposure in the clinic. A flexible nasoendoscopy should be performed in these scenarios to accurately assess the presence and size of the defect.
Fig. 6.
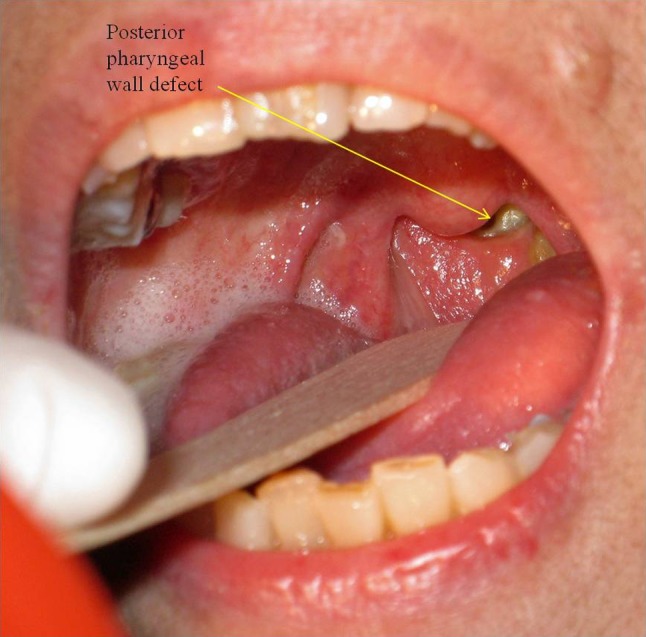
Posterior pharyngeal wall defect seen in transoral examination (patient 13)
Radiological assessment may also be difficult in these patients. Due to previous radiotherapy, significant scar tissue will limit the extent of soft-tissue swelling on the lateral cervical spine X-rays (Fig. 4). In these less obvious cases, MRI would be the investigation of choice to help differentiate ORN from infection. ORN is represented with hypointense signal on T1-weighted films with contrast enhancement while infections are characterized by hyperintense signals on T2-weighted films [5, 6] (Fig. 1a, b) Hypointensity on T1 indicates loss of marrow fat signal. Contrast enhancement on T1 may be present with soft-tissue inflammatory mass or a low-grade infection. MRI has limited use however, in differentiating patients with infection superimposed on ORN from patients with pure osteomyelitis (T2 hyperintense with contrast enhancement) [5]. In these cases, bone biopsies can help identify ORN.
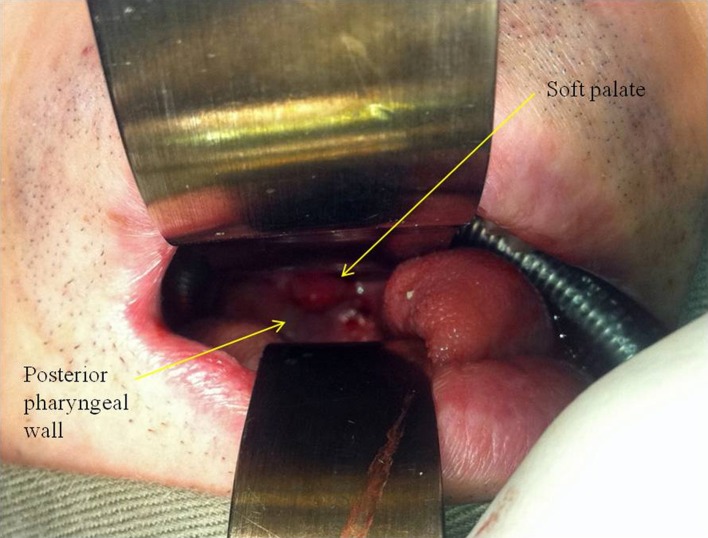
Biopsies should be performed in all patients because tumor recurrence is an important differential diagnosis. This includes a deep biopsy of the C1/2 bone and also a nasopharynx mucosa biopsy. We find the anterior transoral biopsy to be the preferred approach (Fig. 7). CT-guided biopsy through an anterior or transverse approach is risky as it traverses vital structures such as the carotid triangle. This is especially true in post-irradiation necks as the extensive scarring leads to distorted anatomy. The anterior transoral biopsy is the most direct and safe method. We would always begin intravenous augmentin treatment after the biopsy has been performed in suspected infections and will streamline the antibiotic depending on the culture and sensitivity.
Fig. 7.
Anterior transoral biopsy
Conclusion
Patients with previous radiotherapy or surgery for NPC carry a high risk of developing ORN and infection due to the disruption of the cellular homeostasis and the breach of the lymphoid barrier with contamination by the nasopharyngeal flora. The presentation of these complications may be delayed (>10–20 years) and the clinical signs and symptoms can be subtle especially for ORN. The presence of neck pain raises suspicion of osteomyelitis and regular nasopharyngeal examination is recommended during follow-up. Plain radiographs may show limited soft-tissue swelling thus MRI should be used readily for diagnosis. Biopsy must be performed in all cases to rule out tumor recurrence or metastasis and infections should be treated aggressively with antibiotic treatment. Internal stabilization and fusion should be performed in cases of instability and deformity, and flap coverage of nasopharyngeal defect may offer better long-term outcome.
Key points
Delayed onset of cervical spine complications after radiotherapy,
Subtle clinical signs and symptoms (neck pain only, limited soft-tissue swelling on X-ray),
Regular nasopharyngeal examination,
Aggressive use of antibiotics and surgical intervention warranted for infections,
Flap coverage of nasopharyngeal defect may offer better long-term outcome.
Conflict of interest
There are no conflicts of interest and no source of funding.
References
- 1.Rabin BM, Meyer JR, Berlin JW, et al. Radiation-induced changes in the central nervous system and head and neck. Radiographics. 1996;16(5):1055–1072. doi: 10.1148/radiographics.16.5.8888390. [DOI] [PubMed] [Google Scholar]
- 2.Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine (Phila Pa 1976) 1998;23(11):1280–1282. doi: 10.1097/00007632-199806010-00021. [DOI] [PubMed] [Google Scholar]
- 3.Lim AA, Karakla DW, Watkins DV. Osteoradionecrosis of the cervical vertebrae and occipital bone: a case report and brief review of the literature. Am J Otolaryngol. 1999;20(6):408–411. doi: 10.1016/S0196-0709(99)90083-2. [DOI] [PubMed] [Google Scholar]
- 4.Donovan DJ, Huynh TV, Purdom EB, et al. Osteoradionecrosis of the cervical spine resulting from radiotherapy for primary head and neck malignancies: operative and nonoperative management. Case report. J Neurosurg Spine. 2005;3(2):159–164. doi: 10.3171/spi.2005.3.2.0159. [DOI] [PubMed] [Google Scholar]
- 5.King AD, Griffith JF, Abrigo JM, et al. Osteoradionecrosis of the upper cervical spine: MR imaging following radiotherapy for nasopharyngeal carcinoma. Eur J Radiol. 2010;73(3):629–635. doi: 10.1016/j.ejrad.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Kosaka Y, Okuno Y, Tagawa Y, et al. Osteoradionecrosis of the cervical vertebrae in patients irradiated for head and neck cancers. Jpn J Radiol. 2010;28(5):388–394. doi: 10.1007/s11604-010-0440-2. [DOI] [PubMed] [Google Scholar]
- 7.Kouyoumdjian P, Gille O, Aurouer N, et al. Cervical vertebral osteoradionecrosis: surgical management, complications and flap coverage—a case report and brief review of the literature. Eur Spine J. 2009;18(Suppl 2):258–264. doi: 10.1007/s00586-009-0950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JS, Huang CM, Yeh IC, et al. Isolated osteoradionecrosis of the dens mimicking metastasis of nasopharyngeal carcinoma after radiotherapy. J Clin Neurosci Off J Neurosurg Soc Australas. 2010;17(8):1064–1066. doi: 10.1016/j.jocn.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 9.van Wyk FC, Sharma MP, Tranter R. Osteoradionecrosis of the cervical spine presenting with quadriplegia in a patient previously treated with radiotherapy for laryngeal cancer: a case report. J Med Case Reports. 2009;3:7262. doi: 10.4076/1752-1947-3-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Ruiz M, Lopez-Medrano F, Garcia-Montero M, et al. Intramedullary cervical spinal cord abscess by viridans group Streptococcus secondary to infective endocarditis and facilitated by previous local radiotherapy. Intern Med. 2009;48(1):61–64. doi: 10.2169/internalmedicine.48.1548. [DOI] [PubMed] [Google Scholar]
- 11.Tang LM, Chen ST, Chang HS. Tuberculous meningitis in patients with nasopharyngeal carcinoma. Scand J Infect Dis. 1996;28(2):195–196. doi: 10.3109/00365549609049076. [DOI] [PubMed] [Google Scholar]
- 12.Jin JY, Wen N, Ren L, et al. Advances in treatment techniques: arc-based and other intensity modulated therapies. Cancer J. 2011;17(3):166–176. doi: 10.1097/PPO.0b013e31821f8318. [DOI] [PubMed] [Google Scholar]
- 13.Sanger JR, Matloub HS, Yousif NJ, et al. Management of osteoradionecrosis of the mandible. Clin Plast Surg. 1993;20(3):517–530. [PubMed] [Google Scholar]
- 14.Kakarala K, Richmon JD, Durand ML, et al. Reconstruction of a nasopharyngeal defect from cervical spine osteoradionecrosis. Skull Base Off J N Am Skull Base Soc. 2010;20(4):289–292. doi: 10.1055/s-0030-1249244. [DOI] [PMC free article] [PubMed] [Google Scholar]