Abstract
Purpose
To analyze the neurological and mechanical outcomes in 44 consecutive patients treated for a hematological malignancy with epidural localization to assess the place of surgery in the treatment of this pathology.
Methods
Clinical records, CT and MRI scans of 44 patients with epidural localizations of multiple myeloma or lymphoma treated between 1990 and 2005 were analyzed retrospectively. Neurological status, epiduritis and osteolysis volumes, vertebral collapse, and spinal canal compromise were assessed. The neurological outcome was graded according to Frankel and the mechanical outcome was evaluated on the rate of vertebral collapse.
Results
Surgery was performed in 11 patients (25 %) for neurological (n = 9) or mechanical (n = 2) reasons. In five cases, a concomitant biopsy was performed because the etiology of the epiduritis was unknown. Fifteen patients (34.1 %) presented with a neurological deficit secondary to an acute vertebral collapse (n = 4), an epiduritis (n = 7), or both (n = 4). Whatever the treatment (surgical or not), a complete recovery (Frankel E) occurred in 14/15 (93.3 %) after a mean delay of 12 weeks (range 2–24 weeks). During the follow-up, seven collapses occurred. We estimated that a threshold of 30 % of osteolysis was associated with a significant risk of vertebral collapse (P = 0.005).
Conclusions
Hematological malignancies with epidural localization must be treated first medically, even in patients with neurological symptoms. Surgery should be considered only in the cases of acute vertebral collapse, medical treatment failure, or to prevent acute collapse in patients with vertebral osteolysis of more than 30 %.
Keywords: Hematological malignancy, Epiduritis, Spinal cord compression, Osteolysis, Vertebral collapse
Introduction
Neurological complications in spinal localizations of hematological malignancies, such as myeloma and lymphoma, are common. Their incidence varies from 6 to 20 % [1–3]. The hematological disease is often diagnosed by revealing bone or neurological lesions [4, 5]. Spinal epidural compression, radiologically defined as an epidural lesion causing true displacement of the neural structures from its normal position in the vertebral canal [6], may result from vertebral collapse, extradural extension to an adjacent vertebra, or extradural compression without local bone disease [2].
Initial treatment options include surgery and chemotherapy, sometimes associated with radiotherapy. Goals of surgery include decompression of neural structures, pain relief, and spinal stabilization to prevent deformity and allow mobilization [7]. Chemotherapy stops cell proliferation and its consequences on bone or neurological lesions [4, 8, 9]. Local radiotherapy is effective for palliation of bone pain, with fractionated radiotherapy relieving pain in 91–97 % of patients [10].
If the treatment of spinal metastases from solid tumors is well documented in literature, very little data will be available on the place of surgery in the treatment of spinal localizations of hematological malignancies [11–13]. Anterior spinal decompression seems to become the “gold standard” of surgical procedure to treat spinal metastasis [14–16], but laminectomy is still indicated in selected patients [17, 18], and even continues to be preferred by some authors [19, 20]. However, chemotherapy has been shown to provide remarkable activity in hematological malignancies [21], and thus non-operative treatment remains an important option. Moreover, surgery and postoperative recovery may delay the start of any postoperative adjuvant treatment such as chemotherapy.
This study was a retrospective review of 44 consecutive patients who were treated for a hematological malignancy with spinal epidural compression. Neurological and mechanical outcomes were analyzed to assess the place of surgery in the treatment of spinal localizations of hematological malignancies.
Materials and methods
Clinical records of 44 consecutive patients, 19 women and 25 men, with epidural localizations of hematological malignancies were analyzed retrospectively. 33 patients with multiple myeloma and 11 patients with lymphoma were included between 1990 and 2005. The mean age was 58.2 years (range 27–87 years). Table 1 summarizes information pertaining to patient care. The mean duration of follow-up after the diagnosis of hematological malignancy was 38 months (range 11–151 months). 13 patients (29.5 %) died of the hematological malignancy.
Table 1.
Numbers (n) and proportion (%) of hematological malignancies, spinal localizations and rates of neurological deficits, vertebral collapse, and surgery in a series of 44 patients
| Total, n (%) | Lymphoma, n (%) | Myeloma, n (%) | Cervical spine, n (%) | Thoracic spine, n (%) | Lumbar spine, n (%) | Sacrum, n (%) | |
|---|---|---|---|---|---|---|---|
| Entire series | 44 (100 %) | 11 (25 %) | 33 (75 %) | 5 (11.4 %) | 27 (61.4 %) | 11 (25 %) | 1 (2 %) |
| Neurological deficit | 15 (34.1 %) | 7 (15.9 %) | 8 (18.2 %) | 1 (2 %) | 11 (25 %) | 4 (9.1 %) | 0 (0 %) |
| Vertebral collapse | 20 (45.5 %) | 5 (11.4 %) | 15 (34.1 %) | 1 (2 %) | 3 (7 %) | 16 (36 %) | 0 (0 %) |
| Surgical patients | 11 (25 %) | 6 (13.6 %) | 5 (11.4 %) | 0 (0 %) | 9 (20.5 %) | 2 (5 %) | 0 (0 %) |
During the follow-up period, the neurological status was assessed using the Frankel grading system [22]. For each patient, CT and MR images of the whole spine were analyzed at the time of diagnosis and during the follow-up to characterize the spinal lesion. Following data were collected: epidural tumor extension and osteolysis volumes, vertebral collapse, percentage of spinal canal compromise, and degree of vertebral reconstruction. To estimate the volumes (epidural tumor extension and osteolysis), we measured their maximum sizes in each of the three orthogonal planes. Osteolysis volume was estimated using CT scan (Fig. 1). Epidural tumor volume was estimated using T2-weighted MRI scan (Fig. 2). To estimate the extent of epidural compression, the spinal canal cross-sectional area was measured using T2-weighted MRI. The spinal canal dimensions (before epidural compression occurred) were estimated by the average value of cross-sectional areas measured at one spinal level above and below the constricted level, where there was no epidural tumor extension. Cross-sectional area of the spinal canal was then measured at the level where the most severe compression was observed, and compared with the original size of the canal to calculate the percentage of canal compromise (Fig. 3). To maintain consistency, these measurements were taken at the same spinal levels during the follow-up. To analyze the vertebral collapse, we calculated the mean vertebral height on sagittal CT scan reconstructions passing through the spinous process by averaging the measurements obtained from the anterior and posterior portions of the vertebral body. The mean vertebral height of the collapsed vertebra was compared to the mean vertebral height of the adjacent non-collapsed vertebrae (Fig. 4).
Fig. 1.
Osteolysis and vertebral body volumes were estimated by measuring their maximum sizes on a CT scan in each of the three orthogonal planes, approximating the structures to rectangular solids. Height and width were measured on the frontal reconstructions (a, c), and depth was measured on the horizontal reconstruction (b). Percentage of osteolysis (PO) was calculated as follows: 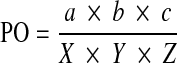
Fig. 2.
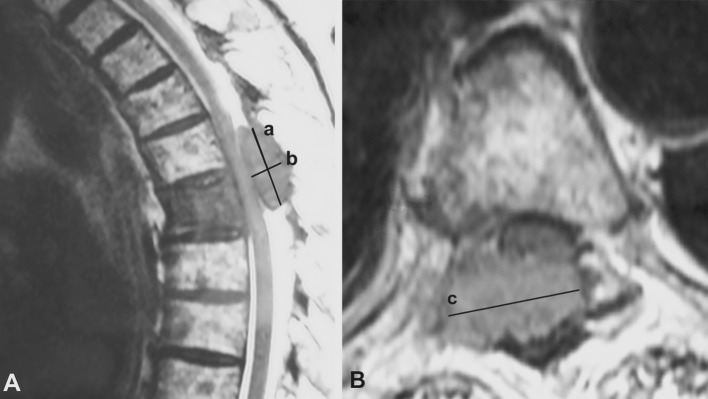
Epidural tumor extension volumes were estimated by measuring their maximum sizes in each of the three orthogonal planes on T2-weighted MRI, approximating the structures to rectangular solids. Height and depth were measured on the sagittal view (a), and width was measured on the axial view (b). 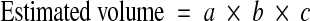
Fig. 3.
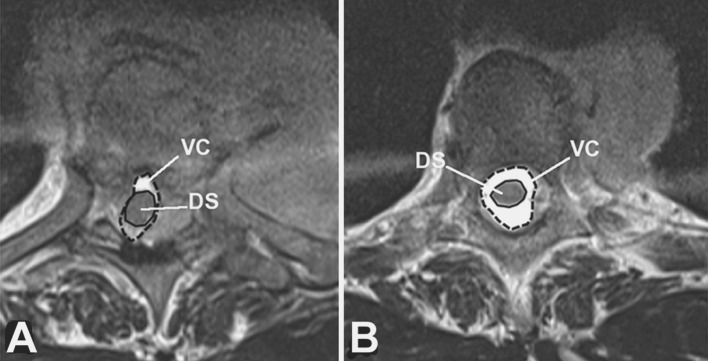
Estimated canal compromise on T2-weighted axial MRI images: comparison between the canal area at the level of canal compromise (a) with canal area of the adjacent non-constricted level (b). DC dural sac, VC vertebral canal
Fig. 4.

The mean vertebral height (MVH) of the collapsed vertebra at level x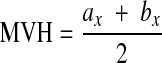 was compared to the mean vertebral height of the adjacent non-collapsed vertebrae at levels x − 1 and x + 1. Vertebral collapse (VC) was calculated from sagittal CT scan reconstructions passing through the spinous process by averaging the measurements obtained from the collapsed vertebra and dividing that by the average of the adjacent non-collapsed vertebrae as follows:
was compared to the mean vertebral height of the adjacent non-collapsed vertebrae at levels x − 1 and x + 1. Vertebral collapse (VC) was calculated from sagittal CT scan reconstructions passing through the spinous process by averaging the measurements obtained from the collapsed vertebra and dividing that by the average of the adjacent non-collapsed vertebrae as follows: 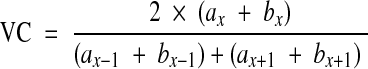
Statistical analysis
Results of categorical data were expressed as number and percentages, those of continuous data as mean ± standard deviation or median with [1st quartile–3rd quartile] depending on the distribution of the variable. Descriptive statistics are given on the global series and according to events of interest: neurological deficit, vertebral osteolysis, and surgical treatment. Distributions were compared by means of non-parametric Mann–Whitney test (quantitative data) or Fisher’s exact test (categorical data). Factors associated to the risk of vertebral collapse were tested by univariate analysis. The receiver operating characteristic (ROC) curve was build to determine the level of vertebral osteolysis, which was the best predictor of vertebral collapse. Logistic regression analysis was used to estimate the risk of collapse due to osteolysis, and adjusted on other parameters. A P value ≤ 0.05 was considered significant. SPSS V.18 and Stata V.11 were used for statistical analysis.
Results
All the patients (n = 44) were treated with chemotherapy, associated with radiation therapy in 28 patients (63.6 %). Surgery was performed in 11 patients (25 %) for neurological (n = 9) or mechanical (n = 2) reasons (Table 2). Decompression surgery was performed in 9 patients at a mean delay of 18.8 days (range 2–35 days) after the appearance of the first neurological symptoms. In four of the five cases treated by decompressive laminectomy, a concomitant biopsy was performed for pathological analysis because the etiology of the epidural tumor extension was unknown. No surgical complications have been observed intra- or postoperatively.
Table 2.
Surgical procedures performed in n = 11 patients treated for a hematological malignancy with epidural localization
| Indications | Total, n (%) | Lumbar spine, n (%) | Thoracic spine, n (%) | Anterior approach | Posterior approach | ||
|---|---|---|---|---|---|---|---|
| Corporectomy + fixationa, n (%) | Fixationb, n (%) | Laminectomyc, n (%) | Laminectomy + fixationd, n (%) | ||||
| Neurological deficit | 9 (81.8) | 2 (18.2 %) | 7 (63.6 %) | 4 (36.4 %) | 0 (0 %) | 5 (45.5 %) | 0 (0 %) |
| Mechanical reasons | 2 (18.2 %) | 0 (0 %) | 2 (18.2 %) | 0 (0 % | 1 (9.1 %) | 0 (0 %) | 1 (9.1 %) |
| Total | 11 (100 %) | 2 (18.2 %) | 9 (81.8) | 4 (36.4 %) | 1 (9.1 %) | 5 (45.5 %) | 1 (9.1 %) |
aAnterior corporectomy with vertebral reconstruction by tricortical iliac crest bone graft and fixation by anterior plate
bFixation by anterior plate with fusion by tricortical iliac crest bone graft to stabilize the spine
cPosterior decompression and biopsy of the epidural tumor extension
dPosterior decompression, fixation by pedicular screws, and posterolateral fusion to treat an acute vertebral collapse
15 patients (34.1 %) presented with a neurological deficit, 14 at the time of diagnosis (Frankel B, 2; C, 11; D, 1), and patient 2 months after the initial diagnosis, secondary to a vertebral collapse (Frankel C). The deficit was secondary to an acute vertebral collapse (n = 4), an epidural tumor extension (n = 7), or the association of a mild collapse and an epidural tumor extension (n = 4). The mean percentage of spinal canal compromise in patients with neurological deficit was 39.3 % (range 19–57 %) compared to 18.4 % (range 11–39 %) in patients without neurological deficit (Table 3). Whatever the treatment (surgical or not), the neurological recovery was excellent: a complete recovery (Frankel E) occurred in 14/15 patients (93.3 %) after a mean delay of 12 weeks (range 2–24 weeks). One patient (Frankel C) partially improved after corporectomy (Frankel D), but her neurological status altered secondarily due to the failure of the hematological malignancy treatment, and she died 10 months after the surgery.
Table 3.
Characteristics of patients treated for a hematological malignancy with epidural localization according to their neurological status
| Patients with neurological deficit | Patients without neurological deficit | Entire series | |
|---|---|---|---|
| Total, n (%) | 15 (34.1) | 29 (65.9) | 44 (100) |
| Mean age ± 1 SD (years) | 61 ± 15 | 56 ± 13 | 58 ± 14 |
| Myeloma/lymphoma | 7/8 | 26/3 | 33/11 |
| Level, n (%) | |||
| Cervical | 1 (6.7) | 4 (13.8) | 5 (11.4) |
| Thoracic | 11 (73.3) | 16 (55.2) | 27 (61.4) |
| Lumbar | 2 (13.3) | 9 (31) | 11 (25) |
| Sacral | 1 (6.7) | 0 (0) | 1 (2.2) |
| Frankel grade, n (%) | |||
| A | 0 (0) | 0 (0) | 0 (0) |
| B | 2 (13.3) | 0 (0) | 2 (4.4) |
| C | 12 (80) | 0 (0) | 12 (27.4) |
| D | 1 (6.7) | 0 (0) | 1 (2.2) |
| E | 0 (0) | 29 (100) | 29 (66) |
| Number of collapsed vertebrae at latest follow-up, n (%) | 8 (53.3) | 14 (48.3) | 22 (50) |
| Number of surgeries at latest follow-up, n (%) | 10 (66.7) | 1 (3.4) | 11 (25) |
| Epidural tumor extension volume (cm3): median [1stQ–3rdQ] | 3 [1–5.2] | 0.6 [0.2–1.5] | 0.95 [0.3–3] |
| Osteolysis volume compared to theoretical vertebra volume %: median [1stQ–3rdQ] | 18 [9–47] | 21 [13–43] | 20 [12–42] |
Spinal canal compromise was similar in operated patients with neurological deficit (n = 9) treated by laminectomy, and those treated by corporectomy, 38 % (range 19–55 %) and 43 % (range 36–57), respectively. In non-operated neurological patients (n = 6) (Frankel B, 1; C, 4; D, 1), the mean epidural tumor extension volume and percentage of spinal canal compromise were 3.9 cm3 (range 0.5–7.8 cm3) and 37 % (range 21–52 %), respectively. At the latest follow-up, after medical treatment, the mean epidural tumor extension volume decreased to 0.22 cm3 (range 0–3.1 cm3) (P = 0.007).
All the patients (n = 44) presented with vertebral osteolysis at the time of diagnosis, associated 13 cases (29.5 %) with vertebral collapse leading to mean vertebral height loss of 39 % (range 20–60 %), and a mean local kyphosis of 8° (range 0–18°). Osteolysis was located in the anterior column in 36 cases (83 %), in the anterior and middle columns in 6 cases (13 %), and in the 3 columns in 2 cases (4 %).
The mean percentage of osteolysis in the non-collapsed vertebrae (n = 31) was 19.5 % (range 11.5–35 %). During the follow-up, seven collapses occurred. Before collapse, the mean percentage of osteolysis in those 7 patients was 46 % (range 25–77 %). From the ROC curve, we estimated that a threshold of 30 % of osteolysis was associated with a significant risk of vertebral collapse (P = 0.005) with a sensitivity of 75 % and a specificity of 74.2 %. Thus, the positive predictive value of at least 30 % osteolysis for the risk of collapse was 53 % and the negative predictive value was 88 %, giving a relative risk (RR) of collapse of 4.6 (95 % CI 1.5–14.6). This strong relationship persisted after the adjustment on gender and pathology (Table 4).
Table 4.
Factors associated with vertebral collapse in patients treated for a hematological malignancy with epidural localization
| Factors | Vertebral collapse (n = 13) | No vertebral collapse (n = 31) | P |
|---|---|---|---|
| Age mean ± SD | 61 | 65 | 0.81 |
| Gender male, n (%) | 8 (62) | 17 (55) | 0.68 |
| Myeloma/lymphoma | 23/8 | 10/3 | 0.85 |
| Level | |||
| Cervical | 1 (8) | 4 (13) | 0.71 |
| Thoracic | 10 (77) | 17 (55) | 0.49 |
| Lumbar/sacral | 2 (15) | 10 (32) | 0.56 |
| Epidural tumor extension volume: median [1stQ–3rdQ] | 0.7 [0.25–3.7] | 1 [0.4–3] | 0.58 |
| Estimated percentage of osteolysis: % (range) | 36.2 [19–55] | 19.5 [11.5–35] | 0.11 |
| Osteolysis ≥ 30 %a, n (%) | 9 (75) | 8 (26) | 0.005 |
aThreshold determined from the ROC curve
Under medical treatment, vertebral reconstruction of the osteolysis occurred and progressed in a centripetal fashion for 6–10 months (average 7 months). It ended with a persistent central void circled with condensed bone.
Discussion
Very little data are available in the literature on the treatment of spinal localizations of hematological malignancies [11, 12]. With 44 patients, the current series is one of the largest reported. However, some limitations of the present study can be identified. The volumes of the vertebral body, the osteolysis, and the epidural tumor extension could not be measured accurately in 3D at the time the CT scans were performed. Thus, rectangular solids were used to approximate these volumes, measuring their largest, highest, and longest dimensions. This approximation overestimates the actual size of the analyzed structures, inducing a bias.
If the commonest complication affecting the central nervous system in hematological malignancies is compression of the spinal cord or cauda equina [2, 3, 23, 24], a clear consensus does not yet exist for deciding which patients should undergo surgical treatment, let alone with what type of surgery. We reported the neurological and mechanical evolution of spinal localizations of hematological malignancies in 44 consecutive patients. Lymphomas and multiple myelomas were analyzed together because (1) they are both hematological malignancies, (2) they are both highly sensitive to chemotherapy, and (3) they both spread to the spine in the same way. However, in our series, the risk of neurological complication was higher in lymphoma patients (7 over 11) than in myeloma patients (8 over 33).
Neurological symptoms were present in approximately one-third of the patients in our series. However, no patients had complete motor and sensory loss (Frankel A). Indications for surgery in the treatment of vertebral localizations of solid tumors include progressive neurological symptoms, pain unresponsive to conservative treatment, need for histologic diagnosis, spinal instability or vertebral collapse, with or without neurologic deficit, and tumor volume reduction [7]. Tumor volume reduction surgery is not indicated in hematological malignancies because chemotherapy has been shown to provide remarkable activity in hematological malignancies [21], and moreover, surgery for metastatic disease of the spine is associated with a 25 % rate of complications [7].
In metastatic epidural compression of the spinal cord, authors advocate that decompression surgery should be started within 24 h, sooner in case of rapid neurological deterioration [25, 26]. However, hematological malignancies respond favorably to non-surgical methods such as chemotherapy and radiation. In our series, after chemotherapy alone, epidural tumor extension volume shrinked in non-operated patients from 3.9 cm3 (range 0.5–12 cm3) to 0.22 cm3 (range 0–3.1 cm3) (P = 0.007). Thus, we believe, as Sinoff and Blumsohn [27] suggested, that spinal compression can respond to chemotherapy alone and that emergency decompressive laminectomy can be avoided for neurological symptoms due to an epidural tumor extension in hematological malignancies. Chemotherapy should be started as soon as possible to reduce the volume of the epidural tumor extension and relieve the neurological symptoms. In the absence of symptom relief, local radiation and/or decompressive laminectomy can be performed secondarily. This is confirmed by our data, in the nine patients operated for a neurological deficit in our series, the mean time between neurological symptoms occurrence and decompressive surgery was 19 days (range 2–35 days). Interestingly, all were Frankel E at the latest follow-up. So, the neurologic recovery was excellent in all the cases even if no emergency surgery was performed.
Etiologic diagnosis is required, however, before starting chemotherapy. The main issue is to be confronted to progressive neurological deficit secondary to an epidural tumor extension of unknown origin. These cases require a minimal work-up to identify a primary tumor or a hematological malignancy, beginning with various blood tests, including serum protein electrophoresis. Depending on the results of the blood tests, one may have to perform a bone marrow aspirate or biopsy. Full-body CT scan can also be useful to identify primary tumors. In case of negative test results, a surgical procedure must be realized for biopsy and decompressive laminectomy.
Surgery is the treatment of choice for mechanical reasons, when the neurological symptoms are due to an acute vertebral collapse. In these cases, the collapse induces anterior spinal cord bony compression and might induce spinal instability. Laminectomy can be beneficial in some patients [12], but anterior decompression by corporectomy and reconstruction with osteosynthesis is the treatment of choice to correct the mechanical and the neurological issues [28–31].
Spinal stability and mechanical failure is another concern. We estimated that the risk of vertebral collapse is significantly higher when the vertebral osteolysis is more than 30 % of the vertebra (P = 0.005). To avoid vertebral collapse, vertebroplasty or kyphoplasty and/or osteosynthesis should be performed to stabilize the spine and relieve pain if the local or general conditions allow it. If not, the patient can be treated conservatively (bed rest, then bracing) until vertebral reconstruction is confirmed on CT scans. But since the mean delay of reconstruction is 7 months, bed rest and bracing might not be suitable for such a long period of time, fixation without fusion might fail. That is why vertebroplasty with possibly percutaneous posterior osteosynthesis might be a more suitable procedure.
Conclusion
In this retrospective series of 44 patients, we observed that all patients with neurological complications due to spinal localizations of hematological malignancies recovered eventually, even though no surgical decompression was performed. Consequently, when confronted to an epidural tumor extension of unknown origin with spinal cord compression, one should first search for a hematological malignancy, and then start the appropriate medical treatment. Surgical treatment must be considered in case of acute vertebral collapse, or if the diagnosis is uncertain and a biopsy is needed, or if the neurological symptoms increase under medical treatment of the hematological malignancy. If the patient has enough life span, vertebroplasty, kyphoplasty and/or osteosynthesis might be suitable to prevent the possibility of collapse and to maintain the spinal alignment, when presenting with vertebral osteolysis of more than 30 % of the vertebra. To obtain better patient care and prevent patients from unnecessary surgeries, multidisciplinary team care is necessary.
Conflict of interest
No conflict of interest.
References
- 1.Callander NS, Roodman GD. Myeloma bone disease. Semin Hematol. 2001;38:276–285. doi: 10.1016/S0037-1963(01)90020-4. [DOI] [PubMed] [Google Scholar]
- 2.Benson WJ, Scarffe JH, Todd ID, Palmer M, Crowther D. Spinal-cord compression in myeloma. Br Med J. 1979;1:1541–1544. doi: 10.1136/bmj.1.6177.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blade J, Rosinol L. Complications of multiple myeloma. Hematol Oncol Clin North Am. 2007;21:1231–1246. doi: 10.1016/j.hoc.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Nau KC, Lewis WD. Multiple myeloma: diagnosis and treatment. Am Fam Physician. 2008;78:853–859. [PubMed] [Google Scholar]
- 5.Blade J, Cibeira MT, Fernandez de Larrea C, Rosinol L. Multiple myeloma. Ann Oncol. 2010;21:vii313–vii319. doi: 10.1093/annonc/mdq363. [DOI] [PubMed] [Google Scholar]
- 6.Barron KD, Hirano A, Araki S, Terry RD. Experiences with metastatic neoplasms involving the spinal cord. Neurology. 1959;9:91–106. doi: 10.1212/WNL.9.2.91. [DOI] [PubMed] [Google Scholar]
- 7.Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 1999;24:1943–1951. doi: 10.1097/00007632-199909150-00014. [DOI] [PubMed] [Google Scholar]
- 8.Smith ML, Newland AC. Treatment of myeloma. QJM. 1999;92:11–14. doi: 10.1093/qjmed/92.1.11. [DOI] [PubMed] [Google Scholar]
- 9.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 10.Leigh BR, Kurtts TA, Mack CF, Matzner MB, Shimm DS. Radiation therapy for the palliation of multiple myeloma. Int J Radiat Oncol Biol Phys. 1993;25:801–804. doi: 10.1016/0360-3016(93)90308-I. [DOI] [PubMed] [Google Scholar]
- 11.Chataigner H, Onimus M, Polette A. Surgical treatment of myeloma localized in the spine. Rev Chir Orthop Reparatrice Appar Mot. 1998;84:311–318. [PubMed] [Google Scholar]
- 12.Brenner B, Carter A, Tatarsky I, Gruszkiewicz J, Peyser E. Incidence, prognostic significance and therapeutic modalities of central nervous system involvement in multiple myeloma. Acta Haematol. 1982;68:77–83. doi: 10.1159/000206956. [DOI] [PubMed] [Google Scholar]
- 13.Tancioni F, Navarria P, Pessina F, Attuati L, Mancosu P, Alloisio M, Scorsetti M, Santoro A, Baena R. Assessment of prognostic factors in patients with metastatic epidural spinal cord compression (MESCC) from solid tumor after surgery plus radiotherapy: a single institution experience. Eur Spine J. 2012;21:146–148. doi: 10.1007/s00586-012-2232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundaresan N, Digiacinto GV, Hughes JE, Cafferty M, Vallejo A. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645–650. doi: 10.1227/00006123-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Hammerberg KW. Surgical treatment of metastatic spine disease. Spine (Phila Pa 1976) 1992;17:1148–1153. doi: 10.1097/00007632-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bickels J, Dadia S, Lidar Z. Surgical management of metastatic bone disease. J Bone Joint Surg Am. 2009;91:1503–1516. doi: 10.2106/JBJS.H.00175. [DOI] [PubMed] [Google Scholar]
- 17.Akeyson EW, McCutcheon IE. Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg. 1996;85:211–220. doi: 10.3171/jns.1996.85.2.0211. [DOI] [PubMed] [Google Scholar]
- 18.Akhaddar A, Albouzidi A, Elmostarchid B, Gazzaz M, Boucetta M. Sudden onset of paraplegia caused by hemorrhagic spinal epidural angiolipoma. A case report. Eur Spine J. 2008;17:S296–S298. doi: 10.1007/s00586-008-0591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer HC. Posterior decompression and stabilization for spinal metastases. Analysis of sixty-seven consecutive patients. J Bone Joint Surg Am. 1997;79:514–522. doi: 10.2106/00004623-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Rompe JD, Eysel P, Hopf C, Heine J. Decompression/stabilization of the metastatic spine. Cotrel-Dubousset-Instrumentation in 50 patients. Acta Orthop Scand. 1993;64:3–8. doi: 10.3109/17453679308994516. [DOI] [PubMed] [Google Scholar]
- 21.Barlogie B, Shaughnessy J, Munshi N, Epstein J. Plasma cell myeloma. In: Beutler E, Lichtman MA, Coller BS, Kipps T, Seligsohn U, editors. Williams Hematology. 8. New York: McGraw-Hill; 2010. [Google Scholar]
- 22.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 23.Bacci G, Savini R, Calderoni P, Gnudi S, Minutillo A, Picci P. Solitary plasmacytoma of the vertebral column. A report of 15 cases. Tumori. 1982;68:271–275. doi: 10.1177/030089168206800313. [DOI] [PubMed] [Google Scholar]
- 24.Feldmann JL, Guedri M, Ohana N, Menkes CJ, Amor B. Solitary spinal plasmacytoma. Ann Med Interne (Paris) 1984;135:259–264. [PubMed] [Google Scholar]
- 25.Quraishi NA, Gokaslan ZL, Boriani S. The surgical management of metastatic epidural compression of the spinal cord. J Bone Joint Surg Br. 2010;92:1054–1060. doi: 10.1302/0301-620X.92B8.22296. [DOI] [PubMed] [Google Scholar]
- 26.Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317:18–21. doi: 10.1136/bmj.317.7150.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinoff CL, Blumsohn A. Spinal cord compression in myelomatosis: response to chemotherapy alone. Eur J Cancer Clin Oncol. 1989;25:197–200. doi: 10.1016/0277-5379(89)90008-4. [DOI] [PubMed] [Google Scholar]
- 28.Fidler MW. Anterior decompression and stabilisation of metastatic spinal fractures. J Bone Joint Surg Br. 1986;68:83–90. doi: 10.1302/0301-620X.68B1.3941146. [DOI] [PubMed] [Google Scholar]
- 29.Harrington KD (1988) Anterior decompression and stabilization of the spine as a treatment for vertebral collapse and spinal cord compression from metastatic malignancy. Clin Orthop Relat Res 233:177–197 [PubMed]
- 30.Manabe S, Tateishi A, Abe M, Ohno T. Surgical treatment of metastatic tumors of the spine. Spine (Phila Pa 1976) 1989;14:41–47. doi: 10.1097/00007632-198901000-00008. [DOI] [PubMed] [Google Scholar]
- 31.McLain RF. Spinal cord decompression: an endoscopically assisted approach for metastatic tumors. Spinal Cord. 2001;39:482–487. doi: 10.1038/sj.sc.3101194. [DOI] [PubMed] [Google Scholar]



