Abstract
Purpose
To investigate the clinical efficacy and feasibility of one-stage surgical treatment for upper thoracic spinal tuberculosis by internal fixation, debridement, and combined interbody and posterior fusion via a posterior-only approach.
Methods
Fourteen patients (eight males, six females) with upper thoracic tuberculosis whose lesions were confined to two adjacent segments were admitted to our hospital. Their ages ranged from 23 to 72 years (average, 50 years). The American Spinal Injury Association (ASIA) impairment scale was used to assess neurological function. ASIA classification showed that preoperatively, one patient was grade A, two patients were grade B, eight patients were grade C, and three patients were grade D. All patients were treated with one-stage surgical treatment by internal fixation, debridement, and combined interbody and posterior fusion via a posterior-only approach. Patients were evaluated preoperatively and postoperatively by measurement of thoracic kyphotic angles using Cobb angle evaluation, determination of erythrocyte sedimentation rate (ESR), evaluation of ASIA impairment scale, and radiological examination.
Results
Operation time ranged from 70 to 135 min, (average, 110 min). Intraoperative blood loss ranged from 200 to 950 mL (average, 450 mL). All patients were followed up for 22 to 48 months postoperatively (average, 31.5 months). No sinus tract formation, cerebrospinal meningitis, or recurrence of tuberculosis occurred. All patients had significant postoperative improvement in ASIA classification scores. The thoracic kyphotic angles were significantly decreased to 12°–26° postoperatively, and at final follow-up were 13°–28°. The ESR recovered to normal within 6 months postoperatively in all patients. Bone fusion was achieved within 3–8 months (average, 5.5 months).
Conclusions
One-stage surgical treatment for upper thoracic spinal tuberculosis by internal fixation, debridement, and combined interbody and posterior fusion via a posterior-only approach can be an effective and feasible treatment method.
Keywords: Upper thoracic vertebrae, Spinal tuberculosis, Bone graft, Internal fixation, Posterior
Introduction
As HIV infection and drug resistance increases, spinal tuberculosis has increased in both developing and developed countries [1]. Patients with upper thoracic spinal tuberculosis often suffer from severe spinal cord damage and kyphotic deformity requiring surgical treatment. Anterior debridement and bone grafting and either anterior or posterior internal fixation have been performed by many researchers as an effective treatment for thoracic spinal tuberculosis [2–5]. However, this procedure may carry a risk of cardiopulmonary complications and prolong recovery time. In addition, exposure of the upper two thoracic vertebral segments remains a great challenge to surgeons [6]. Orthopedic operations should be well tolerated, minimally invasive, and associated with only minor postoperative complications. The purpose of this study was to evaluate the clinical efficacy and feasibility of one-stage surgical treatment of upper thoracic tuberculosis by internal fixation, debridement, and interbody bone fusion via a posterior-only approach.
Materials and methods
The study was approved by the ethics board committee of our hospital. Between May 2004 and January 2009, 34 patients with upper thoracic vertebral tuberculosis were admitted to our department, and 14 patients whose lesions were confined to two adjacent segments were involved in this study. Criteria included tuberculous spondylitis that was mainly confined to one side of the vertebrae without a wide range of tuberculosis abscessation. The patients were eight men and six women with a mean age of 50 years (range 23–72 years) and kyphosis as indicated by a Cobb angle of 34° to 66° (average, 48°). The diagnosis of tuberculosis spondylitis was based on clinical presentation, radiologic findings (plain radiographs, computed tomography [CT], and magnetic resonance imaging [MRI]), and hematologic and pathological examinations. Clinical presentation manifested thoracodorsal pain, night sweats, low-grade fever, weight loss, thoracic kyphosis, and neurological dysfunction. Diseased segments were observed at T1–T2 in two patients, T2 in one patient, T2–T3 in four patients, T3–T4 in five patients, and T4 in two patients. The American Spinal Injury Association (ASIA) impairment scale [7] was applied to evaluate preoperative neurological dysfunction; one patient was of grade A, two patients were of grade B, eight patients were of grade C, and three patients grade D. The erythrocyte sedimentation rate (ESR) ranged from 47 to 78 mm/h (average, 60 mm/h) (Table 1). Paralysis was progressively aggravated in 13 patients; three had mild pleural thickening, and two had partial destruction in one side of the vertebral pedicle.
Table 1.
Clinical data of patients
| Case no. | Age/gender | Follow-up (months) | Diseased segments | Kyphotic Cobb’s angle (°) | ASIA | ESR (mm/h) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preop | Postop | Final follow-up | Preop | Final follow-up | Preop | Three months postop | ||||
| 1 | 60/M | 31 | T2–T3 | 43 | 14 | 15 | C | E | 55 | 5 |
| 2 | 43/M | 23 | T3–T4 | 38 | 15 | 17 | C | E | 78 | 13 |
| 3 | 55/F | 48 | T3–T4 | 55 | 16 | 17 | A | D | 66 | 11 |
| 4 | 47/M | 27 | T4 | 35 | 12 | 13 | C | E | 51 | 10 |
| 5 | 23/M | 37 | T2–T3 | 41 | 13 | 13 | C | E | 52 | 8 |
| 6 | 49/F | 26 | T3 | 66 | 25 | 27 | B | D | 73 | 6 |
| 7 | 32/M | 34 | T1–T2 | 46 | 14 | 16 | C | D | 59 | 6 |
| 8 | 61/F | 42 | T3–T4 | 34 | 13 | 14 | D | E | 69 | 13 |
| 9 | 72/M | 29 | T4 | 59 | 19 | 22 | B | D | 68 | 10 |
| 10 | 66/F | 22 | T2–T3 | 47 | 18 | 20 | C | E | 47 | 6 |
| 11 | 38/M | 40 | T3–T4 | 42 | 17 | 17 | D | E | 63 | 7 |
| 12 | 52/F | 28 | T2 | 53 | 22 | 23 | C | E | 56 | 12 |
| 13 | 59/F | 24 | T2–T3 | 50 | 19 | 20 | D | E | 45 | 14 |
| 14 | 44/M | 30 | T1–T2 | 62 | 26 | 28 | C | E | 52 | 9 |
Indication for this study was that lesions were confined to one segment or two adjacent segments, were without a wide range of tuberculosis abscessation, and could be thoroughly debrided during the preoperative evaluation, besides having either of the following situations: (1) the presence of significant vertebral collapse caused by bone destruction and spinal instability. (2) Severe kyphotic deformity (Cobb angle of >30º). (3) Spinal cord compression by paravertebral/epidural abscesses, and severe or progressive neurologic dysfunction. (4) The formation of a large hollow or significant sequestrum.
Preoperative procedure
Three to five weeks prior to the operation, the patients were administered the antituberculosis drugs isoniazid (5–10 mg/kg/day, no more than 300 mg/day), rifampicin (5–10 mg/kg/day, no more than 300 mg/day), and ethambutol (15 mg/kg/day, no more than 500 mg/day). Enhancement of nutrition and correction of anemia and hypoproteinemia were carried out. The extent of vertebral lesions and range of abscessation were determined by X-ray, CT, and MRI. We use preoperative halo traction for patients with severe kyphosis deformity, but not all patients undergo preoperative traction. If there is rigid oppression in the spinal canal or progressive neurologic dysfunction during halo traction, halo retraction must be terminated. It must also be terminated if patients cannot tolerate continuous traction.
Preoperative halo traction was usually adopted within 2 weeks, and the weight was 3–5 kg. Surgery was performed when the anemia and hypoproteinemia had been corrected according to ESR and the temperature had returned to normal or had significantly decreased.
Operative procedure
The patients were placed in prone position under general anesthesia with somatosensory-evoked potential monitoring. Intraoperative halo traction of 3 kg was applied to lessen upper thoracic kyphosis deformity and maintain spinal stability. Through a midline incision, extraperiosteal dissection at both non-fusion and fusion levels was performed. The posterior spinal construction was exposed, including the spinous processes, lamina, facet joints, and transverse processes. The range of exposure at the level of decompression was expanded to include the bilateral costotransverse articulations and 3–5 cm of the medial ribs. With C-arm fluoroscopy assistance, pedicle screws or hooks were placed at least two levels superior and inferior to the level of decompression. The Vertex Reconstruction System (Sofamor Danek, Medtronic, USA) was used in all 14 patients. First, a temporary rod on the mildly affected side of the lesion was placed to avoid spinal cord injury during decompression and focal debridement. Screws were also placed in diseased vertebrae if the upper part of the vertebral body was not destroyed. Second, costotransversectomy and removal of the articular process, pedicle, and transverse process on the more severely affected side of the lesion segment was performed to drain prevertebral abscesses and expose diseased vertebral bodies. The thoracic nerve roots on the focal side were sacrificed for better exposure. After the posterior longitudinal ligament was separated from the dural sac with a nerve dissector, a nerve root retractor was used to protect the spinal cord. A scalpel was used to incise the posterior longitudinal ligament. Spatulas of various sizes and angles were used from a posterolateral approach to remove lesions including sequestra, abscesses, and granulation tissues. The abscesses were drained by suction and curettage as thoroughly as possible. If bilateral vertebrae were severely damaged, the temporary rod was switched to the other side, and the same debridement and decompression procedure was performed. Correction of the kyphosis deformity was accomplished by installing contoured permanent rods and exertion of decompression at the middle anchoring point with a cantilever bending maneuver. The appropriately sized autologous bone or allograft bone block (Tricortical bone) was imbedded in the interbody. After internal fixation, debridement and interbody thoracic fusion (IFDITF) were performed, strip-sized autogenous bone or allograft bone was imbedded in the posterior body to fuse the segments that underwent decompression and focal debridement. Streptomycin (1.0 g) and isoniazid (0.3 g) were locally deposited, and drainage and placement of incisional sutures were performed postoperatively. The debrided material underwent bacterial culture and histopathologic examination.
Postoperative management
Patients remained in bed for 2 weeks and then walked around under the effective support of a cervical thoracic neck brace for 6–8 months. Nutritional improvement and support therapy were regularly performed. The drainage tube was pulled out when volume was <30 mL per 24 h, which occurred within 3 days postoperatively in most patients. According to the general condition, early application of physical therapy and rehabilitation training were performed to prevent blood clots and restore nerve function. Antituberculosis chemotherapy was administered for 12–18 months postoperatively, and the patients were instructed to undergo monthly routine blood and hepatic function examinations to monitor for any adverse reactions to the antituberculosis agents. ASIA classification, ESR, and CRP were evaluated monthly, and X-ray and CT were examined every 3–6 months.
Statistical analysis
A paired t test was used to evaluate preoperative and postoperative ESR, ASIA classification, and thoracic kyphosis according to the Cobb angle. Customarily, a P value of <0.05 was considered statistically significant using SPSS version 17.0 for Windows. Discrepancy of the normal distribution was evaluated by a rank-sum test with a significance level of 0.05.
Results (Table 1)
Surgical condition and neural recovery
The average operative time was 110 min (range 70–135 min), and average blood loss was 450 mL (range 200–950 mL). No perioperative complications related to instrumentation or decompression occurred. The duration of postoperative follow-up was 31.5 months (range 22–48 months). No patients developed wound infection, sinus formation, or infectious meningitis, and none experienced tuberculosis relapse. Symptoms of tuberculosis disappeared. All patients had significant improvement in the postoperative ASIA classification score (P < 0.001).
Laboratory data
Postoperative pathologic examination proved the presence of tuberculosis in all patients. The ESR and CRP returned to normal within 3 months postoperatively. The preoperative mean ESR value was 59.6 mm/h (range 45–78 mm/h), which normalized within 3 months postoperatively in all patients (P < 0.001).
Radiological data
The average thoracic Cobb angle was significantly decreased at 17.4° (range 12°–26°) postoperatively and was 18.7°(range 13°–28°) at final follow-up. The loss of correction was only 1.3°. There were statistically significant differences compared with the preoperative measurements (P < 0.001), but no significant difference between the postoperative thoracic Cobb angle and that at final follow-up (P > 0.001).
CT was performed as a routine examination to evaluate bone fusion. Achievement of bone fusion was based on Lee’s standard [8]: adjacent surface without gaps, obvious trabecular bone through the graft surface, and displacement of ≤3° on dynamic X-ray films. All patients obtained intervertebral and posterior bone fusion within 48 months (mean 5.5 months).
A 47-year-old patient with spinal tuberculosis had back pain for 6 months and incomplete paralysis for 1 month (ASIS grade C). The lesion had been resistant to chemotherapy for 6 months. Preoperative radiography showed destructive segments located at T4 (Figs. 1, 2). Preoperative CT and MRI showed the almost total disappearance of T4 vertebral body, the presence of a local abscess, and a kyphotic Cobb angle of 35°. The spinal cord was severely compressed (Figs. 3, 4). The patient underwent one-stage surgical treatment for upper thoracic spinal tuberculosis by internal fixation, debridement, and combined interbody and posterior fusion via a posterior-only approach. Postoperative radiography showed that fixation was in good position, kyphosis had improved significantly, and the Cobb angle was 12° (Figs. 5, 6). At the 6-month follow-up, the patient’s ASIA grade had recovered to E (Fig. 7). CT showed bone fusion and recovery of normal thoracic kyphosis (Fig. 8).
Fig. 1.
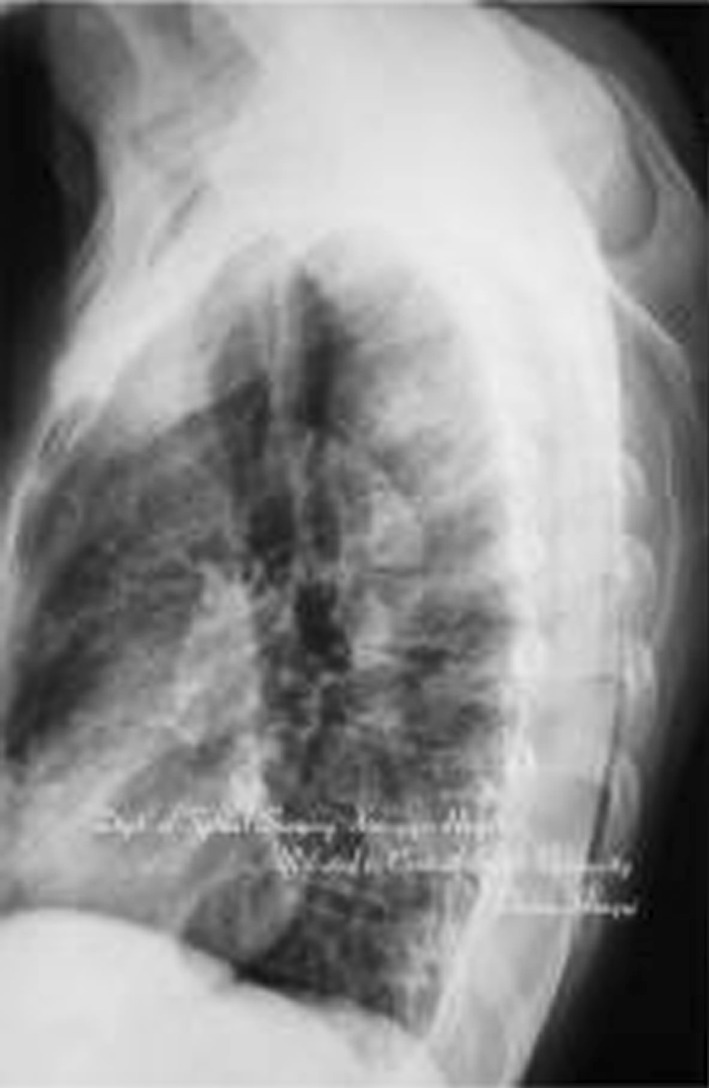
Preoperative lateral X-ray film. Destructive segments located at T4
Fig. 2.
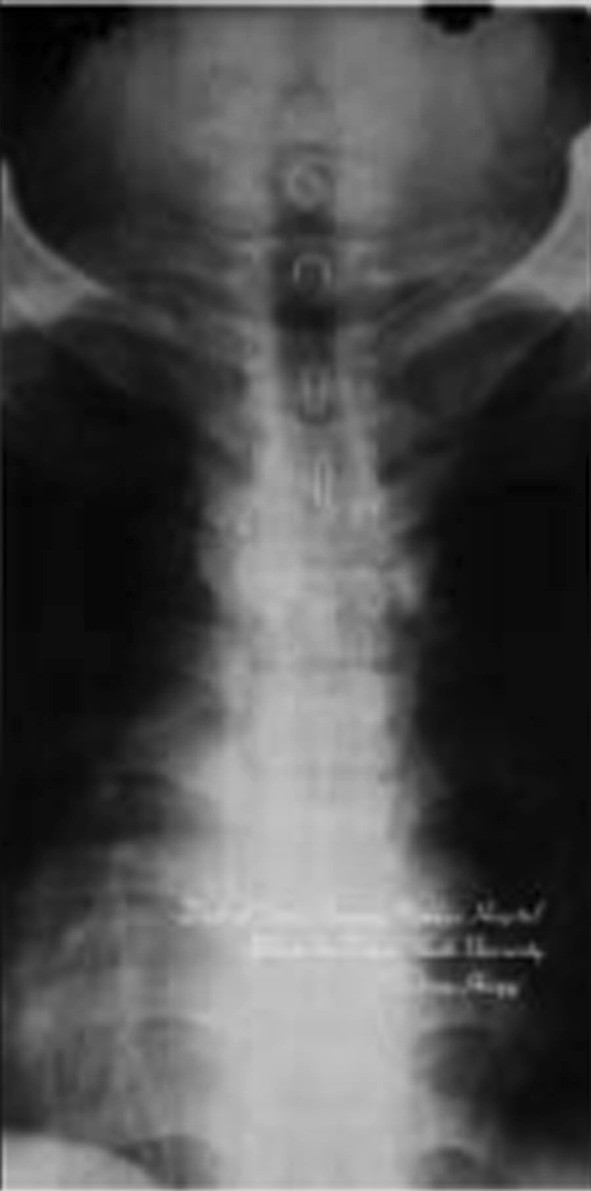
Preoperative anteroposterior X-ray film
Fig. 3.
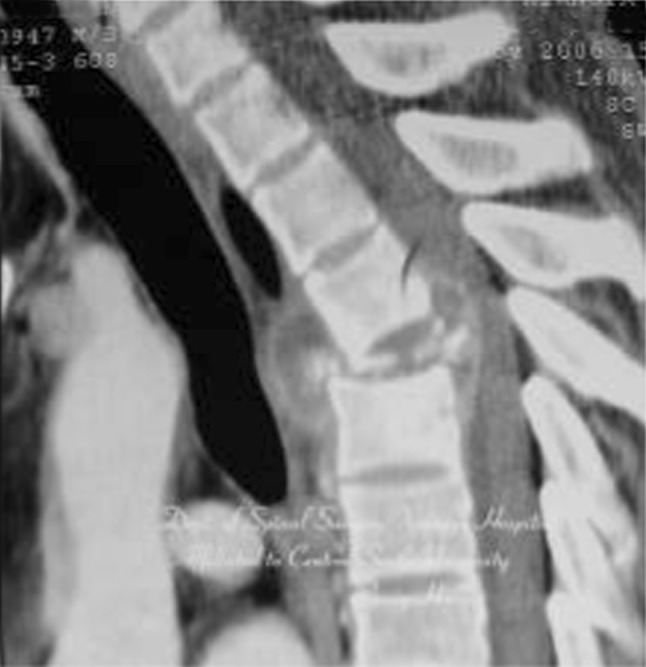
Preoperative CT. The almost total disappearance of T4 vertebral body
Fig. 4.
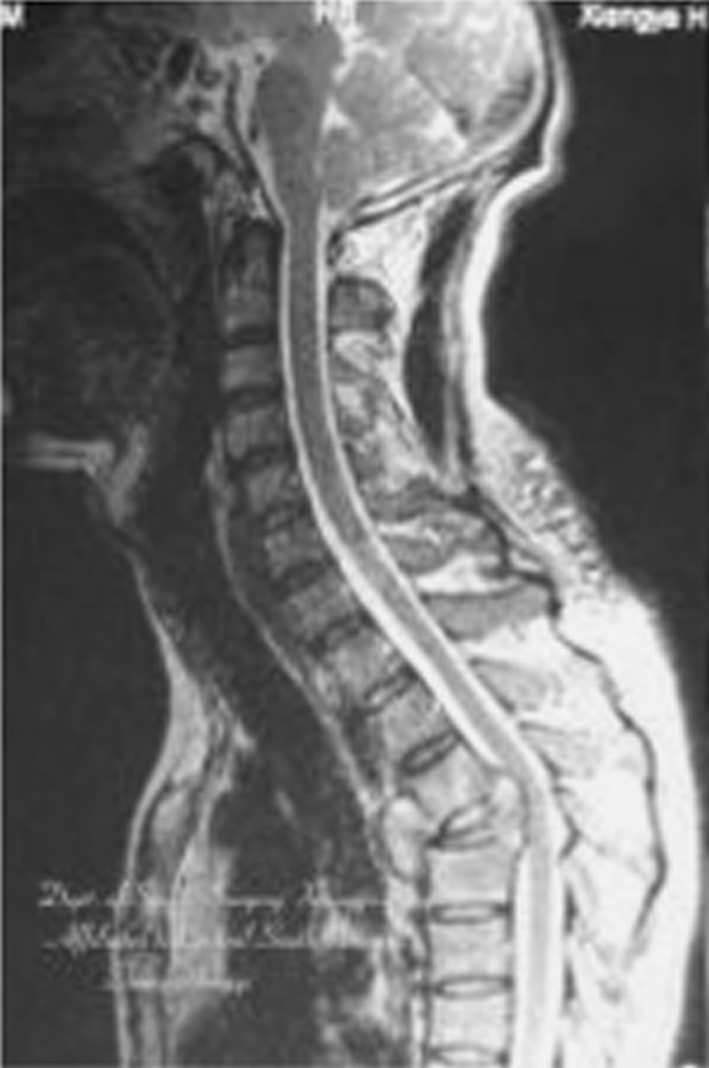
Preoperative MRI. The spinal cord was severely compressed by the local abscess. The local kyphotic Cobb angle was 35°
Fig. 5.
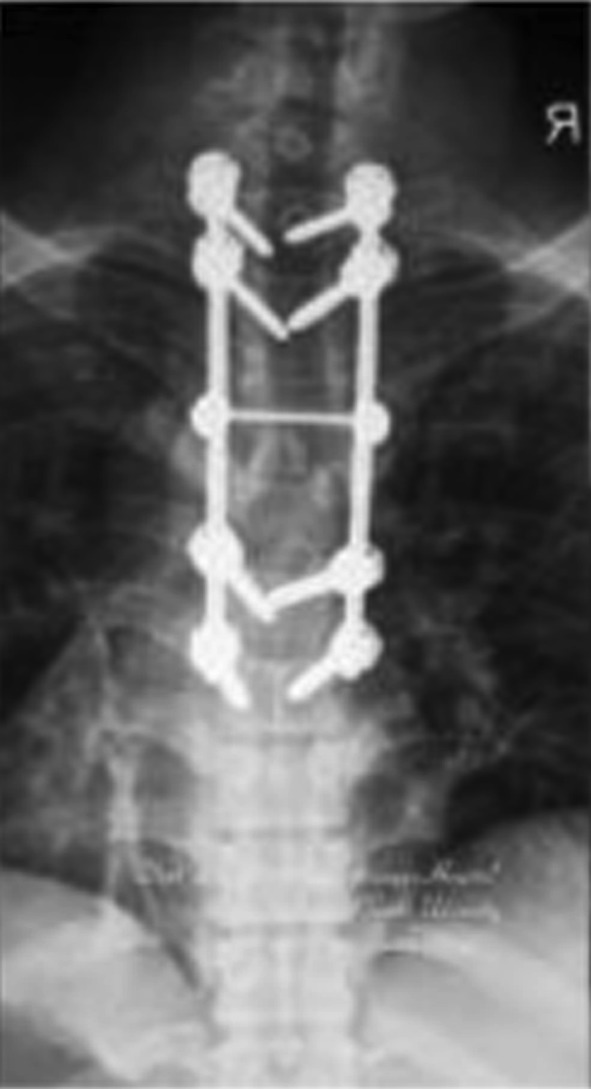
Postoperative anteroposterior X-ray film. Internal fixation was in good position
Fig. 6.
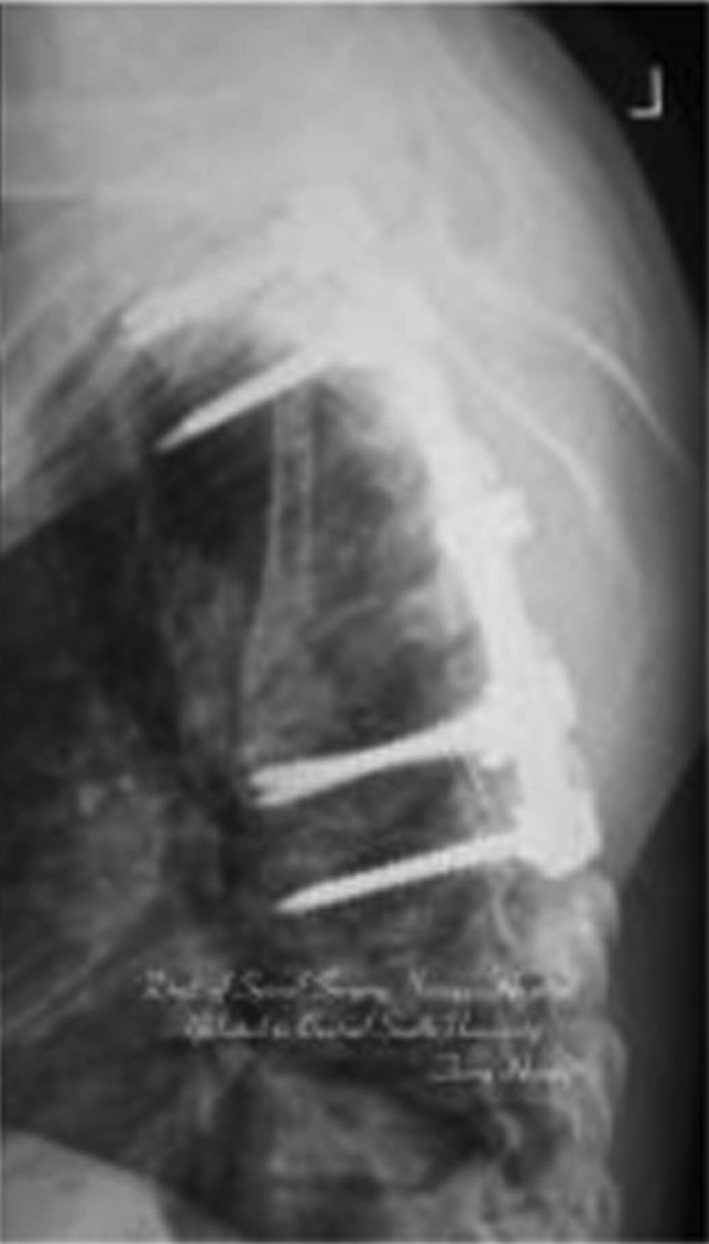
Postoperative lateral X-ray film. Local kyphosis had improved significantly, and the Cobb angle was 12°
Fig. 7.
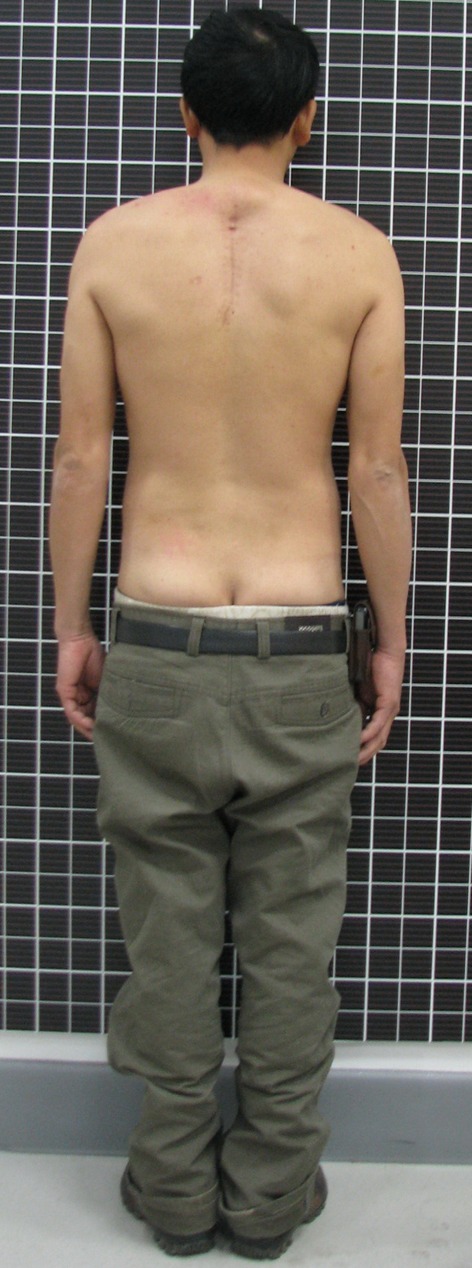
6-month follow-up. The patient’s ASIA grade had recovered to E
Fig. 8.
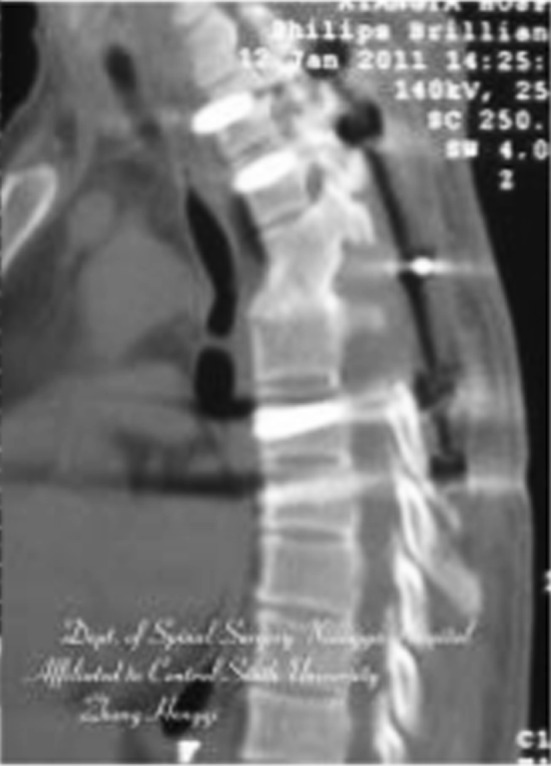
Postoperative CT. Solid bone fusion in diseased segments
Discussion
The purpose of spinal surgery involves removal of the focus, complete decompression of nerves, correction of spinal deformity, and reconstruction of spinal stability [1]. The radiographic criteria of thoracic spinal instability were as follows: (1) sagittal translation was greater than 2.5 mm in lateral radiographs. (2) Subluxation or dislocation of the facet joint at the apex of the kyphus. (3) Translation of the vertebral column in coronal plane, and (4) Segmental kyphosis angle was greater than 30° in the lateral radiographs. Because spinal tuberculosis mostly involves the spinal anterior column, surgical treatment through an anterior approach was long considered to be the “gold standard” because it can provide direct access to vertebral body lesions, allowing for efficient reconstruction of the anterior spinal column [9]. Nevertheless, in upper thoracic tuberculosis, anterior exposure of the lesions is blocked by the thoracic bones, clavicle, costal bone, and mediastinal organs [10]. It is difficult to reveal the upper thoracic vertebrae by thoracotomy because of obstruction of complex anatomical structures [6]. Xiao [11] used an anterolateral transthoracic approach for upper thoracic vertebral tuberculosis. This procedure requires massive transection of muscle tissue and causes great injury. The procedure of thoracotomy requires going through the pleural cavity, which may result in perioperative complications including respiratory insufficiency, hemopneumothorax, pneumonia, and vascular risk [12–14]. Thoracotomy is considered to be contraindicated in patients with extensive pleural adhesions. Jiang et al. [15] used an anterior trans-sternal approach to treat upper thoracic vertebral tuberculosis while avoiding the pleural cavity. However, the approach involved complex and vital structures and injured the recurrent laryngeal nerve, vagus nerve, phrenic nerve, thoracic duct, and other important structures, and the consequences were disastrous. The thoracic posterolateral surgical approach supplies a limited operative field, and it is inconvenient to install vertebral body screws; moreover, the orthopedic effect and stability of internal fixation are dissatisfactory. Although video-assisted thoracoscopic surgery (VATS) is a minimally invasive procedure with which to expose the anterior thoracic column, VATS is associated with a steep learning curve for orthopedic surgeons to master the thoracoscopic surgical technique [16]. It is also inconvenient to perform debridement and instrumentation for the upper thoracic vertebrae.
An “easy, effective, and minimally invasive” surgical treatment for upper thoracic spinal tuberculosis to achieve simultaneous debridement, deformity correction, and bone graft fixation has been debated. Over the years, many scholars believed that posterior laminectomy for removal of the lesion destroyed the normal structure of the posterior column, making the spinal column more unstable. Many have also supposed that the posteriorly exposed space was limited, the lesion may not be completely clear, and there is a risk of spinal cord injury. Thus, most scholars do not advocate adoption of the posterior approach for thoracic tuberculosis [3, 15]. Removal of a single costotransverse joint and facet joint slightly affects spinal stability for the following reasons. (1) Activities of the thoracic spine are limited and spinal stability is maintained by the supporting role of the ribs and fixed effect of the sternum [17]. (2) Fixation of pedicle screws with three columns supplies good support force, including anti-torsion and anti-buckling capabilities, corrects the deformity, and stabilizes the lesion level. (3) With intertransverse and posterior bone grafting, reconstruction of the stability of the posterior column is actually achieved. Some scholars have expressed concern that lesions confined to the vertebral body could spread to the lamina of the vertebra and spinous process to result in infectious meningitis. However, standard antituberculosis chemotherapy can prevent recurrence and metastasis of tuberculosis [4, 18, 19], while the current relevant literature and our study show that such complications are rare [19, 20]. Compared with patients treated with anterior approach, patients treated with posterior approach are more conducive to drainage of residual lesions postoperatively in the supine position because of gravity.
The results of this study revealed that compared with other surgical procedures, surgical treatment of upper thoracic spine tuberculosis has unique advantages. (1) Debridement, spinal cord decompression, correction of deformities, bone grafting, and internal fixation can be completed in one incision and surgical position and iatrogenic spinal injury avoided while turning over, which is similar to the best results obtained via anterior decompression [21, 22]. (2) Without one-lung ventilation, the interference of pulmonary function is relatively small, and there are fewer postoperative complications such as atelectasis, lung infection, and chylothorax because of fewer traumas. Even if there are severe pleural adhesions, the operation can be performed safely. (3) Under direct vision, spinal canal decompression from the anterior, lateral, and posterior aspects (decompression at the 270°) can be thoroughly performed. Removal of the ribs, transverse process, and small joints on one side can supply enough operative space for exposure of lesions to perform IFDITF (internal fixation, debridement and interbody thoracic fusion). In our study, neurological function of patients with paraplegia was significantly improved postoperatively. (4) Pedicle screws used in surgery can not only strengthen the spinal three-column stability with the normal physiological curvature of the spine, but can also be pressurized to correct kyphosis. Use of the “bowstring principle” to further relieve spinal cord compression and intraoperative pressure on the graft bone mass solidifies the graft [23]. However, for patients with spinal tuberculosis with large prevertebral abscesses or destruction of consecutive multi-segmental vertebrae, it is difficult to achieve satisfactory debridement by the posterior approach.
Conclusion
Surgical treatment of upper thoracic spinal tuberculosis by internal fixation, debridement, and combined interbody and posterior fusion via a posterior-only approach is feasible. It is minimally traumatic, has a high correction rate, and prevents the progression of kyphosis. The decompression of the spinal cord can be satisfactory. To date, the clinical and radiographic outcomes have been good. Of course, further study with a large number of patients and longer follow-up will be required.
Conflict of interest
None.
References
- 1.Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br. 2010;92(7):905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 2.Tamura M, Saito M, Machida M, et al. A transsternoclavicular approach for the anterior decompression and fusion of the upper thoracic spine. J Neurosurg Spine. 2005;2(2):226–229. doi: 10.3171/spi.2005.2.2.0226. [DOI] [PubMed] [Google Scholar]
- 3.Benli IT, Acaroglu E, Akalin S, et al. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J. 2003;12(2):224–234. doi: 10.1007/s00586-002-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang HQ, Guo CF, Xiao XG, et al. One-stage surgical management for multilevel tuberculous spondylitis of the upperthoracic region by anterior decompression, strut autografting, posterior instrumentation, and fusion. J Spinal Disord Technol. 2007;20(4):263–267. doi: 10.1097/01.bsd.0000211281.68400.1b. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, Xiao ZM, Zhan XL, et al. Anterior transsternal approach for treatment of upper thoracic vertebral tuberculosis. Orthop Surg. 2010;2(4):305–309. doi: 10.1111/j.1757-7861.2010.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pettiford BL, Schuchert MJ, Jeyabalan G, et al. Technical challenges and utility of anterior exposure for thoracic spine pathology. Ann Thorac Surg. 2008;86(6):1762–1768. doi: 10.1016/j.athoracsur.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 7.Dituno J. Rehabilitation assessment and management in the acute spinal cord injury (SCI) patient. In: Narayan RK, Wilberger JE, Povlishock JT, editors. Neurotrauma. New York: McGraw-Hill; 1996. pp. 1259–1266. [Google Scholar]
- 8.Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20(3):356–361. doi: 10.1097/00007632-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Mihir B, Vinod L, Umesh M, et al. Anterior instrumentation of the cervicthoracic vertebrae: approach based on clinical and radiologic criteria. Spine. 2006;31(9):e244–e249. doi: 10.1097/01.brs.0000214883.11874.80. [DOI] [PubMed] [Google Scholar]
- 10.Seol HJ, Chung CK, Kim HJ. Surgical approach to anterior decompression in the upper thoracic spine. J Neurosurg. 2002;97(3 suppl):337–342. doi: 10.3171/spi.2002.97.3.0337. [DOI] [PubMed] [Google Scholar]
- 11.Xiao ZM, He ML, Zhan XL, et al. Anterior transsternal approach for a lesion in the upper thoracic vertebral body. J Neurosurg Spine. 2010;13(4):461–468. doi: 10.3171/2010.4.SPINE09808. [DOI] [PubMed] [Google Scholar]
- 12.Ikard RW. Methods and complications of anterior exposure of the thoracic and lumbar spine. Arch Surg. 2006;141(10):1025–1034. doi: 10.1001/archsurg.141.10.1025. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell MF, Glassman SD, Dimar JR, II, Puno RM, John-son JR. Perioperative complications of anterior procedures on the spine. J Bone Joint Surg Am. 1996;78(6):839–847. doi: 10.2106/00004623-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Stulík J, Vyskocil T, Bodlák P, et al. Injury to major blood vessels in anterior thoracic and lumbar spinal surgery. Acta Chir Orthop Traumatol Cech. 2006;73(2):92–98. [PubMed] [Google Scholar]
- 15.Jiang H, Xiao ZM, Zhan XL, et al. Anterior transsternal approach for treatment of upper thoracic vertebral tuberculosis. Orthop Surg. 2010;2(4):305–309. doi: 10.1111/j.1757-7861.2010.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson JP, Filler AG. Mc Bride DQ. Endoscopic thoracic discectomy. Neurosurg Focus. 2000;9(4):E11. [PubMed] [Google Scholar]
- 17.Panjabi MM, White IIIA. Physical properties andfunctional biomechanics of the spine. In: White IIIA, Panjabi MM, editors. “Clinical Biomechanicsof the Spine”. Philadelphia: JB Lippincott; 1990. pp. 1–84. [Google Scholar]
- 18.Medical Research Council Working Party on Tuberculosis of the Spine (1999) Five-year assessment of controlled of short-course chemotherapy regimens of 6, 9, 18 months’ duration for spinal tuberculosis in patients ambulatory from the start or undergoing radical surgery: fourteenth report of the Medical Research Council Working Party on tuberculosis of the spine. Int Orthop 23(2):73–81 [DOI] [PMC free article] [PubMed]
- 19.Zhang HQ, Wang YX, Guo CF. One-stage posterior focus debridement, fusion and instrumentation in the surgical treatment of cervicothoracic spinal tuberculosis with kyphosis in children: a preliminary report. Childs Nerv Syst. 2011;27(5):735–742. doi: 10.1007/s00381-010-1319-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Sung JK, Park YM. Single-stage transpedicular decompression and posterior instrumentation in treatment of thoracic and thoracolumbar spinal tuberculosis: a retrospective case series. J Spinal Disord Tech. 2006;19(8):595–602. doi: 10.1097/01.bsd.0000211241.06588.7b. [DOI] [PubMed] [Google Scholar]
- 21.Feyza KG, Erhan EN, Serdar B, et al. Thoracic and lumbar tuberculous spondylitis treated by posterior debridement, graft placement, and instrumentation: a retrospective analysis in 19 cases. J Neurosurg Spine. 2005;3(6):450–458. doi: 10.3171/spi.2005.3.6.0450. [DOI] [PubMed] [Google Scholar]
- 22.Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38(5):926–933. doi: 10.1097/00006123-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HQ, Chen LQ, Liu SH, et al. Posterior decompression with kyphosis correction for thoracic myelopathy due to ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament at the same level. Neurosurg Spine. 2010;13(1):116–122. doi: 10.3171/2010.3.SPINE09237. [DOI] [PubMed] [Google Scholar]


