Abstract
Purpose
We intend to report the largest series of spinal epidural cavernomas (SEC), discuss their clinical features, imaging characteristics, surgical findings, outcome analysis and compare them with similar reports in the literature.
Methods
Among the cases of spinal tumors treated surgically by the authors, there have been nine cases of SEC’s. All the data were collected prospectively and the cases have been followed after surgery up to the time of this analysis.
Results
There were six female and three male patients with the ages ranging between 13 and 74 years. The lesions were located in the thoracic spine (4 cases), lumbar spine (4 cases) and one at the sacral level. Clinical presentations included acute spinal pain and paraparesis in two, low back pain and radiculopathy in five, and slowly progressive myelopathy in the other two cases. The lesion was iso-intense with the spinal cord in T1W images and hyperintense in T2W images and showed strong homogeneous enhancement after contrast medium injection in most of our cases. In the presence of hemorrhage inside the lesion, it was hyperintense in both T1W and T2W MR sequences as in our case 6. In the single case presenting with acute hemorrhage, epidural hematoma was the only finding, our case 1. Complete surgical removal was achieved in all our cases, and confirmed by postoperative MRI.
Conclusion
SEC is hard to be differentiated from other epidural spinal lesions before intervention but should be considered in the list of differential diagnosis regarding its favorable outcome.
Keywords: Cavernous angioma, Magnetic resonance imaging, Spinal epidural cavernoma, Spinal epidural lesion
Introduction
Cavernous angiomas or cavernomas are uncommon vascular malformations of the central nervous system. They may occur in any part of the neuraxis [1, 4, 12, 23, 28], but are most commonly found in the supratentorial cerebral compartment [3, 10, 28]. In the spine, they are usually located in the vertebral bodies and comprise 5–12 % of all spinal hemangiomas [2, 4, 7, 12, 23, 25]. Solitary spinal epidural cavernous angiomas (SSECA) are rare lesions [5, 6, 8, 9, 11, 13, 18, 21, 22, 24]. They are most commonly located in the posterior aspect of the thoracic epidural space and less frequently found in the foraminal and extraforaminal regions [4, 10, 11, 24]. The authors intend to present and discuss the variable clinical presentations and image findings of nine such cases and compare them with those reported in the literature.
Materials and methods
Among the patients operated for spinal tumor between January 2000 and May 2011, there were nine cases of SSECA’s. Their data were gathered prospectively which will be described in brief. The relevant literature is reviewed.
Result
There were six female and three male patients with the mean age of 45.3 years and the age range of 13–74 years. The lesions were located in the thoracic region (4 cases), lumbar region (4 cases) and sacral level (1 case). The clinical presentations included acute pain and paraparesis in two cases, low back pain (LBP) and radiculopathy in five cases, and slowly progressive myelopathy in the other two. Plain radiography was performed in all patients but could not reveal any remarkable pathological changes. Computed tomography (CT) scan of the affected spinal levels was performed in five cases which showed vertebral body scalloping in three and erosion of the lamina in two. All these patients had brain and whole spinal MRI prior to or after surgical intervention searching for multiple lesions, and post-operative MRI in the follow-up period for control of the tumor recurrence. Total surgical excision of the lesion could be achieved in all the patients. Complete recovery of the symptoms appeared gradually after surgery in all the patients except one. Summary of these nine cases are shown in Table 1.
Table 1.
Summary of nine cases of spinal epidural cavernous angioma
| Cases | Gender/age (year) | Level | Symptoms | MRI | Outcome | ||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T1 with Gd | |||||
| 1 | F/74 | T5–9 | Mid thoracic pain + acute myelopathy + epidural hematoma | Mixed signal | Mixed signal | Non-homogeneous enhancement | Partially improved |
| 2 | M/26 | T8 | Thoracic pain + progressive paraparesis | Isointense | Hyperintense | Homogeneous enhancement | Completely improved |
| 3 | F/53 | L2 | Rt L3 Radiculopathy | Isosignal | Hypersignal | Ring enhancement | Completely improved |
| 4 | M/45 | L2 | Lt lower limb pain + toe weakness | Isointense | Hyperintense | Homogeneous enhancement | Completely improved |
| 5 | M/52 | T7–8 | Back pain + progressive paraparesis | Isointense | Hyperintense | Homogeneous enhancement | Completely improved |
| 6 | F/13 | T6–8 | Acute myelopathy | Hyperintense | Hyperintense | Homogeneous enhancement | Completely improved |
| 7 | F/41 | L1–2 | LBP weakness of both legs | Isointense | Hyperintense | Homogeneous enhancement | Completely improved |
| 8 | F/59 | L4–5 | LBP, Rt L5 radiculopathy | Isointense | Hyperintense | Homogeneous enhancement | Completely recovered |
| 9 | F/40 | S1/S2 | LBP, Rt L5/S1 radiculopathy | Isointense | Hyperintense | Homogeneous enhancement | Completely recovered |
F female, M male, Rt right, Lt left, T thoracic, L lumbar, LBP low back pain
Case illustration
Case 1
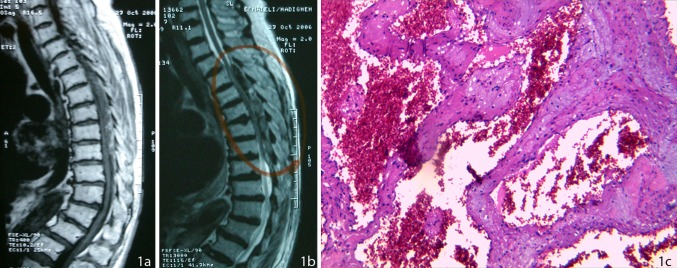
A 74-year-old woman presented with history of acute onset of severe paraparesis 3 days prior to her admission. There was no history of spinal trauma, infection or anticoagulant therapy. On admission, her lower limbs were spastic with hyperreflexia and bilateral extensor plantar reflexes. She had a muscle power of 1/5, a sensory deficit up to the level of T8 and bladder retention. MRI of the thoracic spine revealed an acute epidural hematoma at T5–T9 levels, compressing the spinal cord mostly on the dorsal aspect (Fig. 1a, b). Contrast material injection, showed non-homogeneous enhancement in the periphery of the lesion. A T5–T9 laminectomy revealed an extradural hematoma containing a dark red organized elastic lesion within it and was removed completely. Histopathological diagnosis was compatible with epidural hematoma and a cavernous angioma (Fig. 1c). MRI of the brain, cervical and lumbar spine performed after 2 weeks was normal and patient recovered partially after a year, with improvement of the muscle strength of lower limbs to 3/5, even though she was unable to walk unassisted.
Fig. 1.
a Sagittal T1W MRI of the thoracic spine showing a heterogeneous epidural mass compressing the spinal cord at T5–T9 level, b sagittal T2W MRI showing the heterogeneous epidural lesion compressing the spinal cord at the same level, and c dilated capillaries with a thin wall and a simple endothelial lamina with thin adventitia. Fibrous scar tissue with scattered hemosiderin calcification located in between the vascular spaces
Case 5
A 52-year-old man was admitted with back pain and progressive paraparesis of 4 months duration. Neurological examination showed spastic paraparesis with bilateral sustained clonus and gait disturbance. A pinprick sensory deficit was detectable up to the level of T7. A well-defined posterior epidural mass could be seen in MRI of the thoracic spine at T7–8 level. It was isointense in T1W and hyperintense in T2W images which enhanced strongly after contrast material injection, and compressing the spinal cord (Fig. 2a, b, d). A lobulated dark mass was removed completely via laminectomy of T7 and T8 levels. Histopathological examination revealed a cavernous angioma (Fig. 2c). Postoperative course was uneventful and all the symptoms regressed completely. Brain, cervical and lumbosacral spine MR images were normal.
Fig. 2.
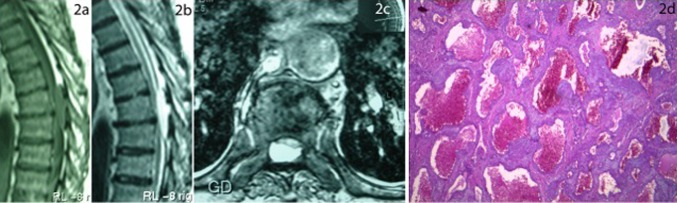
a An isointense epidural mass compressing the spinal cord at T7–T8 level, visible in sagittal T1W MRI of the thoracic spine, b T2W MRI showing a hyperintense epidural mass compressing the spinal cord at the same level, and c axial section of T1W MR after paramagnet contrast material injection showing the thoracic spinal cord being compressed ventrally. d Photomicrograph of the extradural mass showing endothelial-lined cavernous channels supported by a stroma which is relatively rich in cellular content, characterizing a cavernous angioma. Note some blood in the irregular vascular spaces. (Hematoxylin and Eosin, ×165)
Case 8
A 59-year-old woman presented with LBP of 6 months and left L5 sciatalgia of 2 weeks duration, which were intractable to medical treatment. There was no neurological deficit in physical examination. Lumbar MRI revealed an epidural lobulated mass located ventrally at L4–L5 level, compressing the thecal sac. The lesion was isointense in T1W and hyperintense in T2W images (Fig. 3a, b) with marked enhancement after contrast material injection. The lesion extended into the neural foramina. A non-contrast CT scan of the lumbar spine revealed a mass in the left L5 neural foramen. The lesion had a comparable density to the L4–5 inter-vertebral disc with scalloping of L4 vertebral body (Fig. 3c). Complete L4 and partial L5 laminectomy was performed and a lobulated dark red lesion could be removed totally. Histopathological diagnosis was cavernous angioma. The postoperative course was uneventful and MR images of the brain, cervical and thoracic spine were all normal.
Fig. 3.
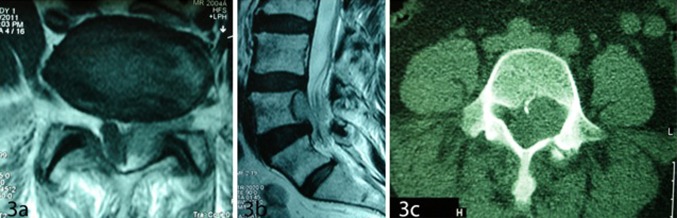
a Axial T1W MRI of the lumbar spine showing an isointense epidural mass at L4–L5 level compressing the thecal sac, b sagittal T2-weighted MRI of the lumbar spine showing an epidural mass at L4–5 level compressing the thecal sac, and c axial CT scan of L4 vertebrae showing scalloping of vertebral body in left side
Discussion
Genesis
Spinal cavernous angiomas are rare developmental vascular malformations and are clearly differentiated from vascular neoplasms [1, 2, 4, 7, 10, 22]. They arise from the localized arrested development of blood vessel progenitors, are composed of dilated vascular spaces walled by thin endothelial cells, and there is no evidence that they may grow by mitotic activity [6, 9, 17]. Although they do not represent true neoplasms, they are dynamic lesions, and intralesional hemorrhage, thrombosis, organization, cyst formation and involution of the caverns, all contribute to the changes in size and nature of these lesions [2, 6, 9, 19, 20]. They can occur in sporadic or familial forms [1, 3, 4, 6, 22]. Although rare, de novo development of the lesion has been described [5].
Cavernous angiomas occurring in the spinal epidural space (SEC)
Prevalence of location
Cavernous angiomas of the spine vary in size from a few millimeters to greater than 1 cm in diameter [7, 19]. SSECA’s are yet very rare lesions with about 104 reported cases in the literature. Hatiboglu et al. [11] reviewed 80 cases of SSECA reported previously back to 1929. Since then, a further 24 cases have been reported [5, 6, 8, 9, 12, 13, 18, 21, 22, 24] including our 9 cases. Being two major referral centers, we had the chance to encounter this rather large cohort of patients with spinal epidural cavernoma. SSECA may occur anywhere along the spinal canal, but appear to have a predilection for the thoracic levels, with decreasing frequency at the cervical, lumbar and sacral levels. Of the reported cases, 58 % were in the thoracic, 26 % in the cervical, 16 % in the lumbar and almost none in the pure sacral location [7, 8, 18, 21]. Our series include one in the sacral S1/2 level which was mistakenly operated as a L5/S1 disc disease and the SEC was neglected in the images. The thoracic region is the most common site of SSECA. The involvement of a specific region of the spine by SSECA is thought to relate to the number of vertebrae contained therein. Reviewing the 104 reported cases of SSECA, cervical, thoracic and lumbosacral involvement occurred in 27, 60, and 17 cases, respectively. Thoracic involvement occurred at a higher than expected rate (5 % per/vertebra vs. the 4 % expected rate), whereas involvement in the lumbar and the cervical area was close to the predicted figures. The higher incidence of cavernous angioma in the posterior thoracic spine is thought to be related with two factors; (a) the larger available epidural space and (b) the lower resistance in the posterior portion of the spinal canal. These two hypotheses may also be explanations for the mainly posterolateral localization of the lesions in the spinal epidural space [8, 11, 15, 21, 23]. The spinal canal is less capacious in the thoracic area in comparison with the cervical and lumbosacral levels, allowing less space for an expanding epidural lesion, and thus a higher likelihood of epidural lesions becoming clinically symptomatic.
Age and sex prevalence
Most patients are in the 30- to 60-year-old age range, with a peak around 40 years [7, 8, 11, 25], even though there have been reports including patients 23 months [6] to 81 years [13] old. According to the literature, 70 % of these lesions have been occurred in women [7, 8, 11]. The age and sex prevalence of our cases have been in the same range (Table 1).
Gross feature
They are usually confined to two or more spinal levels, and have an oval shape, sometimes flattened toward the spinal cord rather than distorting it [15, 25]. SSECA are well delineated and often have a capsule or pseudo-capsule [3, 26]. Lesions of the lumbosacral region are usually round or ovoid, located in the ventral and/or ventro-lateral epidural space or just within the peri-radicular space [9, 26, 28] as in our case 3 and 8. Extra-foraminal extension is very rare [26].
Clinical manifestation
The clinical presentation of SSECA depend on the location, growth rate, and biological behavior [8, 11, 14–16, 20, 21] and consist of spinal pain, radiculopathy, progressive paraparesis, or acute paraplegia [14, 23]. The most common clinical presentation of SSECA is slowly progressive myelopathy [3, 4, 10, 15, 17, 21]. Slowly progressive symptoms may be either due to local pressure effect of the lesion on the adjacent spinal cord or due to small repeated episodes of bleeding [15, 17, 28]. This type of symptoms has occurred in our cases 4, 5, and 7. Intermittent symptoms with variable periods of remission are probably related to episodes of small hemorrhage within the lesion [3] as observed in our second patient. Acute presentation can be either due to extradural hemorrhage [3, 4, 16, 21, 22], or thrombotic occlusion within the structure of the cavernoma causing sudden increase in the bulk of the lesion [4, 14, 16]. Our first case presented with acute spinal epidural hematoma and cord compression, and case No. 6 presented with acute myelopathy due to intralesional hemorrhage. Patients may present with radiculopathy due to either extension of the lesion into the intervertebral foramen [7], or into the ventral and lateral compartments mimicking the symptoms of disc herniation [4, 5, 7, 8, 10, 15] as in our cases 3, 4, 8 and 9. Sphincter dysfunction is a late clinical finding of SEC [3, 11] but occurred in our cases 1 and 6.
Imaging characteristics
SSECA may have some characteristic MRI features. The lesion is iso-intense with the spinal cord in T1W and hyperintense in T2W MR images and showed strong homogeneous enhancement after contrast material injection [2, 3, 6–8, 11, 13, 14, 17, 19, 23, 24]. In the presence of hemorrhage inside the lesion, it can be hyperintense in both T1W and T2W MR images [2–5, 8–10, 13, 19–21, 23] as in our case 6. These lesions may enhance strongly and homogeneously after contrast injection, although variable enhancement patterns such as slight global or peripheral enhancement have also been reported [23] similar to our case 3. Ring enhancement may be due to the necrosis and degeneration at the center of the lesion [2, 23]. In acute hemorrhagic cases, epidural hematoma is often the only finding [5] as in our case 1 and may present as spontaneous spinal epidural hematoma [27].
There are some differences between the MR characteristics of intramedullary and epidural spinal cavernous angiomas:
SEC usually enhance homogeneously after contrast injection in MRI, whereas intra-axial lesions do not enhance homogeneously [5, 25].
The rim of hypointensity resulting from hemosiderin deposit, usually visible in intramedullary cavernous angioma is not seen in epidural lesions [9, 10, 20].
Central heterogeneous reticulated signal (popcorn-like core), detectable in MRI of intramedullary lesions is not seen in extradural cavernous angiomas [20].
The main differential diagnoses of SEC regarding the imaging characteristics include; neurogenic tumors, metastasis, lymphoma, meningioma, multiple myeloma, extraosseous Ewing’s sarcoma, disc fragment, and epidural angiolipoma [2, 4, 7, 8, 11, 13].
Clinical and surgical features
SEC’s are apparently dynamic lesions with tendency to bleed or to grow, with subsequent spinal cord compression, necessitating complete surgical removal as the treatment of choice. The goal of treatment is total en bloc excision during the first operation [5, 9, 11, 12, 14–17, 21, 22], however, severe intraoperative bleeding and anterior or intrathoracic extension of the lesion are the main trouble making factors [11, 14, 15, 17]. Bleeding is seldom a problem with intramedullary cavernous angiomas during surgery, however, severe intraoperative bleeding may occur in spinal epidural cavernous angiomas [17]. Therefore, adequate exposure is necessary to prevent massive intraoperative bleeding, especially in the lumbar lesions, where the lesion resembles a disc fragment [26, 28]. In our series, complete surgical removal was achieved in all cases and confirmed by postoperative MRI. Surgical treatment should be performed before worsening of the patient’s neurological deficits [5, 14]. All our patients recovered completely after excision of the lesion, except case one, who underwent a delayed operation.
In lesions with extension out of the spinal canal and some remnants after surgery, radiotherapy and radiosurgery has been advised as the adjuvant mode of therapy [15, 18, 22, 25]. No adjuvant therapy was needed in our cases.
It is generally believed that the presence of spinal cord cavernous angiomas warrants imaging of the entire neuraxis in order to exclude the relatively significant incidence of multiple lesions in these patients; however, this may be unnecessary in patients with SEC [23, 25]. In our series, all other imaging were negative regarding multiple cavernomas.
Conclusion
SEC are rare benign lesions which may present with a variety of clinical symptoms and different types of MR characteristics as in this series. Patient’s clinical outcome depends on the severity of the preoperative neurological status, therefore, early surgical treatment is recommended to prevent irreversible neurological deficits. Authors re-emphasize the need to consider cavernous angioma in the list of differential diagnosis of every spinal epidural masse.
Conflict of interest
None.
Abbreviations
- SEC
Spinal epidural cavernoma
- SSECA
Solitary spinal epidural cavernous angioma
- MRI
Magnetic resonance imaging
- LBP
Low back pain
- CT
Computed tomography
References
- 1.Acciari N, Padovani R, Pozzati E, Gaist G, Manetto V. Spinal cavernous angioma: a rare cause of subarachnoid hemorrhage. Surg Neurol. 1992;37:453–456. doi: 10.1016/0090-3019(92)90134-9. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi N, Kojima K, Kasai H. Review of spinal epidural cavernous hemangioma. Neurol Med Chir (Tokyo) 2003;43:471–476. doi: 10.2176/nmc.43.471. [DOI] [PubMed] [Google Scholar]
- 3.Appiah CA, Knuckey NW, Robbins PD. Extradural spinal cavernous hemangioma: case report and review of the literature. J Clin Neurosci. 2001;8:176–179. doi: 10.1054/jocn.2000.0756. [DOI] [PubMed] [Google Scholar]
- 4.Carlier R, Engerand S, Lamer S, Vallee C, Bussel B, Polivka M. Foraminal epidural extraosseous cavernous hemangioma of the cervical spine. Spine. 2000;25:629–631. doi: 10.1097/00007632-200003010-00016. [DOI] [PubMed] [Google Scholar]
- 5.Caruse G, Galarza M, Borghesi I, Pozzati E, Vitale M. Acute presentation of spinal epidural cavernous angioma: case report. Neurosurgery. 2007;60:E575–E576. doi: 10.1227/01.NEU.0000255345.48829.0B. [DOI] [PubMed] [Google Scholar]
- 6.Cho JH, Chung YN, Wang KC, Cho BK. Spinal cavernous hemangioma causing sudden paraplegia in a 23-month-old kid. J Korean Neurosurg Soc. 2006;40:273–276. [Google Scholar]
- 7.D’Andrea G, Ramundo OE, Trillo G, Roperto R, Isidori A, Ferrante L. Dorsal foramenal extraosseous epidural cavernous hemangioma. Neurosurg Rev. 2003;16:292–296. doi: 10.1007/s10143-003-0275-8. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Xu YK, Li L, Yang RM, Ye XH, Zhang N, Yu T, Lin BQ. MRI diagnosis and preoperative evaluation for pure epidural cavernous hemangiomas. Neuroradiology. 2009;1:741–747. doi: 10.1007/s00234-009-0555-2. [DOI] [PubMed] [Google Scholar]
- 9.Floeth F, Riemenschneider M, Herdmann J. Intralesional hemorrhage and thrombosis without rupture in a pure spinal epidural cavernous angioma: a rare cause of acute lumbar radiculopathy. Eur Spine J. 2010;19(supp 12):193–196. doi: 10.1007/s00586-010-1345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal A, Singh AK, Cupta V, Tatake M. Spinal epidural cavernous haemangioma: a case report and review of literature. Spinal cord. 2002;40:200–202. doi: 10.1038/sj.sc.3101248. [DOI] [PubMed] [Google Scholar]
- 11.Hatiboglu MA, Iplikcioglu AC, Ozcan D. Epidural spinal cavernous hemangioma: case report. Neural Med Chir (Tokyo) 2006;46:455–458. doi: 10.2176/nmc.46.455. [DOI] [PubMed] [Google Scholar]
- 12.Khalatbari MR, Hamidi M, Moharamzad Y. Pediatric intramedullary cavernous malformation of the conous medullaris: case report and review of the literature. Childs Nerv Syst. 2011;27:507–511. doi: 10.1007/s00381-010-1350-4. [DOI] [PubMed] [Google Scholar]
- 13.Leu NH, Chen S, Chou JM. MR features of posterior spinal epidural cavernous hemangioma: a case report. Chin J Radiol. 2006;31:127–131. [Google Scholar]
- 14.Minh NH. Cervicothoracic spinal epidural cavernous hemangioma: case report and review of the literature. Surgical Neurol. 2005;64:83–85. doi: 10.1016/j.surneu.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Nagi S, Megdiche H, Bouzaidi K, Haouet S, et al. Imaging features of spinal epidural cavernous malformations. J Neuroradiol. 2004;31:208–213. doi: 10.1016/S0150-9861(04)96993-3. [DOI] [PubMed] [Google Scholar]
- 16.Padovani R, Tognetti F, Proietti D, et al. Extradural Cavernous hemangioma. Surg Neurol. 1962;18:463–465. doi: 10.1016/0090-3019(82)90191-4. [DOI] [PubMed] [Google Scholar]
- 17.Padovani R, Acciari N, Giulioni M, et al. Cavernous angiomas of the spinal district: surgical treatment of 11 patients. Eur Spine J. 1997;6:298–303. doi: 10.1007/BF01142674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park ES, Park JS, Kim E. Spinal epidural cavernous angioma: a case report. Kor J Spine. 2008;5:99–101. [Google Scholar]
- 19.Santoro A, Piccirilli M, Bristot R, et al. Extradural spinal cavernous angioma: report of seven cases. Neurosurg Rev. 2005;28:313–319. doi: 10.1007/s10143-005-0390-9. [DOI] [PubMed] [Google Scholar]
- 20.Saringer W, Nobauer I, Haberler C, Ungersbock K. Extraforaminal, thoracic, epidural cavernous hemangioma: case report with analysis of magnetic resonance imaging characteristics and review of the literature. Acta Neurochir. 2001;143:1293–1297. doi: 10.1007/s007010100028. [DOI] [PubMed] [Google Scholar]
- 21.Sarikaya-Seiwert S, Gierga K, et al. Solitary spinal epidural cavernous angiomas in children presenting with acute neurological symptoms caused by hemorrhage: report of 2 cases. J Neurosurg Pediatr. 2010;5:89–93. doi: 10.3171/2009.7.PEDS09203. [DOI] [PubMed] [Google Scholar]
- 22.Satpathy DK. Spinal epidural cavernous hemangioma with myelopathy: a rare lesion. Neurol India. 2009;57:88–90. doi: 10.4103/0028-3886.48805. [DOI] [PubMed] [Google Scholar]
- 23.Shin JH, Lee HK, Rhim SC. Spinal epidural cavernous hemangioma: MR findings. J Comput Assist Tomogr. 2001;25:257–261. doi: 10.1097/00004728-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Sohn MJ, Lee DJ, Jeon SR, Khang SK (2009) Spinal radiosurgical treatment for thoracic epidural cavernous hemangioma presenting as radiculomyelopathy: technical case report. Neurosurgery 64:E 1202 [DOI] [PubMed]
- 25.Talacchi A, Spinnato S, Alessandrini F, Iuzzolinop P, Bricolo A. Radiologic and surgical aspects of pure spinal epidural cavernous angiomas: report on 5 cases and review of the literature. Surg Neurol. 1999;52:198–203. doi: 10.1016/S0090-3019(99)00064-6. [DOI] [PubMed] [Google Scholar]
- 26.Tekkok IH, Akpinar G, Gungen Y. Extradural lumbosacral cavernous hemangioma. Eur Spine J. 2004;13:469–473. doi: 10.1007/s00586-003-0658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P, Xin XT, Lan H, et al. Spontaneous cervical epidural hematoma during pregnancy: case report and literature review. Eur Spine J. 2011;20(Suppl 2):S176–S179. doi: 10.1007/s00586-010-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zevgaridis D, Buttner A, Weis S, Hamburger C, Reulen HJ. Spinal epidural cavernous hemangioma: report of three cases and review of the literature. J Neurosurg. 1998;88:903–908. doi: 10.3171/jns.1998.88.5.0903. [DOI] [PubMed] [Google Scholar]