Abstract
Purpose
Surgical treatment of thoracolumbar osteomyelitis consists of radical debridement, reconstruction of anterior column either with or without posterior stabilization. The objective of present study is to evaluate a case series of patients with osteomyelitis of thoracic and lumbar spine treated by single, posterior approach with posterior instrumentation and anterior column reconstruction.
Methods
Seventeen patients underwent clinical and radiological evaluation pre and postoperatively with latest follow-up at 19 months (8–56 months) after surgery. Parameters assessed were site of infection, causative organism, angle of deformity, blood loss, duration of surgery, ICU stay, deformity correction, time to solid bony fusion, ambulatory status, neurologic status (ASIA impairment scale), and functional outcome (Kirkaldy-Willis criteria).
Results
Mean operating time was 207 min and average blood loss 1,150 ml. Patients spent 2 (1–4) days in ICU and were able to walk unaided 1.6 (1–2) days after surgery. Infection receded in all 17 patients postoperatively. Solid bony fusion occurred in 15 out of 17 patients (88 %) on average 6.3 months after surgery. Functional outcome was assessed as excellent or good in 82 % of cases. Average deformity correction was 8 (1–18) degrees, with loss of correction of 4 (0–19) degrees at final follow-up.
Conclusions
Single, posterior approach addressing both columns poses safe alternative in treatment of pyogenic vertebral osteomyelitis of thoracic and lumbar spine. It proved to be less invasive resulting in faster postoperative recovery.
Keywords: Vertebral osteomyelitis, Thoracic spine, Lumbar spine, One-stage surgery, Instrumentation
Introduction
Vertebral osteomyelitis is an infrequent disease, with the incidence of 1:100,000–1:250,000 [1–4], and a relatively high mortality rate ranging from 2 to 17 % [4–9]. Treatment of vertebral osteomyelitis is mainly conservative, consisting of long-term antibiotic therapy in accordance with causative microorganism and bracing. On the other hand, there is still a subgroup of patients who require either emergency or elective surgery. The indications for surgery are: epidural abscess formation, progressive neurologic deficit, severe destruction of endplates with mechanical instability or segmental kyphosis, septic pseudarthrosis, severe pain and refractoriness to conservative treatment [10–15]. Radical debridement followed by autologous strut-graft interposition, proposed by Hodgson and Sock in early fifties [16] was considered as a golden standard for vertebral osteomyelitis surgery for many years. In the last two decades, however, the rationale changed from: do not put any foreign material in the infected tissue, to: make the segment as stabile as possible [1–3]. Most authors therefore agree that both columns should be addressed either as single- or two-staged surgery [17, 18]. There is still debate whether to employ anterior or posterior route or even to fix spine from both approaches [4, 18].
The aim of the manuscript is to present a case series of patients with osteomyelitis of thoracic and lumbar spine treated by single posterior approach with posterior instrumentation and anterior column reconstruction.
Patients and methods
The records of patients, treated for osteomyelitis of thoracic and lumbar spine between January 2006 and June 2011 were revised. Out of 108 patients, 23 were treated operatively. Two patients were treated by single anterior approach, 4 patients with combined approach and remaining 17 patients underwent posterior instrumentation and anterior column reconstruction with posterior approach only. The average age of 17 patients, with posterior only approach, was 66 years (37–28 years); there were 8 female and 9 male patients. The location of infection was thoracic spine in 8 cases, thoracolumbar junction in 2 cases and lumbar spine in 7 cases (Table 1). In 15 cases, the route of infection was hematogenous and in 2 cases postoperative (herniated lumbar disc surgery one case, instrumented lumbar fusion one case). Prior to surgery three patients were treated for ischemic heart disease, three patients for diabetes mellitus type II, one patient had rheumatoid arthritis and another patient acute myeloid leukemia.
Table 1.
Preoperative data
| No. of patients | Sex | Age at surgery | Location of infection | Ambulatory status (ability to walk unaided) | ASIA impairment score before/after | Neurologic deficit | Causative bacteria |
|---|---|---|---|---|---|---|---|
| 1 | F | 58 | Th12-L1 | Yes | E | No | SA |
| 2 | M | 41 | Th8-Th9 | No | B/D | Paraplegia | SA |
| 3 | F | 70 | Th8-Th9 | Yes | E | No | Strepto.G (beta-hem.) |
| 4 | M | 65 | L1-L2 | Yes | No | Coagulase-Staph. | |
| 5 | F | 66 | Th6-Th7, Th7-Th8 | Yes | E | No | SA |
| 6 | F | 69 | L4-L5 | Yes | Peroneal paresis | Not found | |
| 7 | M | 73 | L4-L5 | Yes | Yes | SA | |
| 8 | F | 79 | Th11-L1 | Yes | E | No | Coagulase-Staph. |
| 9 | M | 58 | Th11-Th12 | Yes | E | No | Serratia marcescens |
| 10 | F | 69 | L4-L5 | Yes | Foot extensors paresis | Not found | |
| 11 | M | 72 | Th6-Th7-Th8 | No | C/D | Spastic paraparesis | SA |
| 12 | F | 74 | Th8-Th9 | Yes | E | No | SA |
| 13 | M | 79 | Th4-Th5 | No | C/D | Paraparesis, urinary bladder symptoms | SA |
| 14 | M | 60 | L4-L5 | Yes | No | Streptocc. C | |
| 15 | M | 37 | Th7-Th8 | Yes | D/E | Spastic paraparesis | Propionibacterium acnes |
| 16 | F | 82 | L4-L5 | Yes | No | SA | |
| 17 | M | 72 | L3-L4 | Yes | No | Propionibacterium acnes |
SA, Staphylococcus aureus; Strepto. G (beta-hem.), Beta hemolytic Streptococcus group G; Coagulase-Staph., Coagulase negative Staphylococcus; Streptocc. C, Streptococcus group C
Surgeries were performed through single midline dorsal access over the affected segments. After blunt retraction of paravertebral muscles, transpedicular screws (XIA, Stryker Spine, Allendale, NJ) were put into adjacent vertebrae and secured with rods. Number of instrumented segments was restricted to minimum and determined in relation to severity of bone destruction and intraoperative observation of bone quality. As a rule, only neighboring segments were instrumented in cases where segments just proximal and distal to affected disc could securely be fixed with pedicle screws. In cases of corpectomy/ies at least two proximal and distal segments were instrumented (Table 1). With the affected segments provisionally stabilized, posterior decompression was performed, followed by anterior decompression consisting of evacuation of the affected intervertebral disc (IVD), debridement of terminal endplates or corpectomy/ies in cases of severely destructed vertebral bodies. In order to avoid dural sac injury, special care was taken while dissecting soft tissues from dural sac. Affected segments were approached only from more affected side. In cord levels, access was enhanced by costotransversectomy and additionally in corpectomy/ies cases by killing the appropriate spinal nerve/s. Either titanium mesh cage (Surgical Titanium Mesh®, DePuy Acromed, Raynham, MA) or PEEK cage (Boomerang®, Medtronic, Minneapolis, MN) (Fig. 1) was used in cases where IVD alone was removed. In cases of corpectomy, expandable titanium cage (VLIFT, Stryker Spine, Allendale, NJ) (Fig. 2) or titanium mesh cage (Surgical Titanium Mesh®, DePuy Acromed, Raynham, MA) (Fig. 3) was used. Bone obtained from posterior elements was put into the cage, at anterior third of IVD or in cases of corpectomy anterior and lateral to the cage. Kyphotic deformity was corrected in cases of disc removal by putting interbody cage of maximal feasible height as anteriorly as possible with posterior compression and in cases of corpectomy/ies by putting expandable cage with appropriate distraction again followed by posterior compression. Drain was left in epidural space until drainage was reduced to less than 50 ml/day for 2 consecutive days.
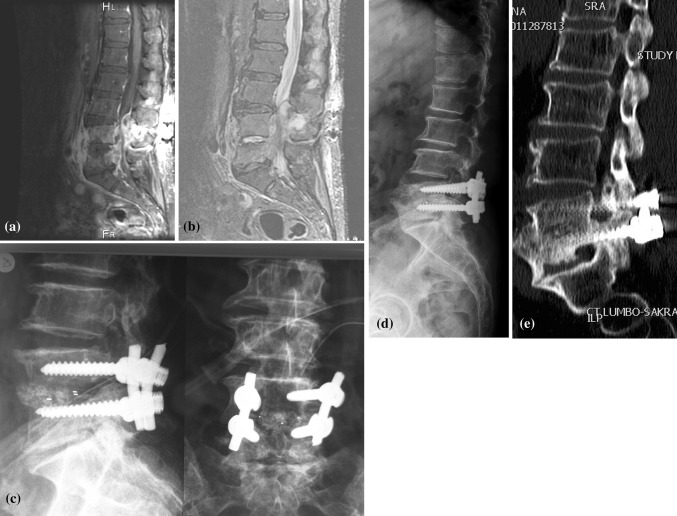
Fig. 1.
A 82-year-old woman with post discectomy osteomyelitis of L4-L5 (Patient no. 16). T1-weighted MRI image showed severely narrowed spinal canal due to large epidural abscess (a) while STIR MRI image showed L4-L5 disc infection (b). Comparison of immediate (c) and late postoperative X-rays (d) showed slight posterior migration of cage. Large fusion mass occurred anteriorly (d). CT image also confirmed anterior fusion mass extending to also L5-S1 disc space (e)
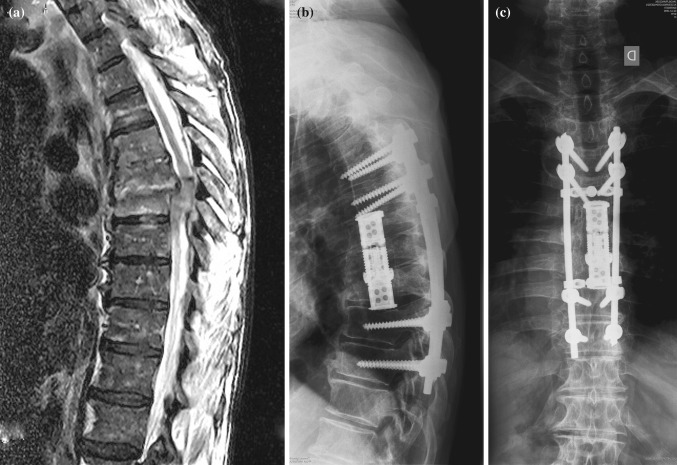
Fig. 2.
A 72-year-old man with osteomyelitis of Th6-Th7-Th8 (Patient no. 11). T2-weighted MRI image preoperatively showed Th7-Th8 deformation and spinal canal narrowing (a). Late postoperative AP (b) and lateral (c) X-rays showed solid bony fusion over three affected segments
Fig. 3.
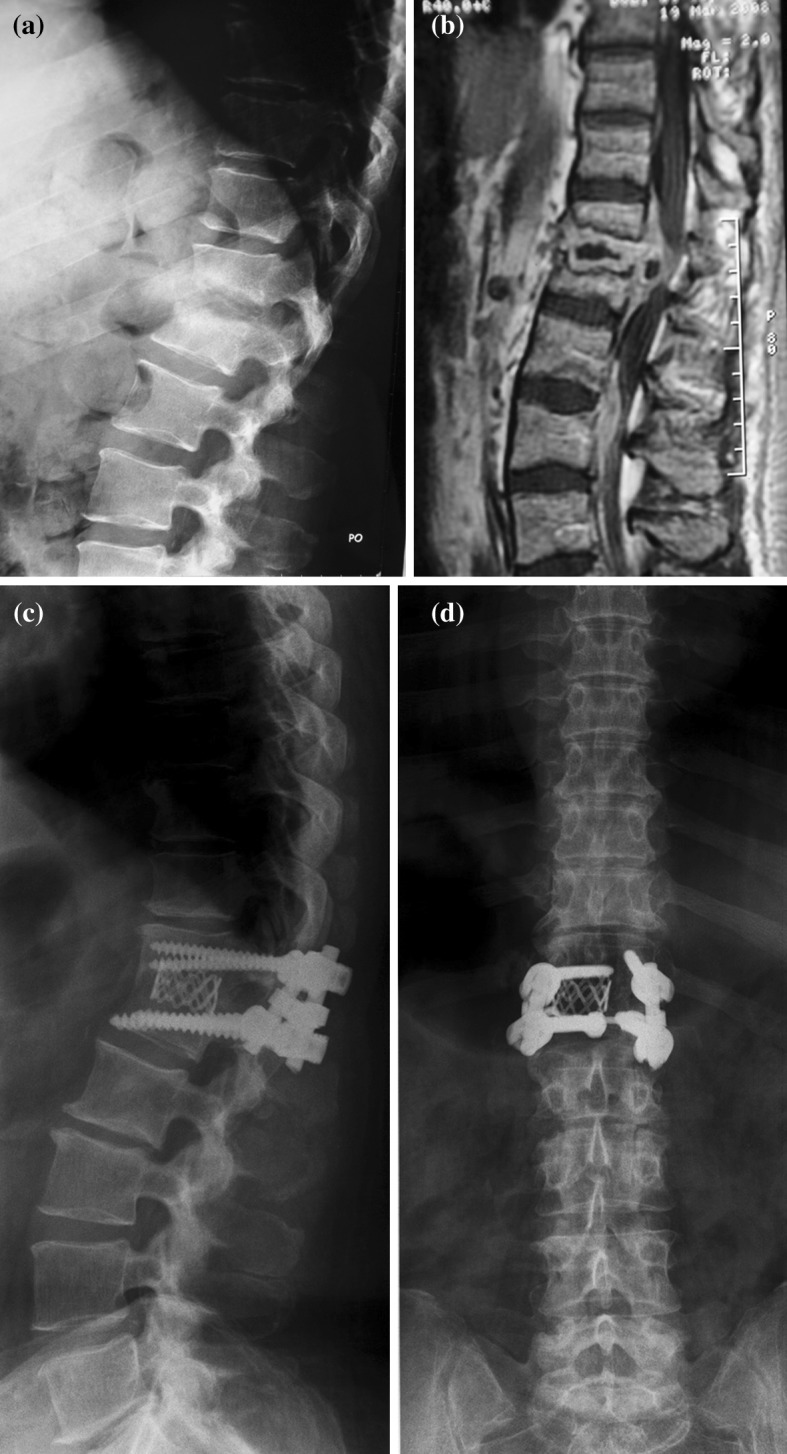
A 58-year-old woman with vertebral osteomyelitis of Th12-L1 and large epidural abscess formation (Patient no. 1). Preoperative lateral X-ray (a), preoperative sagittal T1-weighted MRI (b). AP and lateral X-rays (c, d) 12 months postoperatively demonstrated solid bony fusion
Antibiotic therapy was administered according to sensitivity testing. It was given parenterally for at least 6 weeks. Thereafter, antibiotics were continued orally for 4 weeks or in cases of elevated C-reactive protein and sedimentation rate until their values returned to normal.
Patients were followed at 1, 3, 6 months and 1 year after surgery. Thereafter, the patients were followed-up at yearly intervals. Latest follow-up was on average 19 months (8–56 months) after surgery. Neurologic workup was performed at the admission and repeated during hospitalization and follow-ups. Patients with upper motor neuron impairment were classified according to ASIA impairment classification [19]. Plain radiographs were taken at each follow-up measuring angle of deformity and time to solid bony fusion. In inconclusive cases bony fusion was assessed by CT scan. Clinical data included duration of surgery, blood loss, time spent in the ICU and ambulatory status before and after surgery. Functional outcome, using Kirkaldy-Willis criteria, was determined for each patient at the end of observation period.
Results
Mean time of surgery was 207 (70–360) min and the mean blood loss 1,150 (400–4,600) ml. The average stay in the intensive care unit after surgery was 2 (1–4) days.
Causative bacteria were found in all but two cases, represented by Staphylococcus aureus species (8 cases), followed by Coagulase negative Staphylococci and Propionibacterium acnes (both 2 cases), Beta-hemolytic Streptococcus group G, Serratia marcescens and Streptococcus C (all 1 case). Infection completely resolved after operative and antibiotic intravenous and per-oral therapy in all 17 patients.
Five surgeries were performed as an emergency procedure due to progressive neurologic deficit. There were four patients with upper motor neuron dysfunction, all at least partially improved postoperatively (Table 1). In two cases improvement was seen immediately after surgery, in further two after a period of rehabilitation. At final follow-up three patients could walk on their own, one with assistance. All three patients with radiculopathy made partial recovery after surgery.
All the patients were ambulatory at the end of observation period. Patients without preoperative upper motor neuron symptoms took 1.6 days (1–2 days) to walk unaided (Table 2).
Table 2.
Perioperative and follow-up data
| No. of patient | Location of infection | Level of fixation | Segm. fused | Cage | Duration of surgery (min) | Blood loss (ml) | Ambulatory status (ability to walk unaided) (days post op.) | Observation time (months) | Angle preoperative (°) | Angle postoperative (°) | Angle end (°) | Bone fusion (months) | Kirkaldy-Willis functional outcome | Intensive care unit (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Th12-L1 | Th12-L1 | 1 | Harms | 165 | 400 | 1 | 27 | 12 | 11 | 12 | 3+ | Excellent | 1 |
| 2 | Th8-Th9 | Th8-Th9 | 1 | PEEK | 160 | 1,500 | With assistance only | 16 | 19 | 1 | 20 | 6+ | Good | 3 |
| 3 | Th8-Th9 | Th6-Th12 | 3 | VLIFT | 225 | 1500 | 2 | 14 | 35 | 20 | 22 | 4+ | Excellent | 3 |
| 4 | L1-L2 | L1-L2 | 1 | Harms | 185 | 600 | 2 | 8 | 14 | 5 | 1 | 6 | Good | 4 |
| 5 | Th6-Th7, Th7-Th8 | Th4-Th10 | 4 | VLIFT | 220 | 900 | 2 | 10 | 27 | 11 | 14 | 8 | Good | 2 |
| 6 | L4-L5 | L4-L5 | 1 | Harms | 150 | 500 | 2 | 56 | −8 | −16 | −16 | 5 | Good | 2 |
| 7 | L4-L5 | L4-L5 | 1 | Harms | 155 | 1,500 | 2 | 12 | −17 | −8 | −6 | 6 | Good | 2 |
| 8 | Th11-L1 | Th9-L1 | VLIFT | 360 | 4,600 | 2 | 34 | 10 | 6 | 10 | No | Fair | 4 | |
| 9 | Th11-Th12 | Th9-L3 | 3 | VLIFT | 185 | 700 | 2 | 47 | 18 | 2 | 8 | 3 | Good | 2 |
| 10 | L4-L5 | L4-S1 | 1 | Harms | 185 | 1,000 | 2 | 23 | −18 | −18 | −16 | 5 | Excellent | 4 |
| 11 | Th6-Th7-Th8 | Th4-Th10 | 4 | VLIFT | 285 | 1,500 | 14 | 13 | 20 | 19 | 16 | 6 | Fair | 2 |
| 12 | Th8-Th9 | Th7-Th9 | Harms | 230 | 400 | 1 | 8 | 20 | 16 | 26 | No | Good | 1 | |
| 13 | Th4-Th5 | Th4-Th5 | 1 | VLIFT | 240 | 500 | 11 | 16 | 15 | 8 | 10 | 16 | Excellent | 1 |
| 14 | L4-L5 | L4-L5 | 1 | PEEK | 70 | 500 | 1 | 11 | −5 | −15 | −10 | 4 | Excellent | 2 |
| 15 | Th7-Th8 | Th5-Th10 | 2 | Harms | 300 | 2,000 | 5 | 10 | 30 | 14 | 20 | 8 | Good | 2 |
| 16 | L4-L5 | L4-L5 | 1 | PEEK | 240 | 800 | 1 | 8 | 0 | −7 | −3 | 5 | Fair | 2 |
| 17 | L3-L4 | L3-L4 | 1 | Harms | 165 | 600 | 1 | 6 | 6 | −6 | −1 | 4 | Excellent | 2 |
Surgical procedure resulted in deformity correction of 8 (1–18) degrees on average in 15 patients with fusion. Loss of correction of 4 (0–19) degrees was observed at latest follow-up (Table 2).
Solid bony fusion was achieved in 15 out of 17 patients (88 %) at 6.3 (3–16) months postoperatively. In two patients fusion did not occur at 34 months (Patient no. 8) and 8 months postoperatively (Patient no. 12) (Table 2).
Patient no. 8 presented with vertebral osteomyelitis at age 79 and had severe osteoporosis. After infection resided, she was satisfied with the procedure, however, no solid bony fusion was achieved. 28 months after operation she presented at outpatient clinic with mechanical back pain, where radiography showed pullout of distal pedicular screws. She was a candidate for reoperation, but before that she was hospitalized in a gastroenterology department where she was diagnosed and treated for a gastric cancer.
Patient no. 12 has made a complete recovery and was followed-up until 8th month after surgery. Although she was satisfied with the outcome, she refused further check-ups. At last follow-up, solid bony fusion did not occur and she was therefore considered “non-fused”.
Patient self-assessment of the functional outcome, based on the Kirkaldy-Willis criteria, showed excellent result in six patients, good in eight and fair result in three patients (Table 2).
Three patients died during the observation period, none of the reasons directly connected to vertebral osteomyelitis. Patient no. 4 suffered from leukemia (AML) treated with bone marrow transplantation prior to vertebral osteomyelitis at L1/L2 level. After surgical and antibiotic treatment, he made a beneficial recovery. He had a relapse of leukemia and died 10 months after the procedure due to sepsis originating from a bacterium other than Koagulase negative Staphylococci that caused the spinal infection. He achieved solid bony fusion 6 months after the operation. Patient no. 5 and patient no. 7 died of cardiac reasons 12 and 14 months after surgery. Both recovered completely after surgery, patient’s No. 7 neurologic deficit improved after a period of rehabilitation. They were both satisfied with the treatment, and both achieved solid bony fusion.
Discussion
Surgical treatment of thoracolumbar pyogenic osteomyelitis consists of radical debridement, reconstruction of anterior column with or without posterior stabilization aiming for fast postoperative mobilization [11, 18]. Surgical goals could be achieved via different approaches be it anterior, posterior or combined. Most of the reported case series preferred anterior approach [8, 11, 20] or combined approach executed either as one- or two-staged procedure [8, 10, 13, 21, 22]. Posterior approach addressing both column pathology has been widely accepted in tumor surgery [23, 24], yet there are few reports on posterior approach for vertebral osteomyelitis with majority of reported cases dealing with lumbar spine pathology [25–29]. Reconstruction of anterior column was performed mostly using iliac crest bone graft [27–29] or even employing transdiscal osteotomy with vertebral shortening [25]. Present case series, on the other hand, report on posterior approach for one or multilevel thoracic and lumbar pyogenic osteomyelitis utilizing cages for anterior column reconstruction.
Single posterior approach has few important advantages over most standard approaches. Compared to anterior route, one can avoid entering thoracic and/or abdominal cavity with less morbidity for a patient. This could result in fast postoperative recovery, which was demonstrated in our series as patients left intensive care unit on second postoperative day and walked unaided on first or second postoperative day, except for patients with upper motor neuron lesion who required longer rehabilitation. No patient needed bracing postoperatively.
Surgery time in our series was 207 min on average compared to 427 min for combined anteroposterior two-stage approach [30] and 345 min for single-stage anteroposterior approach [21]. Dai et al. [31] reported a short operative time of 168 min utilizing single anterior approach. Nevertheless, besides the anterior approach-related morbidity, some patients needed postoperative external support after fixation of only anterior column. Blood loss was also substantially lower compared to other studies. Combined single-staged procedure was reported to have blood loss of 1,700 ml [20] and double-stage combined approach of 2,700 ml [32] as opposed to 1,150 ml in our series.
In cases of two or more level corpectomies, spinal nerve/s has/ve to be killed to gain access for cage placement. There were two cases of two level and a case of three level corpectomies (Fig. 2) in present series, all in thoracic spine. While there were no clinical consequences following spinal nerve section in our thoracic cases one might expect paresis of distinct lower limb muscles when utilizing posterior approach in lumbar spine. Therefore, anterior or combined approach is to be considered in multilevel osteomyelitis of lumbar spine. Two other contraindications for single posterior approach could be early staged vertebral osteomyelitis in which there is no spread of infection into the spinal canal and substantial psoas abscess.
In the last decade, many studies advocated utilization of titanium cages for anterior column reconstruction in vertebral osteomyelitis without increase of infection recurrence [11, 31–34]. The question is posed, however, whether to use PEEK material in septic osteomyelitis of the spine. Only few reports, with total of 20 patients, exist on successful treatment of vertebral osteomyelitis with PEEK cages [35–37]. Three out of 17 patients in our series were treated with PEEK cages resulting in resolution of infection in all cases. Although small in number, our cases suggest that PEEK cages could be used for anterior column reconstruction in purulent conditions of thoracolumbar spine.
Time to solid bony fusion and percentage of fused cases in present study closely correlate to data from literature. 88 percent of patients achieved solid bony fusion on average 6.3 months postoperatively. Most series report solid bony fusion to occur in over 90 percent of cases [11, 13, 30, 38] and time to fusion from 7.5 to 8.6 months [30, 38, 39]. Kyphosis correction averaged 8 degrees in present series as compared to 12.5 degrees in other series [21]. Deterioration of kyphosis correction of 4 degrees at the latest follow-up did not have clinical consequences in any of the patients.
There are drawbacks and limits to the present study. The cohort is diverse concerning the location, extent of pathology and causative organism. The study is retrospective in its basis and includes no control group, which enabled only descriptive analysis.
Conclusion
Posterior approach poses a safe alternative in treatment of thoracolumbar osteomyelitis. It offers adequate exposure for thorough decompression, debridement, posterior instrumentation and anterior column reconstruction. It has an advantage of being less invasive thereby enabling a patient fast postoperative recovery.
Conflict of interest
None.
References
- 1.Acosta FL, Jr, Chin CT, Quinones-Hinojosa A, Ames CP, Weinstein PR, Chou D. Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus. 2004;17:2. doi: 10.3171/foc.2004.17.6.2. [DOI] [PubMed] [Google Scholar]
- 2.Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Nontuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral center. Spine. 2006;31:2695–2700. doi: 10.1097/01.brs.0000244662.78725.37. [DOI] [PubMed] [Google Scholar]
- 3.Cramer J, Haase N, Behre I, Ostermann PAW. Spondylitis und spondylodiszitis. Trauma Berufskrankheit. 2003;5:336–341. doi: 10.1007/s10039-003-0771-7. [DOI] [Google Scholar]
- 4.Frangen TM, Kälicke T, Gottwald M, Andereya S, Andress HJ, Russe OJ, Muller EJ, Muhr G, Schinkel C. Die operative Therapie der Spondylodiszitis. Eine Analyse von 78 Patienten. Der Unfallchirurg. 2006;109:743–753. doi: 10.1007/s00113-006-1084-7. [DOI] [PubMed] [Google Scholar]
- 5.Schinkel C, Gottwald M, Andress HJ. Surgical treatment of spondylodiscitis. Surg Infect. 2003;4:387–391. doi: 10.1089/109629603322761445. [DOI] [PubMed] [Google Scholar]
- 6.Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM. Non tuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral centre. Spine. 2006;31:2695–2700. doi: 10.1097/01.brs.0000244662.78725.37. [DOI] [PubMed] [Google Scholar]
- 7.Nolla JM, Ariza J, Gomez-Vaquero C, Fiter J, Bermejo J, Valverde J, Escofet DR, Gudiol F. Spontaneous pyogenic vertebral osteomyelitis in non-drug-users. Semin Arthritis Rheum. 2002;31:271–278. doi: 10.1053/sarh.2002.29492. [DOI] [PubMed] [Google Scholar]
- 8.Linhardt O, Matussek J, Refior HJ, Krödel A. Long-term results of ventro-dorsal versus ventral instrumentation fusion in the treatment of spondylitis. Int Orthop. 2007;31:113–119. doi: 10.1007/s00264-006-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woertgen C, Rothoerl RD, Englert C, Neumann C. Pyogenic spinal infections and outcome according to the 36-Item Short Form Health Survey. J Neurosurg Spine. 2006;4:441–446. doi: 10.3171/spi.2006.4.6.441. [DOI] [PubMed] [Google Scholar]
- 10.Dimar JR, Carreon LY, Glassman SD, Campbell MJ, Hartman MJ, Johnson JR. Treatment of pyogenic vertebral osteomyelitis with anterior debridement and fusion followed by delayed posterior spinal fusion. Spine. 2004;29:326–332. doi: 10.1097/01.BRS.0000109410.46538.74. [DOI] [PubMed] [Google Scholar]
- 11.Fayazi AH, Ludwig SC, Dabbah M, Bryan Butler R, Gelb DE. Preliminary results of staged anterior debridement and reconstruction using titanium mesh cages in the treatment of thoracolumbar vertebral osteomyelitis. Spine J. 2004;4:388–395. doi: 10.1016/j.spinee.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Gasbarrini AL, Bertoldi E, Mazzetti M, Fini L, Terzi S, Gonella F, Mirabile L, Barbanti Brodano G, Furno A, Gasbarrini A, Boriani S. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9:53–66. [PubMed] [Google Scholar]
- 13.Korovessis P, Petsinis G, Koureas G, Iliopoulos P, Zacharatos S. One-stage combined surgery with mesh cages for treatment of septic spondylitis. Clin Orthop Relat Res. 2006;444:51–59. doi: 10.1097/01.blo.0000203449.51769.7f. [DOI] [PubMed] [Google Scholar]
- 14.Priest DH, Peacock JE., Jr Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South Med J. 2005;98:854–862. doi: 10.1097/01.smj.0000168666.98129.33. [DOI] [PubMed] [Google Scholar]
- 15.Schuster JM, Avellino AM, Mann FA, Girouard AA, Grady MS, Newell DW, Winn HR, Chapman JR, Mirza SK. Use of structural allografts in spinal osteomyelitis: a review of 47 cases. J Neurosurg. 2000;93:8–14. doi: 10.3171/spi.2000.93.1.0008. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson AR, Stock FE. Anterior spinal fusion a preliminary communication on the radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44:266–275. doi: 10.1002/bjs.18004418508. [DOI] [PubMed] [Google Scholar]
- 17.Heyde CE, Boehm H, El Saghir H, Tschoke SK, Kayser R. Surgical treatment of spondylodiscitis in the cervical spine: a minimum 2-year follow-up. Eur Spine J. 2006;15:1380–1387. doi: 10.1007/s00586-006-0191-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16:1307–1316. doi: 10.1007/s00586-006-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waring WP, 3rd, Biering-Sorensen F, Burns S, Donovan W, Graves D, Jha A, Jones L, Kirshblum S, Marino R, Mulcahey MJ, Reeves R, Scelza WM, Schmidt-Read M, Stein A. 2009 review and revisions of the international standards for the neurological classification of spinal cord injury. J Spinal Cord Med. 2010;33:346–352. doi: 10.1080/10790268.2010.11689712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krödel A, Krüger A, Lohscheidt K, Pfahler M, Reflor HJ. Anterior debridement, fusion, and extrafocal stabilization in the treatment of osteomyelitis of the spine. J Spinal Disord. 1999;12:17–26. [PubMed] [Google Scholar]
- 21.Safran O, Rand N, Kaplan L, Sagiv S, Floman Y. Sequential or simultaneous, same day anterior decompression and posterior stabilization in the management of the vertebral osteomyelitis of the lumbar spine. Spine. 1998;23:1885–1890. doi: 10.1097/00007632-199809010-00018. [DOI] [PubMed] [Google Scholar]
- 22.Jain AK, Dhammi IK, Prashat B, Sinha S, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Jt Surg B. 2008;90:1477–1481. doi: 10.1302/0301-620X.90B11.20972. [DOI] [PubMed] [Google Scholar]
- 23.Stener B. Complete removal of vertebrae for extirpation of tumors. Clin Orthop. 1989;245:72–82. [PubMed] [Google Scholar]
- 24.Tomita K, Kawahara N, Baba H, Tsuchiya H, Fujita T, Toribatake Y. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine. 1997;22:324–333. doi: 10.1097/00007632-199702010-00018. [DOI] [PubMed] [Google Scholar]
- 25.Halpern EM, Bacon SA, Kitagawa T, Lewis SJ. Posterior transdiscal three-column shortening in the surgical treatment of vertebral discitis/osteomyelitis with collapse. Spine. 2010;35:1316–1322. doi: 10.1097/BRS.0b013e3181e9acb2. [DOI] [PubMed] [Google Scholar]
- 26.Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926–933. doi: 10.1097/00006123-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Hempelmann RG, Mater E, Schön R. Septic hematogenous lumbar spondylodiscitis in elderly patients with multiple risk factors: efficacy of posterior stabilization and interbody fusion with iliac crest bone graft. Eur Spine J. 2010;19:1720–1727. doi: 10.1007/s00586-010-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JS, Suh KT. Posterior lumbar interbody fusion with an autogenous iliac crest bone graft in the treatment of pyogenic spondylodiscitis. J Bone Jt Surg B. 2006;88:765–770. doi: 10.1302/0301-620X.88B6.17270. [DOI] [PubMed] [Google Scholar]
- 29.Zaveri GR, Mehta SS. Surgical treatment of lumbar tuberculous spondylodiscitis by transforaminal lumbar interbody fusion (TLIF) and posterior instrumentation. J Spinal Disord Tech. 2009;22:257–262. doi: 10.1097/BSD.0b013e31818859d0. [DOI] [PubMed] [Google Scholar]
- 30.Fukuta S, Miyamoto K, Masuda T, Hosoe H, Kodama H, Nishimoto H, Sakaeda H, Shimizu K. Two-stage (posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine. 2003;28:302–308. doi: 10.1097/01.BRS.0000083318.40123.5E. [DOI] [PubMed] [Google Scholar]
- 31.Dai L, Chen W, Jiang L. Anterior instrumentation for the treatment of pyogenic vertebral osteomyelitis of thoracic and lumbar spine. Eur Spine J. 2008;17:1027–1034. doi: 10.1007/s00586-008-0661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruf M, Stoltze D, Merk HR, Ames M, Harms J. Treatment of vertebral osteomyelitis by radical debridement and stabilization using titanium mesh cages. Spine. 2007;32:275–280. doi: 10.1097/01.brs.0000261034.83395.7f. [DOI] [PubMed] [Google Scholar]
- 33.Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002;15:149–156. doi: 10.1097/00024720-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Liljenqvist U, Lerner T, Bullmann V, Hackenberg L, Halm H, Winkelmann W. Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J. 2003;12:606–612. doi: 10.1007/s00586-003-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yong HP, Jong DP, Young-Geun C, Sang-Ho L. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: autologous iliac bone strut versus cage. J Neurosurg Spine. 2009;8:405–412. doi: 10.3171/SPI/2008/8/5/405. [DOI] [PubMed] [Google Scholar]
- 36.Walter J, Kuhn SA, Reichart R, Kalff R, Ewald C. PEEK cages as a potential alternative in the treatment of cervical spondylodiscitis: a preliminary report on a patient series. Eur Spine J. 2010;19:1004–1009. doi: 10.1007/s00586-009-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mondorf Y, Gaab MR, Oertel JM. PEEK cage cervical ventral fusion in spondylodiscitis. Acta Neurochir. 2009;151:1537–1541. doi: 10.1007/s00701-009-0486-z. [DOI] [PubMed] [Google Scholar]
- 38.Cavuşoğlu H, Kaya RA, Türkmenoğlu ON, Tuncer C, Colak I, Aydin Y. A long-term follow-up study of anterior tibial allografting and instrumentation in the management of thoracolumbar tuberculous spondylitis. J Neurosurg Spine. 2008;8:30–38. doi: 10.3171/SPI-08/01/030. [DOI] [PubMed] [Google Scholar]
- 39.Blondel B, Fuentes S, Pech-Gourg G, Metellus P, Dufour H. Minimally invasive osteosynthesis in septic conditions. Neurochirurgie. 2011;57:15–20. doi: 10.1016/j.neuchi.2011.01.001. [DOI] [PubMed] [Google Scholar]