Abstract
Purpose
The aim of this study was to discuss the clinical presentation, imaging findings, treatments received, and outcome of therapies for patients with epidural spinal cord compression caused by thyroid spinal metastases, with the goal of emphasizing the importance of surgery in this setting and discussing therapeutic plan for treating these patients.
Methods
A total of 22 patients with spinal cord compression due to thyroid tumor spinal metastases who received surgery in our department were identified from 2004 to 2011. The series of 22 patients collected from our institution over the past 7 years was used to discuss treatment options for thyroid cancer spinal metastases on the basis of literature review and our own extensive experience.
Results
The mean age of the patients in this study was 57 years (range 37–78 years). The duration of the preoperative symptoms was 1–24 months, with an average of approximately 6 months. All patients attained improvement of at least one level of the Frankel classification after surgery. Two patients received more than one operation at our institution. Two patients died during follow-up, two patients had stable disease, and all other patients maintained a disease-free status during follow-up.
Conclusions
As thyroid tumor spinal metastases have a favorable prognosis, a radical therapeutic attitude should be considered in decision-making. Dorsal spinal decompression through curettage and stabilization can preserve or restore neurological function for most patients. For patients who have more than one metastatic lesion of the spine, surgeries can be sequentially performed based on the urgency of the case. In addition to treatment of primary disease, surgery and bisphosphonate treatment are the most important therapies for these patients.
Keywords: Retrospective study, Spinal metastatic tumor, Thyroid cancer, Spinal cord compression, Surgery
Introduction
It is well known that thyroid cancer has a favorable prognosis. Thyroid cancer patients with metastatic spinal tumors are expected to have relatively longer survival than patients with other types of cancers and metastatic spinal tumors. Patients with differentiated thyroid carcinoma (DTC) have a 10-year survival rate of 80–95 %, and even patients who present with distant metastatic disease may have prolonged survival. In one series of 44 such patients, the 20-year survival rate was 43 % [1]. However, most orthopedists are unsure whether aggressive surgical resection should be employed for thyroid metastatic spinal lesions. With such a potentially high survival rate, treatment of these patients should be more aggressive [2, 3]. Various treatment methods are used to treat these patients, including surgical excision, PVP, 131I, radiotherapy, chemotherapy, bisphosphonates, and other new therapies. However, pain intolerance, resistance to conservative treatment, vertebral compression fractures and segmental instability, and response failure to chemotherapy and 131I treatment together with progressive neurologic deficits and para- or tetraplegia collectively make operative intervention mandatory. Spinal cord compression occurs more frequently in patients with thyroid cancer than in those with other types of bone-seeking cancers, with an occurrence rate of 28 % compared to 10 and 8 % for prostate and breast cancers, respectively [4]. Several studies have shown that surgical treatment options are both important and effective for these patients [2, 3, 5]. However, clinicians are still hesitant to choose surgery when faced with vertebral metastases. The question of whether surgery is too aggressive for patients with spinal metastatic disease is still a controversial issue. Few studies have presented large case series of surgical patients with thyroid cancer spinal metastases. Here, we present our clinical experience with the diagnosis and management of 22 patients with spinal cord compression caused by thyroid tumor spinal metastases.
Patients and methods
A total of 22 patients with spinal cord compression caused by thyroid tumor spinal metastases who received an operation in our department were identified from 2004 to 2011 (Table 1). The indications for surgery were unendurable pain and neurologic deficit or the potential of neurologic deficit in the area affected by the tumor. The clinical and operative notes, radiographic images, and pathological reports of all patients who received surgery in our hospital for thyroid cancer spinal metastases were reviewed. In this study, we discuss the treatment options for thyroid cancer spinal metastases on the basis of literature review and our own extensive experience. This study was approved by the hospital ethics committee and informed consent was obtained from the participants.
Table 1.
Analysis of 22 cases of thyroid cancer spinal metastases
| Patients | Age (year)/sex | LC | Presentation | Preoperation | Postoperation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F-S | Tx | Resection mode | Instrument | F-S | Pathologic | F-U (month) | Last status | ||||
| #1 | 63/F | C4–5 | 4 months of pain and neurologic deficit | D | OP | Total | TM/AP/PF | E | Papillary | 61 | NED |
| #2 | 45/M | C1, 2, 5 | 1 months of swallowing difficult, voice hoarse | C | OP+131I | Total | TM/PF | E | Papillary | 21 | NED |
| #3 | 65/F | C2 | 1 year of pain and neurologic deficit | C | OP | Subtotal | PF | E | Papillary | 18 | NED |
| #4 | 72/F | C5, 6 | 2 years of pain and neurologic deficit | C | OP | Subtotal | TM/AP | D | Papillary | 24 | NED |
| #5 | 69/F | C5, 6 | 1 month of neurologic deficit | C | OP | Total | TM/AP/PF | D | Medullary | 8 | NED |
| #6 | 37/F | T7 | 8 months of pain and 6 months of neurologic deficit | C | EMB+OP | Total | AV | D | Follicular | 78 | AWD |
| #7 | 63/F | T8 | 2 months of pain | D | EMB+OP | Total | TM/PF | E | Follicular | 63 | DOD |
| #8 | 50/M | T7 | 3 months neurologic deficit | A | EMB+OP+131I | Total | TM/PF | E | Follicular | 56 | NED |
| #9 | 46/F | T10 | 9 months of pain and neurologic deficit | C | EMB+OP+131I | Total | TM/PF | E | Follicular | 46 | NED |
| #10 | 57/F | T9 | 6 months of pain and neurologic deficit | C | EMB+OP | Total | TM/PF | D | Papillary | 41 | NED |
| #11 | 54/M | T3, 9 | 4 years of pain and 20 days of neurologic deficit | C | EMB+OP | Total | PF | D | Follicular | 27 | NED |
| #12 | 29/F | T6 | 4 months of pain and 3 months of neurologic deficit | C | EMB+OP | Total | PF | E | Follicular | 29 | AWD |
| #13 | 74/F | T3 | 3 months of pain and neurologic deficit | B | EMB+OP | Total | TM/PF | D | Follicular | 19 | NED |
| #14 | 78/M | T2 | 3 months of pain and 20 days of neurologic deficit | B | EMB+OP | Total | PF | E | Papillary | 9 | NED |
| #15 | 63/F | T1 | 10 months of pain | C | EMB+OP | Subtotal | PF | D | Follicular | 10 | NED |
| #16 | 57/M | T10 | 3 years of pain and 1 month of neurologic deficit | C | EMB+OP+131I | Total | TM/PF | D | Follicular | 8 | NED |
| #17 | 59/M | T12 | 1 month of pain and 1 week of neurologic deficit | A | EMB+OP | Total | TM/PF | A | Poorly differentiated follicular | 4 | NED |
| #18 | 58/F | L2, 3 | 1 year of pain | C | EMB+OP | Total | TM/PF | C | Poorly differentiated follicular | 22 | DOD |
| #19 | 60/F | L5 | 1 year of pain and neurologic deficit | C | EMB+OP | Total | TM/PF | E | Follicular | 33 | NED |
| #20 | 37/M | L1 | 1 month of pain | E | EMB+OP+131I | Total | TM/PF | E | Follicular | 10 | NED |
| #21 | 42/F | S1 | 6 months of pain | E | EMB+OP | Total | PF | E | Follicular | 55 | NED |
| #22 | 63/F | S1–2 | 2 years of pain and 2 months of neurologic deficit | C | EMB+OP | Total | PF | D | Follicular | 14 | NED |
AP anterior plating, AV artificial vertebral, AWD alive with disease, DOD dead of disease, EMB arterial embolization, LC location, F female, F-U follow up, F-S Frankel classification score, M male, MET metastases, NED no evidence of disease, OP operation, PF posterior fixation, Subtotal subtotal resection, Total total spondylectomy, TM titanium mesh, Tx treatment
Results
Patient descriptions
Twenty-two patients were identified for our study (14 female, 8 male). The mean age was 57 years (range 37–78 years). All patients underwent a total thyroidectomy for the primary cancer before or after spinal surgery. Seventeen patients had a history of a thyroid mass; however, 10 of the tumors were subsequently found to be benign. In the remaining five patients, spinal metastases were the first presentation of thyroid cancer. Only 2 cases occurred in the sacrum, and the other 20 cases were first found in the mobile spine (5 in the cervical spine, 12 in the thoracic spine, and 3 in the lumber spine). Fifteen patients were also found to have a single lesion of the spine upon initial examination at our institution. One patient (Patient #5) was referred for local recurrence after receiving treatment at another institution.
Clinically, all of the spinal metastasis tumors had a similar presentation. The most common symptoms were pain and neurologic deficit. Pain was the first symptom to occur and was the most frequently reported. Neurological symptoms were also common and occurred in 16 of the 22 (73 %) patients. Symptoms included muscle weakness, difficulty swallowing, bowel or bladder incontinence, constipation, paresthesia, paraplegia, etc. The duration of the preoperative symptoms ranged from 1 to 24 months, with an average of approximately 6 months.
Radiographic findings
The lesions commonly presented with osteolysis and did not involve the intervertebral space. In addition, the lesions were commonly located in the lumbar and thoracic spine (15 out of 22), and almost all of the lesions were found in the vertebral body.
Computed tomography (CT) can show bone destruction, sclerosis, or the absence of pedicles by tumor infiltration. It is used to assess the extent of metastatic lesions and is particularly efficient at evaluating sites that are difficult to evaluate with other approaches.
Magnetic resonance imaging (MRI) provides more details of bone and soft tissues, and is therefore best applied when spinal cord compression and involvement of the tumor is suspected.
However, the use of single-photon emission computed tomography (SPECT)/CT can dramatically increase both the sensitivity and specificity of CT/MRI. It can tell doctors whether there are other metastatic lesions. Therefore, 8F-fludeoxyglucose positron emission tomography (18F-FDG-PET) is likely to be a useful investigative tool due to the high proliferative rates of these tumors.
Histological findings
The prognosis of patients with thyroid cancer is highly related to the pathological type. The overall risk of synchronous distant metastasis is 4 %, and is lowest for papillary (2 %) and highest for follicular (11 %) and Hürthle cell (12 %) carcinoma [1]. In our series, papillary carcinoma accounted for 6 (27 %) cases, while 15 (68 %) cases were follicular carcinoma. Only one case was medullary thyroid carcinoma, and it appeared that patients with follicular carcinoma were much more likely to develop distant metastases, whereas it was easy to detect lesions in the cervical spine of papillary carcinoma patients (Table 1).
Treatments
Due to the high risk and limitations of the technique and experience of the interventional radiologist, only patients with metastatic lesions below the cervical spine (17 patients) received selective arterial embolization before surgery, which was used to minimize intraoperative blood loss. Twenty-seven surgeries were collectively performed on these patients. All 22 patients were treated by curettage and reconstruction of the spine. All patients received total resection of the tumor, with the exception of Patients #3, #4, and #19, in whom partial resection was performed because of heavy bleeding and important structural involvement. For lumbar and thoracic metastases, dorsal spinal decompression and stabilization is the standard surgical technique. In cervical metastases, the currently preferred method is clearly a ventral/combined approach of decompression and ventral stable-angle plate osteosynthesis. Titanium mesh filled with bone cement was used to replace the involved vertebral body, which is more stable and markedly less expensive than other materials. This technique is also easily performed and no side effects of the bone cement were observed. All patients were treated with bisphosphonate after surgery and administered incadronate disodium at a dose of 10 mg in 500 mL saline as a 2 h intravenous (i.v.) infusion once a month.
Follow-up and outcome
The average follow-up duration of the patients in this study was 30 months (range 4–78 months). Two patients died during follow-up. One patient (Patient #18) died 1 year after surgery because of poor health, while the other patient (Patient #7) died from lung metastases 62 months post-surgery. In addition, Patients #1 and #8 received more than one surgery. In particular, one male patient (Patient #8) received four surgeries at our institution during the last 56 months of follow-up. His first metastatic lesion was in T7, which caused paraplegia. After curettage of the T7 lesion and reconstruction of the spine, the paraplegia was completely reversed. However, 2 months later, another lesion was discovered in L4, which caused paraplegia again. This patient subsequently received a second surgery and completely recovered. Unfortunately, 10 months after the second operation, a C3 metastatic lesion was discovered, and a third operation was performed. The patient resumed a normal lifestyle until the T7 and L4 lesions reoccurred 30 months later. A fourth operation was subsequently performed, but the patient lost partial use of his lower extremities thereafter. The patient remains under follow-up (Fig. 1).
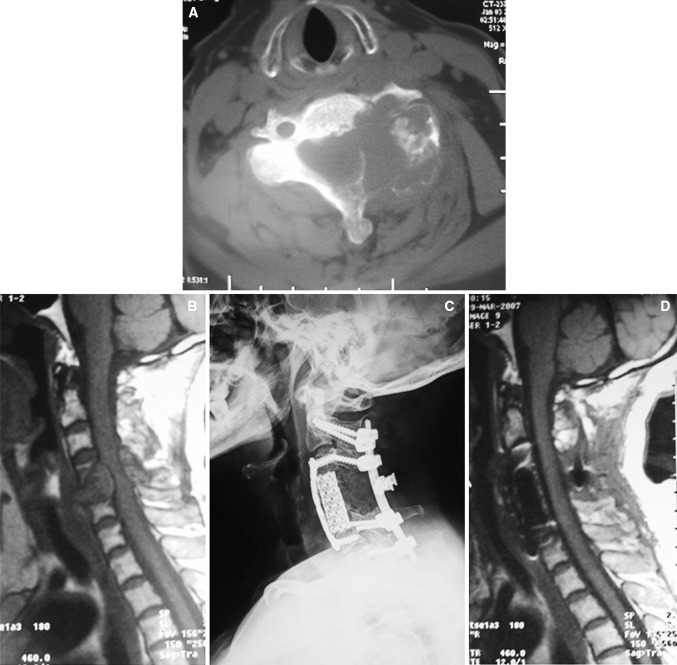
Fig. 1.
A 63-year-old woman (case 1). Preoperative computed tomography (CT) scan (a) and magnetic resonance imaging (b) allowed evaluation of tumor size and spinal involvement. The patient underwent a total spondylectomy of the anterior and posterior combination. A post-surgical plain radiograph (c) showed reconstruction with an anterior titanium plate and titanium mesh filled with bone cement and a posterior screw–rod system. (d) Magnetic resonance imaging at the 3-month follow-up where no relapse was detected. No worsening of the neurologic status has been observed and the patient is continuing follow-up
All of our patients had an improvement of at least one level in the Frankel classification system (Table 1) and substantial pain relief post-surgery. No side effects from bisphosphonate treatment were observed in any of the patients, although fever (not higher than 39 °C) was commonly observed. Loss of renal function and jaw osteonecrosis was also not observed in the patients. Although the follow-up time was not long enough, no significant difference in survival or local recurrence rates was observed, regardless of 131I administration. With the exception of the two patients who died during follow-up, two patients survived with stable disease and all of the other patients had no local recurrence (Fig. 2).
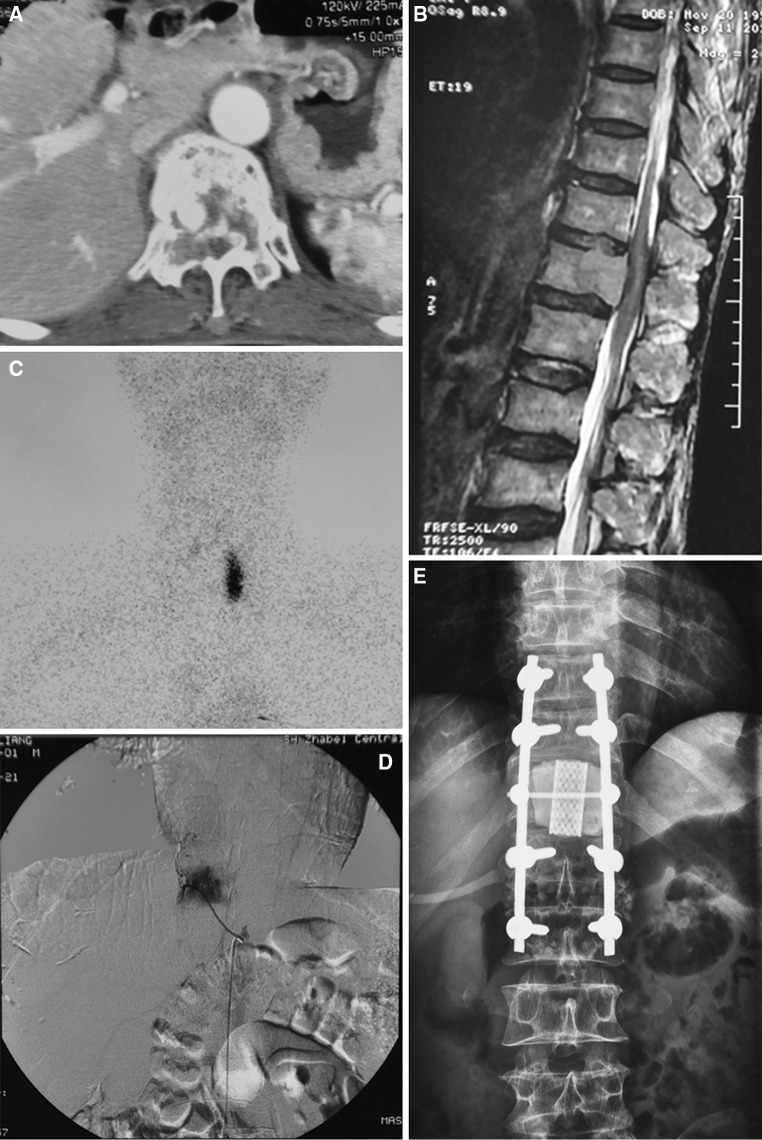
Fig. 2.
A 59-year-old man (case 17). Preoperative computed tomography (CT) scan (a) and magnetic resonance imaging (b). c The left thyroid gland showed high Tc-99 m uptake. Pre-surgical embolization (d). Post-surgical X ray (e). A titanium mesh filled with bone cement was used as a replacement of the involved vertebral body. Extra bone cement was used to consolidate the structure
Discussion
Thyroid cancer is a tumor type with a favorable prognosis. For thyroid carcinoma, greater than 5 years between diagnosis and surgery for spinal metastases represents a good prognostic factor [6]. Survival is also significantly associated with the histology of primary tumor [5]. We subdivide these patients into three categories due to different pathological types. We define more than one operation, dead and alive with disease as bad prognoses. There were five cases with bad prognosis in the follicular group, while there was only one patient in the papillary group during follow-up. However, lacking sufficient papillary cases (only 6 cases), there was no statistically significant evidence showing that papillary thyroid tumor has a more favorable prognosis in spinal metastases (Fisher probabilities, P = 0.623). The outcome difference among different pathologic types should be analyzed on a greater cohort.
Of these 22 patients, 17 had a history of thyroid surgery. Ten of the patients were found to have a benign thyroid mass after thyroidectomy. Therefore, in cases where a patient with a spinal tumor has a history of thyroid mass, thyroid cancer spinal metastases should be considered even if the pathologic report of thyroid neoplasm was benign. The ideal treatment of thyroid cancer spinal metastases requires a multidisciplinary collaboration, because the cancer should be viewed as a systemic disease that may necessitate a multifactor treatment regimen, including surgery, radiotherapy, chemotherapy, radioidine (131I), bisphosphonates, and other new therapies.
It is well known that a vertebrectomy, en bloc spondlectomy, and even intralesional resection can lead to substantial blood loss, which in turn can lead to life-threatening hemorrhaging and increase the transfusion requirements during surgery. Blood loss during spinal surgery also interferes with the surgeon’s ability to obtain a clear view of the surgical field, which can lead to increased operative times, increased risk of intraoperative complications, postoperative intraspinal hematomas, and delayed wound healing. Preoperative embolization of spinal tumors is an effective procedure that can reduce intraoperative blood loss, palliate pain, and improve neurologic symptoms [7]. Because large bias existed in our statistics of blood loss and only patients with metastatic lesions below the cervical spine received selective arterial embolization before surgery, we cannot give a formal data to support our view by comparing the blood loss of patients who did or did not receive preoperation embolization. However, in our clinical experience, we found that preoperative embolization could dramatically reduce intraoperative blood loss of other metastatic tumors. Therefore, we recommend preoperative embolization for every patient if possible.
Surgical treatment of thyroid cancer has been found to be important and effective in several recent studies [2, 3, 5, 8, 9]. Operations for patients with thyroid cancer spinal metastases should be more aggressive, which is in contrast to more traditional opinions in the field. Aggressive surgical management of spinal metastatic disease can prevent fracture with spinal cord injury and can improve neurological function, as well as provide marked pain relief. The most important goal of surgical resection in patients with thyroid cancer spinal metastases is to preserve or restore neurological function. Even if the patient’s neurological function is damaged, surgery has the potential to reverse it. All of the patients treated in our study had an improvement of at least one level in Frankel classification. Twenty patients had preserved or restored ambulation after surgery, while the number is only four preoperation (χ2 = 23.467, P < 0.001). The improvement in Frankle level has dramatic clinical significance. Therefore, the benefit is not only apparent to the patients’ family, but also to society, because many patients received restoration of their ability to care for themselves. There are many surgical therapies that can be applied, such as curettage, vertebrectomy, total en bloc spondylectomy, PVP, and others. The choice of surgical treatment is a difficult decision, because many factors should be considered. All the thyroid metastases present should be discussed with the oncologists to decide about a best therapeutic plan. The good responders should be treated with minimal surgery, only for functional purpose (fixation, decompression), while the poor responders or patients having neurological deficits should be submitted to more aggressive surgery (debulking, gross total). In cases of a solitary metastasis, a total vertebrectomy can be performed after radical resection of the primary tumor when the patient’s prognosis is good. Total en bloc spondylectomy is an ideal, but not a practical surgical option for spinal metastases patients. Some studies have shown that total en bloc spondylectomy with sufficient margin can provide favorable local control of spinal metastases of thyroid carcinoma during a patient’s lifetime [8]. Haomiao et al. evaluated the outcome of excisional surgeries (en bloc/debulking) of 131 patients receiving spinal cord metastatic treatment and found that en bloc surgery could achieve a lower local recurrence rate than debulking surgery. That means en bloc resection would reduce the morbidity associated with local recurrence and revision surgery compared with curettage. However, the two procedures resulted in a similar survival outcome, neurological salvage, and incidence of complications [9]. Moreover, total spondylectomy is a procedure with strict principles that are difficult to fully achieve. Therefore, total resection of the tumor by intralesional surgery and debulking are still the most frequently applied surgical procedures. All 22 patients in this study were treated by curettage. For patients who have more than one metastatic lesion of the spine, operations can be sequentially performed based on the urgency of the case.
For most patients, dorsal spinal decompression through curettage and stabilization can be applied as a main surgical technique for treating spinal metastases [10]. Dorsal decompression without stabilization should only be performed as a palliative procedure in patients with an inoperative tumor, those who have a poor prognosis, or if the estimated postoperative segmental stability seems to be sufficient [11]. However, conventional surgical decompression of the spinal cord with or without instrumentation often results in unsatisfactory neurological recovery and local recurrence. In addition, the rate of local recurrence was 2.5 %. Taku et al. suggested that when complete resection was not possible, intraoperative radiotherapy (IORT) should be considered [12]. For patients without neurological damage or spinal instability, percutaneous verteplasty (PVP) and kyphoplasty can be used as palliative therapies to maintain stabilization of the compromised vertebrae and prevent fracture with spinal cord injury, relieve pain, and improve survival and quality-of-life [13].
A postoperative therapeutic plan should be well designed with the oncologist, and 131I treatment should be used as first-line treatment. However, studies using radioactive iodine therapy for bone metastases have shown that the metastases are generally resistant to 131I and may require other approaches [14]. Brown et al. [15] reported no 10-year survivors in a group of patients with bone metastases treated with 131I, but a 54 % 10-year survival rate for patients with lung metastases. Moreover, a previous retrospective study evaluated the therapeutic outcome, total administered radioiodine activity, and side effects in 107 patients with initial bone metastases [16]. This study concluded that initial bone metastases can be treated with curative intent in select differentiated thyroid carcinoma (DTC) patients up to 45 years of age, particularly in patients with less than three bone metastases. Based on the author’s experience, 131I may not be effective in treating bone metastases, and therefore it is important to cure the overall disease and control local recurrence in the thyroid gland and soft tissue metastases. Thus, it is still useful in the clinic for the treatment of spinal metastases.
In recent studies, bisphosphonates have been shown to be highly effective in preventing bone resorption, hypercalcemia, pathological fracture, and even reducing local recurrence. Orita et al. [17] studied 50 patients with bone metastases from differentiated thyroid carcinoma who were divided into two groups, where one group did not receive bisphosphonate therapy and the other received zoledronic acid (ZA) therapy. Treatment with ZA was effective in reducing skeletal-related events (SREs) or delaying their appearance in patients with bone metastases from differentiated thyroid carcinoma. Bisphosphonates are frequently used at our institution for treating thyroid carcinoma. In addition, all patients in this study were administered incadronate disodium at a dose of 10 mg in 500 mL saline as a 2 h i.v. infusion once a month. We found that SREs were apparently controlled, as no patient suffered an SRE during follow-up. Therefore, bisphosphonates are an integral component of the current treatment regimen of spinal metastases and, importantly, side effects should be closely observed.
Few effective therapies are available for patients who do not respond to these described treatment modalities [18]. There are no effective chemotherapeutic agents for any of the histologies of advanced thyroid cancer. However, some studies have reported that single-agent doxorubicin has a response rate of 25–40 % and is the most effective chemotherapeutic agent. Also, there is little evidence to suggest that combination chemotherapy is more effective [19–21]. Therefore, the development of novel therapies, such as biological modifiers or new chemotherapy agents, is urgently needed for this disease.
Conclusions
As thyroid tumor spinal metastases have a favorable prognosis, a radical therapeutic attitude should be considered in decision-making. Dorsal spinal decompression through curettage and stabilization can preserve or restore neurological function for most patients. For patients who have more than one metastatic lesion of the spine, surgeries can be sequentially performed based on the urgency of the case. In addition to treatment of primary disease, surgery and bisphosphonate treatment are the most important therapies for these patients.
Conflict of interest
None.
Abbreviations
- DTC
Differentiated thyroid carcinoma
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- SPECT
Single-photon emission computed tomography
- 18F-FDG-PET
8F-fludeoxyglucose positron emission tomography
- IORT
Intraoperative radiotherapy
- PVP
Percutaneous verteplasty
- ZA
Zoledronic acid
- SREs
Skeletal-related events
Footnotes
D. Zhang and H. Yin contributed equally to this work.
Contributor Information
Dan Zhang, Email: zhangadanbama@hotmail.com.
Jianru Xiao, Phone: +0086021885634, FAX: +008602163720099, Email: 3012005142@smmu.edu.cn.
References
- 1.Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174(5):474–476. doi: 10.1016/S0002-9610(97)00158-X. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Wanis ME, Kawahara N, Murata A, Murakami H, Nambu K, Ueda Y, Tomita K. Thyroid cancer spinal metastases: report on 25 operations in 14 patients. Anticancer Res. 2002;22(4):2509–2516. [PubMed] [Google Scholar]
- 3.Stojadinovic A, Shoup M, Ghossein RA, Nissan A, Brennan MF, Shah JP, Shaha AR. The role of operations for distantly metastatic well-differentiated thyroid carcinoma. Surgery. 2002;131(6):636–643. doi: 10.1067/msy.2002.124732. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 5.Tancioni F, Navarria P, Pessina F, Attuati L, Mancosu P, Alloisio M, Scorsetti M, Santoro A, Baena RR. Assessment of prognostic factors in patients with metastatic epidural spinal cord compression (MESCC) from solid tumor after surgery plus radiotherapy: a single institution experience. Eur Spine J. 2012;21(1):146–148. doi: 10.1007/s00586-012-2232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Geus-Oei Lioe-Fee, Oei Hong-Yoe, Hennemann Georg, Krenning Eric P. Sensitivity of 123I whole-body scan and thyroglobulin in the detection of metastases or recurrence differentiated thyroid cancer. Eur J Nuclear Med Mol Imaging. 2002;29(6):768–774. doi: 10.1007/s00259-002-0781-x. [DOI] [PubMed] [Google Scholar]
- 7.Ozkan E, Gupta S. Embolization of spinal tumors: vascular anatomy, indication, and technique. Tech Vasc Interv Radiol. 2011;14(3):129–140. doi: 10.1053/j.tvir.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Demura S, Kawahara N, Murakami H, Abdel-Wanis ME, Kato S, Yoshioka K, Tomita K, Tsuchiya H. Total en bloc spondylectomy for spinal metastases in thyroid carcinoma. J Neurosurg Spine. 2011;14(2):172–176. doi: 10.3171/2010.9.SPINE09878. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Gasbarrini A, Cappuccio M, Terzi S, Paderni S, Mirabile L, Boriani S. Outcome of excisional surgeries for the patients with spinal metastases. Eur Spine J. 2009;18(10):1423–1430. doi: 10.1007/s00586-009-1111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delank KS, Wendtner C, Eich HT, Eysel P(2011)The treatment of spinal metastases. Dtsch Arztebl Int 108(5):71–79; quiz 80 (Epub 4 Feb 2011) [DOI] [PMC free article] [PubMed]
- 11.Dominkus M, Krepler P, Schwameis E, Kotz R. Surgical therapy of spinal metastases. Orthopade. 1998;27(5):282–286. doi: 10.1007/s001320050232. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Kondo T, Hozumi T, Karasawa K, Seichi A, Nakamura K. Results of posterior surgery with intraoperative radiotherapy for spinal metastases. Eur Spine J. 2005;15(2):216–222. doi: 10.1007/s00586-005-0979-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushchayev S, Kushchayeva Y, Theodore N, Preul MC, Clark OH. Percutaneous vertebroplasty for thyroid cancer metastases to the spine. Thyroid. 2010;20(5):555–560. doi: 10.1089/thy.2009.0420. [DOI] [PubMed] [Google Scholar]
- 14.Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, Francese C, Fontaine F, Ricard M, Parmentier C. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37(4):598–605. [PubMed] [Google Scholar]
- 15.Brown AP, Greening WP, McCready VR, Shaw HJ, Harmer CL. Radioiodine treatment of metastatic thyroid carcinoma: the Royal Marsden Hospital experience. Br J Radiol. 1984;57(676):323–327. doi: 10.1259/0007-1285-57-676-323. [DOI] [PubMed] [Google Scholar]
- 16.Petrich T, Widjaja A, Musholt TJ, Hofmann M, Brunkhorst T, Ehrenheim C, Oetting G, Knapp WH. Outcome after radioiodine therapy in 107 patients with differentiated thyroid carcinoma and initial bone metastases: side-effects and influence of age. Eur J Nucl Med. 2001;28(2):203–208. doi: 10.1007/s002590000420. [DOI] [PubMed] [Google Scholar]
- 17.Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011;21(1):31–35. doi: 10.1089/thy.2010.0169. [DOI] [PubMed] [Google Scholar]
- 18.Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID. Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995;80(7):2041–2045. doi: 10.1210/jc.80.7.2041. [DOI] [PubMed] [Google Scholar]
- 19.Ekman ET, Lundell G, Tennvall J, Wallin G. Chemotherapy and multimodality treatment in thyroid carcinoma. Otolaryngol Clin North Am. 1990;23(3):523–527. [PubMed] [Google Scholar]
- 20.Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56(9):2155–2160. doi: 10.1002/1097-0142(19851101)56:9<2155::AID-CNCR2820560903>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PC, Millar BM, Brierley JD. The management of advanced thyroid cancer. Clin Oncol (R Coll Radiol) 2004;16(8):561–568. doi: 10.1016/j.clon.2004.08.009. [DOI] [PubMed] [Google Scholar]