Abstract
Permanent hearing loss is caused by the irreversible damage of cochlear sensory hair cells and nonsensory supporting cells. In the postnatal cochlea, the sensory epithelium is terminally differentiated, whereas tympanic border cells (TBCs) beneath the sensory epithelium are proliferative. The functions of TBCs are poorly characterized. Using an Axin2lacZ Wnt reporter mouse, we found transient but robust Wnt signaling and proliferation in TBCs during the first 3 postnatal weeks, when the number of TBCs decreases. In vivo lineage tracing shows that a subset of hair cells and supporting cells is derived postnatally from Axin2-expressing TBCs. In cochlear explants, Wnt agonists stimulated the proliferation of TBCs, whereas Wnt inhibitors suppressed it. In addition, purified Axin2lacZ cells were clonogenic and self-renewing in culture in a Wnt-dependent manner, and were able to differentiate into hair cell-like and supporting cell-like cells. Taken together, our data indicate that Axin2-positive TBCs are Wnt responsive and can act as precursors to sensory epithelial cells in the postnatal cochlea.
Keywords: Stem cells, Regeneration, Development, Inner ear, β-Catenin, Hair cells, Mouse
INTRODUCTION
The integrity of hair cells and supporting cells in the mammalian organ of Corti is required for hearing function. Because the postnatal sensory organ lacks the capacity to repair or regenerate, disorders causing cell loss in the cochlear sensory epithelium (SE) result in permanent hearing loss. In mice, cell proliferation in the developing organ of Corti terminates on embryonic day 14 (Ruben, 1967). Although various sensory and non-sensory cell types continue to mature until hearing function begins in the second postnatal week, these cells remain mitotically inactive. Recent evidence suggests, however, that the postnatal mammalian cochlea contains a relatively uncharacterized population of cells that retain a transient proliferative potential, as measured in vitro by cell-derived floating spheres (Malgrange et al., 2002; Oshima et al., 2007; Savary et al., 2008; Shi et al., 2012; Zhang et al., 2007) or by culturing the cells on specific feeder cells (Chai et al., 2012; Sinkkonen et al., 2011; White et al., 2006). Cultured cochlear cell-derived spheres are thought to be derived from stem/progenitor cells, whereas the sphere-derived cells can differentiate in vitro into cells exhibiting hair cell and supporting cell phenotypes (Oshima et al., 2007). However, the proliferative potential of this endogenous pool of cochlear progenitor cells rapidly declines during the first 3 postnatal weeks (Oshima et al., 2007; White et al., 2006). At present, there is limited understanding of both the origin of these cochlear progenitor cells and the mechanisms that regulate their proliferative capacity, although experimental evidence suggests that a subpopulation of cochlear supporting cells behave as progenitor cells in vitro (Sinkkonen et al., 2011; White et al., 2006).
The Wnt/β-catenin signaling pathway is essential in maintaining homeostasis of many tissues (Logan and Nusse, 2004). Active Wnt signaling marks endogenous stem cells in the gastrointestinal system (Barker et al., 2007; Ootani et al., 2009), integumentary system (Jaks et al., 2008) and the mammary gland (Zeng and Nusse, 2010). Expression of Axin2, a downstream target and feedback inhibitor of the Wnt pathway, reliably reports active canonical Wnt signaling in many tissues (Jho et al., 2002; Lustig et al., 2002). By manipulating the Wnt pathway with exogenous agonists, several investigators have reported proliferation and expansion of resident stem/progenitor cell populations (Kalani et al., 2008; Ootani et al., 2009; Zeng and Nusse, 2010). Although components of the Wnt pathway are expressed in the mammalian postnatal cochlea (Daudet et al., 2002; Shah et al., 2009), their roles in mediating Wnt signaling in inner ear progenitor cells are incompletely understood. Here, we tested whether active canonical Wnt signaling marks and promotes proliferation of cochlear progenitor cells. We found that the Wnt target gene Axin2 marks a previously poorly characterized cochlear cell population: tympanic border cells (TBCs). Both in vivo and in vitro, TBCs retain proliferative capacity, which is promoted by Wnt activation and suppressed by Wnt inhibition. By lineage tracing and culturing sorted cells, we found that Axin2-positive TBCs are able to generate new hair cells and supporting cells in vivo and in vitro.
MATERIALS AND METHODS
Mice
Wild-type and Axin2lacZ/+ mice (Jho et al., 2002; Lustig et al., 2002) in CD1 background, Pax2-Cre mice (provided by A. Groves, Baylor College of Medicine, Houston, TX, USA) (Ohyama and Groves, 2004), Actin-GFP mice (stock #007075) (Okabe et al., 1997), Actin-DsRed mice (stock #006051) (Vintersten et al., 2004), R26RmTmG mice (stock #007576) (Muzumdar et al., 2007) and R26RtdTomato mice (stock #007914) (Madisen et al., 2010) (all from the Jackson Laboratory); and Axin2CreERT2 mice (van Amerongen et al., 2012) were used. For lineage tracing, pups were injected intraperitoneally with tamoxifen (0.05-1.00 mg/25 g dissolved in corn oil) (Sigma). Intraperitoneal injection of EdU (50 mg/kg; Invitrogen) was performed once per day for 2 days or twice per day for 3 days. The latter regimen, when combined with tamoxifen, caused ∼33% lethality. Cochleae were processed for cryosectioning and immunostaining as described below, and EdU detection was performed per product protocol using an Alexa Fluor 555 Imaging Kit. All protocols were approved by the Animal Care and Use Committee of Stanford University School of Medicine.
X-gal staining and cryosectioning
Tissues were fixed with 4% paraformaldehyde (Electron Microscopy Services) in phosphate-buffered solution (PBS, pH 7.4) for 30 minutes on ice and subsequently washed with 2 mM MgCl2 (in PBS) before incubation with X-gal reagents at 37°C for 35 minutes. For cryosectioning, cochleae were similarly fixed and stained, then treated in a sucrose gradient (10-30% in PBS). Tissues were serially treated with sucrose/OCT compound (Sakura Finetek) mixture (1:1, 3:7, then 0:1) in a vacuum chamber for 1 hour at room temperature. Tissues were then sectioned at 10 μm and processed for immunohistochemistry. Decalcification with EDTA (0.5 M in PBS) was performed for cochleae from mice aged P7 or older.
Cell sorting
As previously described (Jan et al., 2011), cochleae from P0-P2 Axin2lacZ/+ mice were isolated, with the stria vascularis and spiral ganglia removed before incubation in 0.125% trypsin (Invitrogen; in PBS for 8 minutes) and then in trypsin inhibitor/DNase1 cocktail (1:1; 10 mg/ml; Worthington Biochem). Following trituration, cells were passed through a 40 μm filter and labeled with 3-carboxyumbelliferyl β-D-galactopyranoside (CUG, 1:50 for 25 minutes; Marker Gene Technologies) and propidium iodide (1 μg/ml; Sigma). Wild-type cochleae were used to determine background labeling levels in each sort. Using a BD Aria FACS cytometer (BD Biosciences), we consistently achieved over 90% cell viability and over 93% purity for sorted cells as measured via re-sort analysis. For staining, sorted cells were plated on fibronectin (Sigma)-coated slides (2 hours at room temperature) before fixation and immunohistochemistry.
RT-PCR and qPCR
Total RNA isolation was carried out using a RNeasy Mini Extraction kit (Qiagen), followed by cDNA synthesis using SuperScript III First-Strand Synthesis System kits (Invitrogen). Primer pairs were designed using the online Primer3 software (http://frodo.wi.mit.edu/primer3/) as follows: Sox2 forward, 5′-ATGAACGGCTGGAGCAACGGCA-3′; Sox2 reverse, 5′-TCACATGTGCGACAGGGGCAGT-3′; Axin2 forward, 5′-ATGTTAGAGAGTGAGCGGCAGA-3′; Axin2 reverse, 5′-CTTCAGCATCCTCCTGTATGGA-3′; Sp5 forward, 5′-CCGTCGTACCCTTACGAGTTCT-3′; Sp5 reverse, 5′-ATCTGGCTCTGGTACTGTGCAA-3′; p27Kip1 forward, 5′-AAGCACTGCAGAGACATGGAAG-3′; p27Kip1 reverse, 5′-GTAGAAGAATCGTCGGTTGCAG-3′; Brn3.1 forward, 5′-ACCCAAATTCTCCAGCCTACAC-3′; Brn3.1 reverse, 5′-GGCGAGATGTGCTCAAGTAAGT-3′; Prestin forward, 5′-TACCTCACGGAGCCGCTGGT-3′; Prestin reverse, 5′-GCAGTAATCAGTCCGTAGTCC-3′; β-actin forward, 5′-ACGGCCAGGTCATCACTATTG-3′; β-actin reverse, 5′-AGGGGCCGGACTCATCGTA-3′. qPCR reactions were performed with SYBR Green PCR Master Mix on a 7900HT-Fast Real time PCR system (both Applied Biosystems). The ΔΔCT method with β-actin as the endogenous reference was used (Livak and Schmittgen, 2001). Reactions were carried out in triplicate.
Adherent cultures
Feeder cells were collected by isolating embryonic 18-day-old chicken utricles and removing the SE via thermolysin treatment (0.5 mg/ml in HBSS; Sigma) (Oshima et al., 2010; Warchol, 1999). Dissociated cells were cultured in DMEM/F12 with 10% FBS and propagated cells were grown to 90% confluence and pre-treated with mitomycin C (10 μg/ml, Sigma) prior to use as feeder cells. Adding 100 μl of 70 sorted cells/μl (i.e. 7000 cells/well) onto a 0.97 cm2 surface area was determined to foster clonal colony formation. For colony expansion assays, Actin-DsRed-positive cells were cultured on feeder cells and colonies were quantified after 7 days and then passaged (0.125% trypsin for 3 minutes at 37°C). Quantification of propidium iodide-labeled cells found ∼70% cell death with this passaging protocol.
Enzyme-linked immunosorbent assay (ELISA)
Cochleae cultured in defined conditions were lysed and homogenized. A BCA Protein Assay kit was used to determine protein levels and a FluoReporter lacZ/Galactosidase Quantification kit (both Molecular Probes) was used to determine β-galactosidase activity. Manufacturer’s protocols were followed.
Immunohistochemistry
Tissues were fixed for 30-60 minutes in 4% paraformaldehyde (in PBS, pH 7.4) prior to incubation with primary antibodies overnight at 4°C. We used antibodies against the following markers: myosin 7a (1:400; Proteus Bioscience), Ki67 (1:400; ThermoScientific), phosphohistone 3 (1:400; Millipore), pancytokeratin (1:200; Sigma), parvalbumin (1:1000; Sigma), calretinin (1:1000; Millipore), fibronectin (1:400; BD Biosciences), β-galactosidase (1:10,000; Promega), jagged 1 (1:800; Santa Cruz Biotechnology), Sox2 (1:400; Santa Cruz Biotechnology), espin (1:1000; a gift from A. Hudspeth, Rockefeller University, New York, NY, USA), Prox1 (1:1000; Millipore), E-cadherin, vimentin (both 1:400; Sigma), p27Kip1 (1:400; Fisher Scientific), GFP (1:1000; Abcam) and Brn4 (1:500; a gift from B. Crenshaw, University of Pennsylvania, Philadelphia, PA, USA). The secondary antibodies were conjugated with FITC, TRITC, or Cy5 (1:500; Invitrogen). Images were acquired using epifluorescent or confocal microscopy (Axioplan 2, Zeiss, Germany) and analyzed with Photoshop CS4 (Adobe Systems). Three-dimensional reconstruction was performed using Volocity software (v5.3.0; Improvision).
Whole-organ cultures
Cochleae were cultured as previously described (Chai et al., 2011). Briefly, organs were cultured in growth factor-enriched, serum-free media (GM) consisting of DMEM/F12, N2 (1:100), B27 (1:50; all from Invitrogen), bFGF (1 ng/ml), IGF-1 (50 ng/ml), EGF (20 ng/ml), heparan sulfate (50 ng/ml) and ampicillin (50 μg/ml; all from Sigma) (Oshima et al., 2007) in four-well Petri dishes (Greiner Bio-one) with media replenished every 1-2 days. R-spondin 1 (1 μg/ml; R&D Systems), purified Wnt3a (200 ng/ml), Fz8CRD (25 μg/ml) and EdU (25 ng/ml; Invitrogen) were added. The synthesis and purification of Wnt3a and Fz8CRD have been previously described (DeAlmeida et al., 2007; Willert et al., 2003).
Statistics
Statistical analyses were conducted using Excel (Microsoft) and Origin softwares (OriginLab). Two-tailed, unpaired Student’s t-test and one-way ANOVA were used to determine statistical significance. P<0.05 was considered significant.
RESULTS
Wnt signaling in the postnatal cochlea
We used an Axin2lacZ/+ reporter mouse (Jho et al., 2002; Lustig et al., 2002) to detect active canonical Wnt signaling in the postnatal cochlea. As a feedback inhibitor, Axin2 is expressed in cells with active Wnt signaling (Jho et al., 2002). We established that Axin2lacZ/+ mice had no detectable phenotypes in the auditory system, as the mice had normal cochlear morphology and function (Fig. 1A; supplementary material Fig. S1A). Analysis of cryosections and whole mounts of neonatal cochlea revealed that TBCs underneath the SE on the basilar membrane express LacZ (Fig. 1A,B; supplementary material Fig. S1E). In situ hybridization demonstrated robust Axin2 expression in the TBCs (supplementary material Fig. S1B) (Chai et al., 2011), corroborating the Axin2lacZ reporter gene activity data. TBCs extend from the lateral cochlear wall to the habenula perforata (Fig. 1B; supplementary material Fig. S1C,D). To further characterize the TBCs, we examined expression of markers of proliferation, hair cells, supporting cells, epithelial cells and mesenchymal cells. In contrast to the P1 SE cells, TBCs were proliferative (Ki67 positive) and did not express markers of hair cells (myosin 7a) or supporting cells (jagged 1 and Sox2) (Fig. 1C-E). Furthermore, unlike the SE cells, which expressed E-cadherin and cytokeratin, TBCs were devoid of these epithelial cell markers (Fig. 1F,G). The mesenchymal markers vimentin and fibronectin were also absent in the TBCs (Fig. 1H,I), but they expressed the transcription factor Brn4 at P0 (supplementary material Fig. S2A-C) (Ahn et al., 2009; Phippard et al., 1999). In combination, an absence of markers seen in mature cells of the cochlear SE and the expression of proliferative markers led us to conclude that TBCs are poorly differentiated, actively cycling and distinct from the Axin2-negative mitotically inactive SE.
Fig. 1.
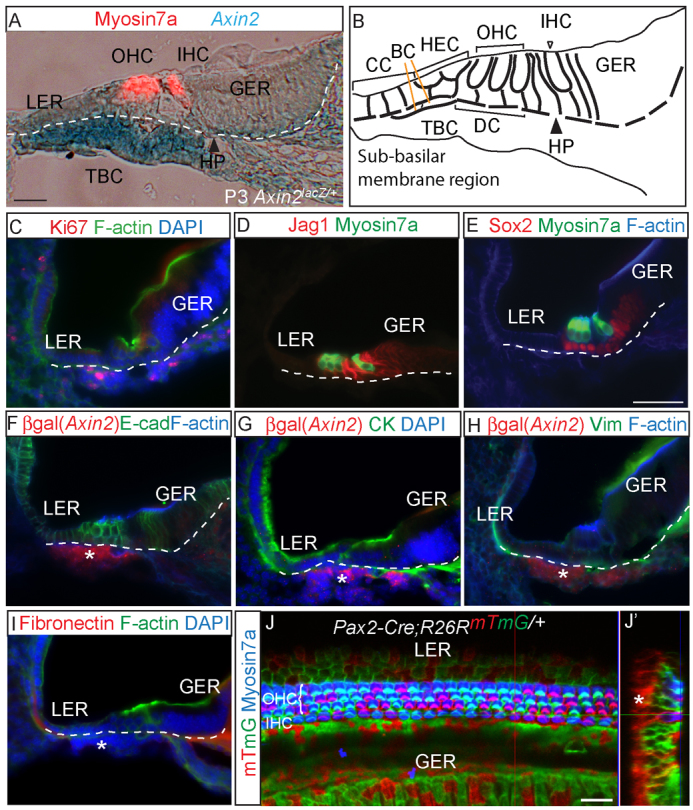
Axin2 expression in tympanic border cells and their lineage origin. (A) Cryosection of X-gal-stained P3 Axin2lacZ/+ cochlea demonstrating intense LacZ (Axin2) expression among tympanic border cells (TBCs) beneath the basilar membrane (dotted line). Myosin 7a marks outer and inner hair cells (OHCs and IHCs). (B) The location of TBCs in the cochlea in relation to the sensory epithelium (SE). (C) TBCs are proliferative and express Ki67. (D,E) Expression of jagged 1 (Jag1) and Sox2 was restricted to SE-supporting cells and absent in TBCs. (F-I) Anti-β-galactosidase antibody was used to detect Axin2-lacZ-positive TBCs (asterisks) in the P1 Axin2lacZ/+ cochlea. Both epithelial (cytokeratin and E-cadherin) and mesenchymal markers [vimentin (Vim) and fibronectin] were absent in TBCs. (J) Whole-mount cochleae from P1 Pax2-Cre;R26RmTmG/+ demonstrating traced mGFP-positive cells in the SE but not in TBCs. (J′) Reconstructed cross-section of z-stack images. Asterisk indicates TBCs. BC, Boettcher cells; CC, Claudius cells; HEC, Hensen’s cells; DC, Deiters’ cells; HP, habenula perforata; LER, lesser epithelial ridge; GER, greater epithelial ridge. Scale bars: 25 μm in A,J,J′; 50 μm in C-I.
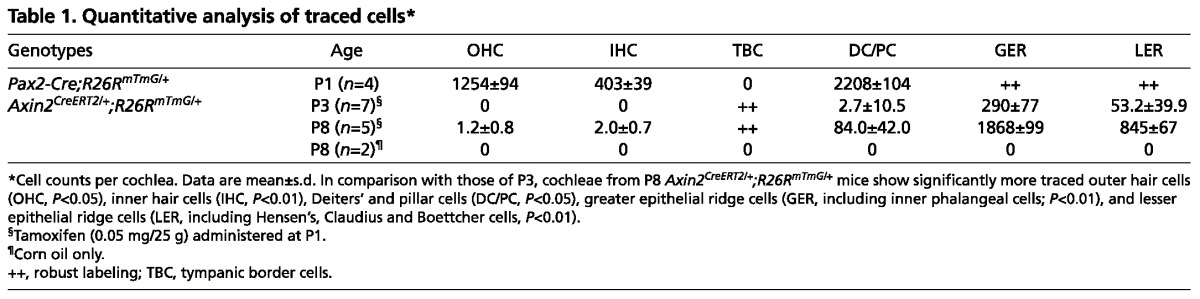
To investigate whether there is a lineage relationship between the SE cells and TBCs, we first examined cochleae from the Pax2-Cre;R26RmTmG/+ (Rosa26-mTmG) transgenic mice (Muzumdar et al., 2007; Ohyama and Groves, 2004). Pax2 is expressed in the otic placode and developing otocyst and Pax2-positive cells have been shown to contribute to the cochlear ductal epithelium (Basch et al., 2011; Ohyama and Groves, 2004). However, their relationship with TBCs is unclear. Crossing the Pax2-Cre mouse strain with the R26RmTmG double reporter strain allowed us to examine the identity of cells derived from Pax2 expressing cells. The presence of Cre recombinase initiates expression of mGFP reporter while switching off the mTomato signal. In the P1 cochlea, mGFP-positive cells occupied the majority of the SE, including hair cells and supporting cells, whereas TBCs were not labeled (Fig. 1J; supplementary material Fig. S1F; Table 1). In contradistinction, Brn4 expression is undetectable from the sensory epithelium and robust in the periotic mesenchymal cells in the embryonic period, and was transiently present in TBCs in the early postnatal period (supplementary material Fig. S2A-C) (Ahn et al., 2009; Phippard et al., 1999). We performed lineage tracing of Brn4-expressing cells using the B4OE-Cre;R26RtdTomato/+ mouse strain, and found a subset of TBCs traced in the P1 cochleae (not shown). These results show that TBCs originate from Brn4-expressing cells outside the cochlear ductal epithelium.
Table 1.
Quantitative analysis of traced cells*
Ongoing proliferation in the early postnatal cochlea
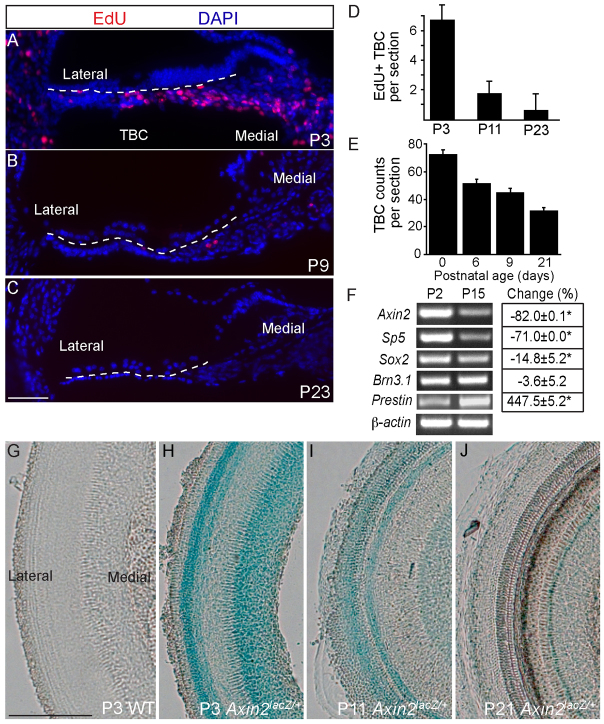
Because the SE continues to mature during the first 3 weeks of postnatal development, we further investigated TBCs during this period. When the thymidine analog 5-ethynyl-2′-deoxyuridine (EdU) was administered for 2 days, robust label uptake was seen in TBCs in the P3 wild-type cochleae (6.8±1.2 EdU-positive TBCs per turn), less frequent uptake in the P9 cochleae (1.8±0.9) and there was rare uptake at P23 (0.7±1.2); at all three ages, there was no uptake into the SE cells (Fig. 2A-D). These data show that TBCs proliferate until the second week of postnatal development, in contrast to the quiescent SE cells. Interestingly, the number of TBCs declined during the first 3 postnatal weeks, and they cease to proliferate between the first and second postnatal week, while the anatomy of this region gradually transitions from a three- to four-cell layered structure at P3 to a one- to two-cell layer by the second postnatal week (Fig. 2A-C,E).
Fig. 2.
Proliferation and Wnt signaling decreased with age in the postnatal cochlea. (A-C) Cryosections of cochleae from mice administered EdU once daily for 2 days. EdU-labeled TBCs decreased from P3 to P9, and further decreased from P9 to P23. (D) Quantification of EdU-positive TBCs (n=3-10). (E) The number of TBCs (between the lateral cochlear wall and habenula perforata) decreases with age, n=6 for each age. (F) Using RT-PCR and qPCR, cochleae demonstrated a significant decrease in the Wnt target genes (Axin2 and Sp5) (P<0.005 for both) between P2 and P15; experiments were carried out in triplicate. Levels of the hair cell marker (Brn3.1) remained constant (P=0.24). Levels of the supporting cell marker (Sox2) decreased (P<0.05) and those of the outer hair cell gene Prestin increased (P<0.001). (G-J) Axin2lacZ expression in the postnatal cochlea decreased with age. Scale bars: 25 μm in A-C; 200 μm in G-J. Data are mean±s.d. Asterisk indicates statistical significance.
Concomitant with the decline in proliferation and quantity of TBCs, mRNA expression levels of the Wnt target genes Axin2 and Sp5 in the cochlea decreased, whereas expression of the outer hair cell gene Prestin (Slc26a5) was elevated. Decreased expression of Sox2, a supporting cell marker, correlated with the natural maturation of the organ of Corti (Fig. 2F). The hair cell-specific gene Brn3.1 is important for hair cell specification (Erkman et al., 1996) and its expression levels did not change significantly. In Axin2lacZ/+ cochleae, LacZ was expressed at P3 and declined over the subsequent 2-3 weeks (Fig. 2G-J; supplementary material Fig. S1C,D).
These experiments show that TBCs beneath the organ of Corti display a robust but transient proliferative potential during the first postnatal week in vivo, after which proliferation rapidly decreases. This decline in proliferation is accompanied by decreased expression levels of Wnt target genes as well as increased expression of mature cell markers. These observations led to the hypothesis that high levels of Wnt signaling might be indicative of progenitor cell features, and we therefore investigated whether Axin2-positive cells can behave as progenitor cells for the SE.
A subset of sensory epithelial cells are derived from Axin2-positive tympanic border cells
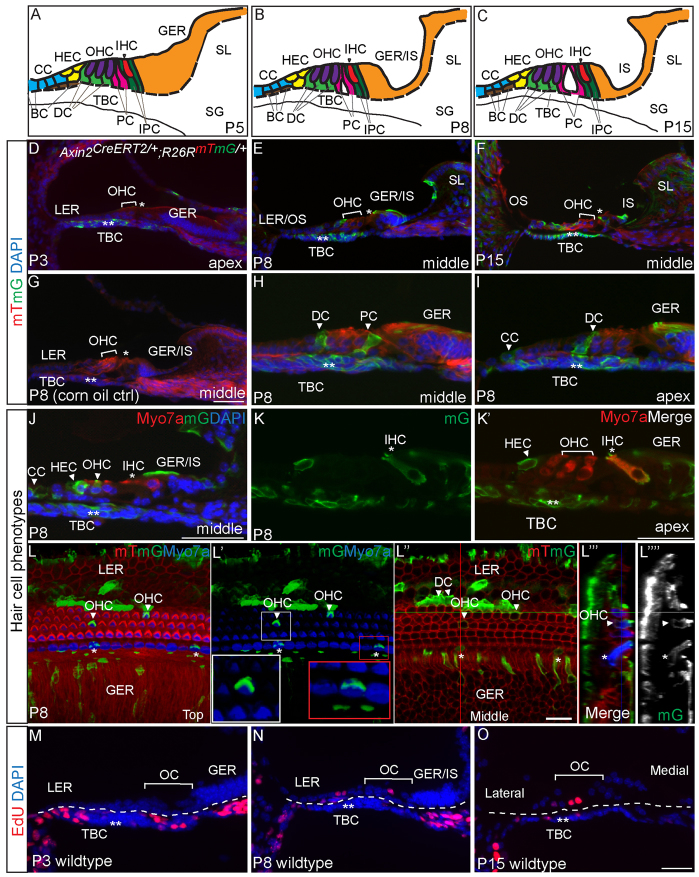
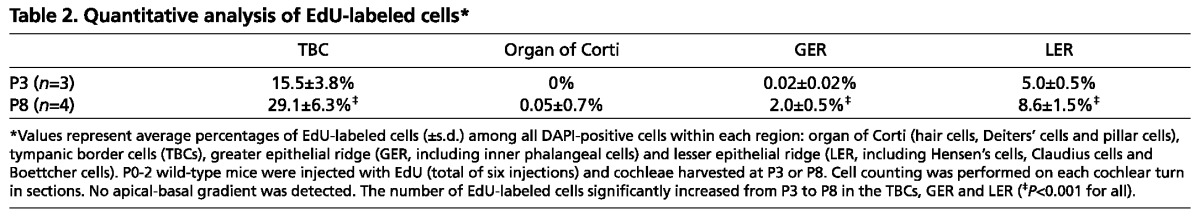
The architecture of the organ of Corti is dynamic during the first 3 postnatal weeks, and SE cell types are distinguished by their anatomic locations and specific markers (Fig. 3A-C). Using lineage-tracing experiments, we tested the hypothesis that TBCs can act as progenitor cells for the cochlear SE in vivo during this period. We generated an Axin2CreERT2 mouse strain (van Amerongen et al., 2012) and crossed it with the R26RmTmG reporter strain to examine cells produced by Axin2-positive TBCs. A single injection of tamoxifen on P1 or P3 activated Axin2-driven Cre recombinase to initiate mGFP reporter activity while switching off the mTomato signal 2 days post injection (DPI) (Fig. 3D; supplementary material Fig. S3A,B; Movies 1, 2). mGFP expression was first observed in the TBCs at 2 DPI and was not detected in cochleae of mice that received drug vehicle alone (Fig. 3D,G). At 5 and 7 DPI, mGFP-positive cells occupied the SE and constituted distinct supporting cell types, which are specified by their anatomical locations (Fig. 3E,H,I; supplementary material Fig. S3C-F). No apical-basal gradient of traced cells was observed (not shown). Quantitative analyses showed that the number of mGFP-positive cells within the population of sensory hair cells, supporting cells, and greater and lesser epithelial ridges (GER and LER) significantly increased from P3 to P8 (P<0.05, Table 1). These cells remained integrated in the P15 cochleae (at 12 DPI) and included Deiters’ and pillar cells (Fig. 3F), which are defined by the expression of Sox2, Prox1, E-cadherin and jagged 1 (Fig. 1D-F; supplementary material Fig. S6F). mGFP-positive cells were also seen among Hensen’s (Sox2 and E-cadherin positive) (Fig. 3J; supplementary material Fig. S3C,H,I), Claudius, Boettcher (both E-cadherin-positive) (Fig. 3I,J; supplementary material Fig. S3D) and GER cells (Sox2, E-cadherin and jagged 1 positive) (Fig. 3E,F,H-L; supplementary material Fig. S3C-F,H,I). These findings suggest that TBCs can migrate into the organ of Corti and adjacent tissues and differentiate into multiple cochlear supporting cell types. On occasion, we found outer and inner hair cells that were mGFP positive (Fig. 3J-L; supplementary material Fig. S3E,F; Movies 1, 2). Hair cells are distinguished by their anatomical locations, expression of myosin 7a and stereociliary hair bundles on their apical surfaces (Fig. 3J-L; supplementary material Fig. S3E,F; Movies 1, 2). Outer hair cell stereocilia are organized in a V-shaped pattern, which is observed in mGFP-positive outer hair cells (Fig. 3L; supplementary material Movies 1, 2). The finding that both inner and outer hair cells were mGFP-positive further supports the idea that TBCs have the potential to generate multiple cell types in the SE.
Fig. 3.
Lineage tracing of Axin2-positive tympanic border cells (TBC). (A-C) Schematics depicting the dynamic cytoarchitecture of the P5, P8 and P15 organ of Corti: formation of the tunnel of Corti and the inner sulcus (IS); resorption of the GER; and a decrease in TBC number. (D-F) Tamoxifen administration to P1 or P3 Axin2CreERT2/+; R26RmTmG/+ mice resulted in selective mGFP labeling of Axin2-positive cells. Two days post-injection (DPI), TBCs became mGFP labeled, with rare mGFP-positive medial GER cells seen. There is a robust increase in the number of mGFP-positive cells in the SE with longer post-injection periods, whereas TBCs remained mGFP positive (Table 1). (G) Corn oil alone failed to induce mGFP reporter activity. (H,I) A subset of each supporting cell subtype was mGFP positive, whereas mGFP-labeled TBCs increased in number from 59.3±10.7% at P3 to 89.7±6.4% at P8 (P<0.001, n=3-4). (J,K) mGFP-positive inner and outer hair cells expressed myosin 7a and exhibited stereocilia-like projections on the apical surface. (L-L″″) 3D-reconstructed images of traced cochlea (7 DPI). Insets in L′ depict higher-magnification views of traced OHC and IHC. L″ shows image taken at the level of hair cells’ cell bodies. L″′ and L″″ are reconstructed images. (M-O) EdU labeling shows that TBCs, and not organ of Corti cells, proliferated between P0 and P2. Six and 13 days after injection, EdU-labeled cells were found within the organ of Corti. Rare EdU-positive, GFP-positive cells were seen in the SE (supplementary material Fig. S3M-O). BC, Boettcher cells; CC, Claudius cells; PC, pillar cells; IPC, inner phalangeal cells; GER, greater epithelial ridge; LER, lesser epithelial ridge; IS, inner sulcus; OS, outer sulcus; SL, spiral limbus; SG, spiral ganglia; OC, organ of Corti; single asterisk indicates IHC; double asterisks indicate TBCs. Scale bars: 25 μm in D-J,M-O; 15 μm in K-L″″.
To examine the possibility that delayed Cre activity could lead to reporter activity within the SE, we administered tamoxifen at P8 and found mGFP expression limited to TBCs 2 DPI (supplementary material Fig. S3J), confirming tight regulation of the Axin2CreERT2 activity. Administration of EdU (50 mg/kg twice daily, P0-2) revealed EdU-positive cells among the TBCs (15.5±3.8%), but none in the organ of Corti at P3 (Fig. 3M; Table 2). Among cells in the lesser (LER) and greater epithelial ridges (GER), 5.0±0.5% and 0.02±0.02% were EdU positive, respectively (n=3). P8 and P15 cochleae revealed that the EdU-labeled cells occasionally contributed to organ of Corti cells (Fig. 3N,O). At P8, EdU labeled significantly more cells: 29.1±6.3% TBCs, 8.6±1.5% LER cells and 2.0±0.5% GER cells [P<0.001 for all (n=4), Table 2]. Among four cochleae, we detected three EdU-positive cells in the organ of Corti. Concurrent EdU and tracing experiments identified occasional EdU, mGFP double-positive SE cells at P8, but also untraced EdU-positive cells within the SE (supplementary material Fig. S3M-O). Together, these data suggest that Axin2-positive TBCs are multipotent progenitor cells in the postnatal mouse cochlea.
Table 2.
Quantitative analysis of EdU-labeled cells*
Isolated Axin2hi cells from the cochlea form clonal colonies
To test the in vitro potential of Axin2-positive TBCs, we used flow cytometry to isolate cochlear Axin2-expressing cells for further analyses (Jan et al., 2011). Using cochleae from P0-2 Axin2lacZ/+ mice, we labeled and isolated the most fluorescent cells (top 16.1±0.6%), expressing high levels of the Wnt target genes Axin2 and Sp5, and the fewest fluorescent cells (bottom 23.2±2.3%), which expressed hair cell (Brn3.1) and supporting cell (p27Kip1 and Sox2) genes (Fig. 4A,B,H; supplementary material Fig. S4A). We designed parameters to isolate the least fluorescent cells to minimize contamination and allow comparisons between the two groups based on the levels of Axin2 expression. These two groups are henceforth referred to as Axin2hi and Axin2lo cells, respectively. To confirm cell purity, we immunostained cells immediately post-sort and found that the Axin2hi cells contained over 93% β-galactosidase-positive cells, 0% myosin 7a-positive hair cells, 0% Prox1-positive supporting cells, 75% Brn4-positive cells and <0.1% other supporting cell types (Sox2-, Jag1- or p27Kip1-positive; n=3 sorts with 2500-5000 DAPI-positive cells analyzed) (Fig. 4C-G; supplementary material Fig. S4B-F).
Fig. 4.
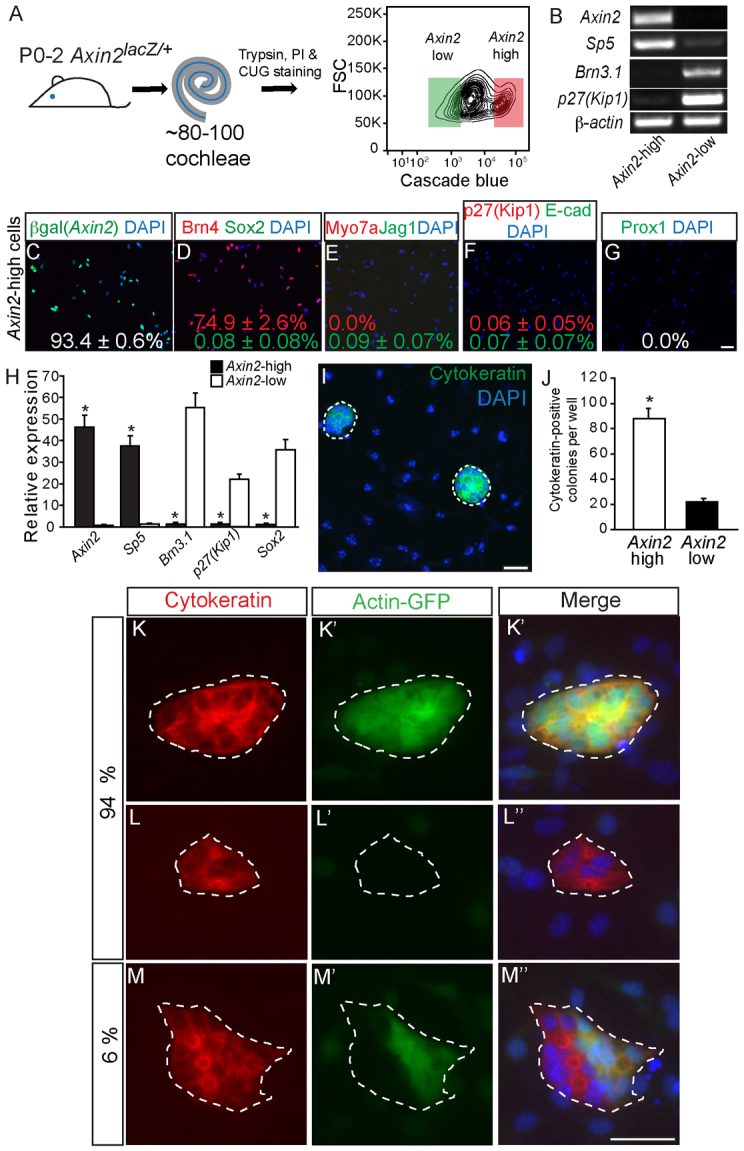
Purified Axin2hi cells display robust proliferative capacity. (A) P0-P2 Axin2lacZ/+ cochleae were dissociated and labeled with propidium iodide (PI) and CUG to allow for isolation of PI-negative (viable), CUG-positive cells. The flow cytometry plot depicts gates used for isolation of the Axin2hi (red) and Axin2lo (green) cells. (B-H) Isolated CUG-positive cells robustly expressed the Wnt target genes Axin2 and Sp5, and not the hair cell marker Brn3.1 or the supporting cell markers p27Kip1 and Sox2 (P<0.01 for all). Experiments were carried out in triplicate. Immunostaining of CUG-positive cells shows 93.4±0.6% β-gal-positive, 74.9±2.6% Brn4-positive, 0.0% myosin 7a-positive hair cells (0.0%) and rare (<0.1%) (Sox2-, jagged 1-, p27Kip1-, E-cadherin or Prox1-positive) supporting cells. (I,J) After 10 days in vitro, Axin2hi cells formed cytokeratin-positive colonies more frequently than Axin2lo cells (88.2±19.4 versus 22.0±6.2, P<0.0001, n=5). (K-M″) Axin2hi cells isolated from both Actin-GFP-positive Axin2lacZ/+ cochleae and Actin-GFP-negative Axin2lacZ/+ cochleae were mixed 1:1 and cultured. After 10 days, 94% of cytokeratin-positive colonies were monochromatic, suggesting Axin2hi colonies were highly clonal (n=3). Scale bars: 25 μm. Data are mean±s.d. Asterisks indicate statistical significance.
We first compared the proliferative capacity of Axin2hi and Axin2lo cells using a colony formation assay: cells were seeded in serum-free, N2/B27-supplemented media onto feeder cells harvested from embryonic chicken utricles (supplementary material Fig. S4G). The use of embryonic otic mesenchymal tissues and deprivation of growth factors has been shown to promote differentiation of inner ear progenitor cells (Chai et al., 2012; Doetzlhofer et al., 2004; Oshima et al., 2010; Sinkkonen et al., 2011; White et al., 2006). After 10 days in vitro, all newly generated colonies were immunopositive for the epithelial marker cytokeratin. Moreover, Axin2hi cells formed fourfold more colonies than the Axin2lo cells (88.2±19.4 versus 22.0±6.2 colonies per 7000 plated cells, P<0.0001) (Fig. 4I,J). To determine whether these colonies arose from individual cells, Axin2hi cells were isolated from cochleae of Axin2lacZ/+ mice that were transgenic for GFP driven by the ubiquitous Actin promoter and mixed (1:1) with those from GFP-negative Axin2lacZ/+ cochleae (supplementary material Fig. S4G). When this mixture of cells was cultured in the above conditions, we found that 94% of the colonies were monochromatic and thus clonal (Fig. 4K-M). These data demonstrate that isolated TBCs from the postnatal cochlea have colony-forming capacity.
Axin2hi cells differentiate into hair cell-like and supporting cell-like cells in vitro
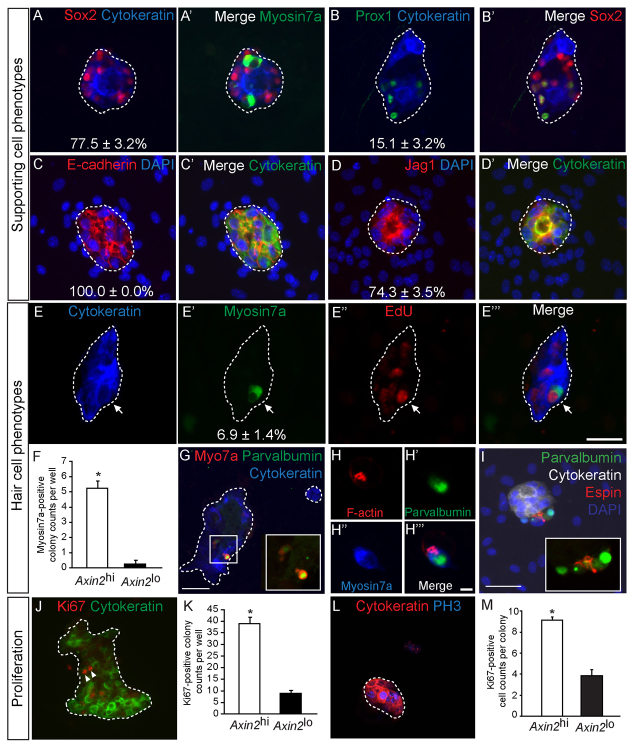
The cochlear SE comprises two main cell types: supporting cells and hair cells. After 10 days, colonies derived from the Axin2hi cells uniformly expressed E-cadherin and cytokeratin, both epithelial markers expressed in the SE (Fig. 5A-D). This observation suggests that the isolated TBCs are able to adopt epithelial characteristics in vitro, similar to their behavior in vivo. In the postnatal organ of Corti, Sox2 is a marker of supporting cells and Prox1 is expressed in two subtypes of supporting cells (Deiters’ cells and pillar cells) (Fig. 1E; supplementary material Fig. S6F) (Bermingham-McDonogh et al., 2006; Oesterle et al., 2008). Colonies derived from Axin2hi cells expressed Sox2 and Prox1 (77.5±3.2% and 18.2±0.6%, respectively) with Prox1-positive cells always co-expressing Sox2 (Fig. 5A-B). In addition, jagged 1 was also highly expressed (74.3±3.5%) in TBC-derived colonies (Fig. 5D). Control experiments ascertain that chicken mesenchymal cells do not express used markers: cytokeratin, Prox1, jagged 1, myosin 7a, Sox2 or E-cadherin (supplementary material Fig. S4H-K). As TBCs do not express Sox2, Prox1, jagged 1, E-cadherin or cytokeratin in vivo (Fig. 1D-G), the finding that their progeny can express these markers in vitro implies that isolated TBCs have an enhanced potential to acquire an epithelial phenotype and display markers that are specific for SE supporting cells.
Fig. 5.
Purified Axin2hi cells acquire hair cell and supporting cell phenotypes. (A-D′) Axin2hi cells formed colonies uniformly expressed cytokeratin and E-cadherin, whereas 77.5±3.2%, 74.3±3.5% and 15.1±3.2% of all cytokeratin-positive colonies expressed Sox2, jagged 1 and Prox1, respectively (n=3 for each, median=22 Sox2-positive cells, 22 jagged 1-positive cells and 4 Prox1-positive cells per colony). (E-E″′) In the presence of EdU, myosin 7a-positive, EdU-positive hair cell-like cells were observed on cytokeratin-positive colonies (arrow) (n=4). (F) Myosin 7a-positive hair cells were observed more frequently among Axin2hi (median indicates one myosin 7a-positive cell per colony) than Axin2lo colonies (P<0.005, n=4). (G) All myosin 7a-positive hair cell-like cells also expressed parvalbumin. (H-H″′) A polarized pattern of filamentous actin on the hair cell-like cells was observed. (I) Two parvalbumin-positive hair cell-like cells with espin expression localized to one pole of the cell. (J,K) Cytokeratin-positive colonies containing actively proliferating Ki67-labeled cells (arrowheads) were noted more commonly in Axin2hi than in the Axin2lo colonies (P<0.001, n=3). (L) Within each Axin2hi colony, a subset of cells expressed the M-phase marker phosphohistone 3 (PH3). (M) There were more Ki67-positive cells in Axin2hi than Axin2lo colonies (P<0.0001, n=3). Scale bars: 25 μm in A-E″′,I,L; 50 μm in G,J; 5 μm in H-H″′. Data are mean±s.d. Asterisks indicate statistical significance.
Within the cochlear SE, hair cells are marked by antibodies to myosin 7a (Hasson et al., 1997) and parvalbumin (Hackney et al., 2005). The apical surfaces of hair cells contain highly ordered stereocilia bundles and cuticular plates, which are enriched in filamentous actin and espin, an actin-bundling protein that is essential for hearing function (Zheng et al., 2000). Myosin 7a-positive hair cell-like cells were infrequently noted among Axin2hi colonies (6.9±1.4%); when present, they were always juxtaposed to Sox2-positive cells (Fig. 5A) and detected in 9.4±0.9% of Sox2-positive colonies. Although purified Axin2lo cells initially expressed high levels of the hair cell marker Brn3.1 (Fig. 4B,H), myosin 7a-positive hair cell-like cells were more frequently observed in the Axin2hi colonies after 10 days in vitro (P<0.005) (Fig. 5F). When EdU was present during the first 3 days of the culture period, some of the hair cell-like cells were EdU labeled, suggesting that Axin2hi cells generated new sensory cells via mitotic divisions (Fig. 5E; supplementary material Fig. S5A), although most myosin 7a-positive cells were EdU negative (supplementary material Fig. S5B). The hair cell-like cells also co-expressed parvalbumin and exhibited stereocilia-like structures, as indicated by a polarized expression pattern of filamentous actin and espin (Fig. 5G-I). Interestingly, we observed that a subset of cells within each type of colony remained proliferative (9.2 Ki67-positive cells per Axin2hi colony versus 3.9 per Axin2lo colony; Fig. 5M). Overall, significantly more Ki67-positive colonies were derived from the Axin2hi than from the Axin2lo cell populations (P<0.001) (Fig. 5J-K). Labeling for the M-phase marker phosphohistone 3 further demonstrated that a subset of cells within Axin2hi colonies was proliferating (Fig. 5L). In summary, these findings demonstrate the heterogeneity of colonies originating from the Axin2-positive TBCs; some progeny differentiated into cell types resembling supporting cells and hair cells of the cochlear SE, whereas others remained proliferative. We next investigated whether proliferation of these colony-forming cells is regulated by Wnt signaling.
Wnt proteins stimulate proliferation of Axin2-positive cells in vitro
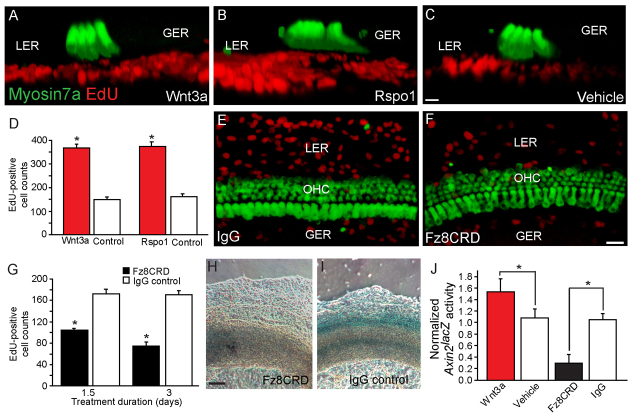
Because Axin2 is a marker of active canonical Wnt signaling, we tested whether addition of Wnt proteins would enhance the proliferative capacity of Axin2-positive TBCs. Wnt3a treatment increased the number of Ki67-positive Axin2hi colonies, whereas addition of the Wnt inhibitor Fz8CRD, a soluble analog of Frizzled receptors that can inhibit the activity of Wnt proteins (DeAlmeida et al., 2007), suppressed the formation of Ki67-positive Axin2hi colonies (Fig. 6A-E; supplementary material Table S1). Ki67-positive colonies were rare in the Axin2lo group and their numbers were not affected by Wnt3a/Fz8CRD treatment, suggesting that the Axin2hi, and not Axin2lo, cells were competent to respond to Wnt proteins. Individual Axin2hi colonies, but not Axin2lo colonies, demonstrated an increase in Ki67-positive cells and in Axin2 expression in response to Wnt3a treatment (Fig. 6A-G; supplementary material Table S1). However, the number of colonies with myosin 7a-positive hair cells did not change significantly with Wnt3a/Fz8CRD treatment (supplementary material Fig. S5C). These data suggest that Wnt proteins promote proliferation and expansion of the Axin2hi cells. To further examine this possibility, we cultured purified Axin2hi and Axin2lo cells from Actin-DsRed-positive Axin2lacZ/+ cochleae in serum-free N2/B27 media containing Wnt3a proteins without other factors. We measured proliferative capacity of the cells by quantifying DsRed-positive colonies and found that the Axin2hi cell lineage had a significantly higher colony-forming capacity than Axin2lo cell lineage (P<0.01, one-way ANOVA). Wnt3a treatment further increased the number of colonies over multiple passages (P<0.05) (Fig. 6H-J). However, Axin2lo colonies failed to respond to Wnt3a treatment. After two cycles of expansion, the number of Axin2hi colonies increased 319-fold in comparison with those from the Axin2lo cell lineage, and this difference further increased to 924-fold with Wnt3a treatment. Similar to their first generation precursors, these expanded colonies from the Axin2hi cell population displayed an epithelial phenotype, expressed cytokeratin, and expressed markers for hair cells (myosin 7a) and supporting cells (Sox2) (Fig. 6K,L). All Sox2-positive and myosin 7a-positive cells formed within colonies were Actin-DsRed positive, indicating that they are derived from mouse tissues and not chicken mesenchymal cells (supplementary material Fig. S5D,E). With expansion via passaging, the percentage of Sox2-positive colonies (∼60%) was maintained with Wnt3a supplementation (supplementary material Fig. S5F), whereas that of myosin 7a-positive colonies declined (first generation=8.9±0.4%, second generation=5.0±1.2%, third generation=1.2±1.5%; none detected in subsequent generations). These findings lend credence to the notion that Wnt proteins act as growth factors to purified Axin2hi TBCs from the cochlea.
Fig. 6.
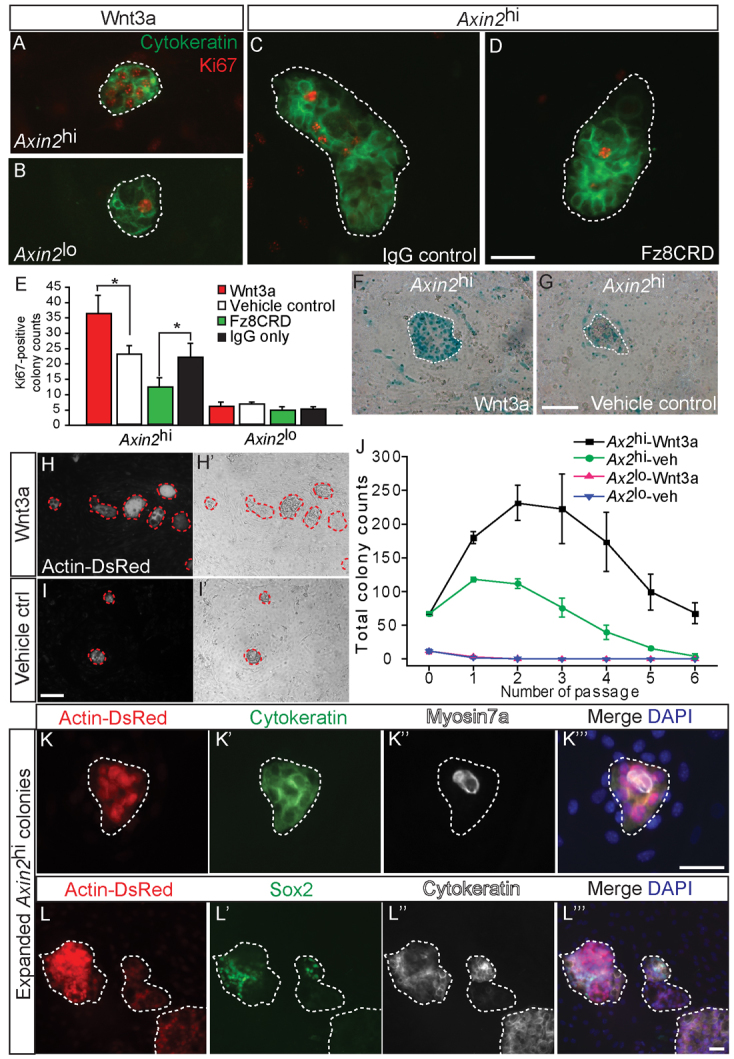
Wnt3a protein acts as a proliferation stimulant on Axin2-positive tympanic border cells and their descendants. (A-E) When cultured with Wnt3a for 10 days, only Axin2hi colonies significantly upregulated Ki67 expression (P<0.05). Conversely, the soluble Wnt antagonist Fz8CRD effectively suppressed Ki67-positive colonies in comparison with IgG-only control (P<0.005, n=3-5). (F,G) Wnt3a-treated Axin2hi colonies robustly expressed Axin2 when compared with vehicle-only control. (H-J) Axin2hi and Axin2lo cells from Actin-DsRed-positive Axin2lacZ/+ cochleae were cultured and DsRed-positive colonies were examined. Axin2hi cells had higher colony forming capacity than Axin2lo cells (P<0.01, one-way ANOVA). Wnt3a treatment significantly increased the Axin2hi and not the Axin2lo colonies (P<0.05, one-way ANOVA, n=3). (K-L″′) Third generation colonies from Wnt3a-treated Axin2hi cells expressed myosin 7a and Sox2 (supplementary material Fig. S5F). Scale bars: 25 μm in A-D,F,G,K-L″′; 50 μm in H-I′. Data are mean±s.d. Asterisks indicate statistical significance.
We further investigated the effects of endogenous Wnt signals on the Axin2-positive TBCs in cochlear explants, where architecture of the organ of Corti is preserved. We manipulated the Wnt pathway with Wnt3a, R-spondin 1 and Fz8CRD. R-spondin 1 is an alternative Wnt agonist that acts synergistically with endogenous Wnt proteins (Kim et al., 2006; Ootani et al., 2009). Wnt3a or R-spondin 1 treatment robustly enhanced TBC proliferation (Fig. 7A-C; supplementary material Fig. S6A-C,F; Movie 3). Quantitative analyses of Ki67- or EdU-positive cells indicated that Wnt activation induced a doubling of the baseline proliferation, and that this mitogenic effect was evident for at least 5 days in vitro (Fig. 7D; supplementary material Table S2). Like isolated Axin2hi cells, TBCs were competent to respond to exogenous Wnt agonists by showing a marked increase in proliferation, which was not observed in the SE. In addition, proliferation among TBCs was highly dependent on Wnt signaling as Fz8CRD effectively reduced EdU-positive and Ki67-positive cells and suppressed Axin2 expression (Fig. 7E-I; supplementary material Fig. S6D,E; Table S2). We used ELISA to quantify lacZ (Axin2) expression in cochlear cultures and found it to be significantly enhanced by Wnt3a treatment (P<0.01) and inhibited by Fz8CRD treatment (P<0.005) (Fig. 7J). Based on these observations, we conclude that the proliferative capacity of Axin2-positive TBCs is dependent on Wnt signaling.
Fig. 7.
Wnt activity modulates proliferation and Axin2 expression in cochlear explants. (A-D) 3D-reconstructed images show that Wnt3a and R-spondin 1 significantly (P<0.001 for both, n=3) and selectively increased EdU labeling among TBCs beneath the sensory epithelium in cultured wild-type cochleae. (E-G) Wnt inhibition with Fz8CRD significantly reduced EdU-labeled TBCs (P<0.0001 for both the 36- and 72-hour treatments, n=3-4) in comparison with IgG control. (H,I) Fz8CRD suppressed LacZ (Axin2) expression in cultured Axin2lacZ/+ cochleae. (J) ELISA studies show that Wnt3a significantly enhanced β-galactosidase activity in cultured Axin2lacZ/+ cochleae (P<0.01), whereas Fz8CRD reduced it (P<0.005, n=3). Scale bars: 15 μm in A-C,E,F; 100 μm in H,I. Data are mean±s.d. Asterisks indicate statistical significance.
DISCUSSION
Sensory hair cells are mechanoreceptors that are required for auditory function; their irreversible loss leads to permanent hearing loss. Previously thought to lack regenerative capacity, the early postnatal cochlea was recently shown to harbor cells with colony-forming capacity and the potential to generate new hair cell-like cells in vitro (Chai et al., 2012; Oshima et al., 2007; Savary et al., 2008; Shi et al., 2012; Sinkkonen et al., 2011; White et al., 2006; Zhang et al., 2007). In defined culture conditions, supporting cells isolated from SE exhibit both of these qualities and therefore are prime candidates as progenitor cells (Sinkkonen et al., 2011; White et al., 2006). In vivo, we and others have found no evidence of label incorporation in the organ of Corti immediately after EdU injection, suggesting that proliferation is either rare or non-existent. Conversely, robust proliferation is seen among TBCs, a distinct, yet poorly characterized, cell population beneath the SE. Also known as mesothelial cells, TBCs were first described in mammals by Claudius (Claudius, 1856), and subsequently in more detail in other species, including humans (Bhatt et al., 2001; Cabezudo, 1978; Keithley et al., 1994). Because of their close association with the basilar membrane, TBCs have been suggested to secrete extracellular matrix proteins (Amma et al., 2003). Others have proposed a biomechanical role for the TBCs, as their number varies along the cat cochlea (Cabezudo, 1978). However, their exact physiological function(s) remain unclear.
Our study provides the first pieces of in vitro and in vivo evidence that Axin2-positive TBCs can behave as progenitor cells in the postnatal cochlea. Wnt/β-catenin signaling is crucial for the maintenance of stem cell niches, thereby promoting stem cell self-renewal in many mammalian systems (Barker et al., 2007; Kalani et al., 2008; Lie et al., 2005; Zeng and Nusse, 2010). The expression of Axin2 is a marker for active Wnt signaling in many cell types (Kalani et al., 2008; Lustig et al., 2002; Zeng and Nusse, 2010). Using Axin2lacZ mice to isolate Wnt-responsive cells in the postnatal cochlea, we discovered that Axin2-positive TBCs have colony-forming capacity. Furthermore, they can differentiate to multiple cell types, including supporting cells and hair cells both in vitro and in vivo. Therefore, our data suggest that TBCs can act as progenitor cells in the neonatal cochlea.
As demonstrated for somatic stem cells in other organ systems (Barker et al., 2010; Ootani et al., 2009; Willert et al., 2003; Zeng and Nusse, 2010), and recently in the embryonic and postnatal cochlea (Chai et al., 2012; Jacques et al., 2012; Shi et al., 2012), Wnt agonists act as growth factors for TBCs. However, Wnt inhibition decreases the self-renewal capacity of TBCs, but it does not affect the frequency of hair cell differentiation, which may operate in a Wnt-independent manner. Interestingly, we frequently observed supporting cells among the progeny of TBCs in vitro as well as in vivo, suggesting that TBCs more readily differentiate into supporting cells than into sensory hair cells in both microenvironments. The mechanisms regulating this differentiation are currently unknown and warrant further investigation.
Our lineage tracing and thymidine analog labeling results further suggest that the postnatal organ of Corti remains dynamic, contradicting the previous assumption that hair cell formation is complete by E16.5 in mice (Kelley, 2007) and failed detection of proliferated cells in the postnatal organ of Corti (Ruben, 1967). Instead, our findings correspond with data from other mammalian species suggesting that postnatal hair cell addition occurs (Kaltenbach and Falzarano, 1994; Mu et al., 1997). Given that our results are surprising, it is important to point out that the incorporation of proliferating cells into the SE is rare (Table 2). Moreover, detecting proliferating cells resulted from six injections of EdU over a 3-day period, in contrast to fewer rounds of potentially more cytotoxic thymidine analogs used previously by others (Lee et al., 2006; Ruben, 1967). One candidate mechanism for such cell addition is migration of TBCs across the basilar membrane. In the embryonic inner ear, gaps in extracellular matrix proteins may mediate the process of delamination when neuroblasts migrate out of the otic epithelium (Davies, 2011). Moreover, neuroepithelial derivatives migrate into the otic epithelium through gaps in the basal lamina (Freyer et al., 2011). These gaps are characterized by the absence of fibronectin, which is also observed in the basilar membrane in the neonatal cochlea (Fig. 1I). Together, our data support the notion that TBCs may also serve as a pool of progenitors for SE cells in the postnatal cochlea.
Although the current study examines the Axin2-positive TBCs as a single cell population, it is more likely that TBCs are a heterogeneous population that consists of cells with different potentials for proliferation and differentiation. Lineage tracing and thymidine analog labeling experiments showed that the progeny of Axin2-positive TBCs in the P1-3 cochleae appear in the SE in older cochleae, while others remain in the TBC region. Likewise, purified Axin2hi cells generated colonies with differentiated SE cells and Ki67-positive proliferative cells in vitro. In vivo, the low number of EdU-positive traced cells may have resulted from the stochastic nature of lineage tracing and the limited efficiency of the EdU labeling assay, which may be better addressed by using mosaic analysis with double markers (MADM) in future experiments (Zong et al., 2005).
Our results raise several important questions, including how is cell fate of individual TBCs determined, what is the mechanism underlying the decrease in TBCs during the first 3 weeks of postnatal development, and what are the roles of the Axin2-positive cells that have been demonstrated to surround the embryonic cochlear duct (Chai et al., 2011)? Do TBCs from older animals retain such progenitor cell potentials? It is plausible to consider applying Wnt agonists to TBCs in the adult cochlea in an attempt to rekindle such progenitor cell potentials. Stimulation of progenitor cell capacity in the mature cochlea is of clinical interest, especially in the context of regenerating the damaged organ of Corti. Although it has been reported that TBCs show increased proliferation post-injury through 3H-thymidine uptake in adult mammals (Roberson and Rubel, 1994; Sobkowicz et al., 1992), no regeneration of sensory cells was noted. The current study raises the possibility that enhancing Wnt signaling may promote regeneration of the damaged inner ear, an approach that has shown promise in the context of bone regeneration (Minear et al., 2010).
In summary, we have characterized tympanic border cells as Wnt-dependent progenitor cells and have demonstrated their potential to become sensory epithelial cells both in vitro and in vivo. We postulate that TBCs may potentially serve as a reservoir of cells that function as a fail-safe mechanism for the intricate organization of the organ of Corti during early postnatal development. This may explain the proliferative nature of these cells and their subsequent decrease in proliferation and decline in number as the organ matures. Nonetheless, there exists a remnant quiescent population of TBCs in the adult cochleae that may serve as potential targets of regenerative therapeutics. However, any future therapy will require our full understanding of the mechanisms that help guide TBCs to migrate and differentiate into functional sensory cells.
Supplementary Material
Acknowledgments
We thank A. Ricci, L. Cunningham, O. Bermingham-McDonogh and members of our laboratories for fruitful discussions; K. Ahn, P. White and T. Hayashi for excellent technical assistance; A. Groves for sharing the Pax2-Cre mouse; and B. Crenshaw for sharing the B4OE-Cre mouse and anti-Brn4 antibodies.
Footnotes
Funding
This work was supported by the Howard Hughes Medical Institute (HHMI) Medical Research Training Fellowship; Stanford Medical Scholars program [T.A.J.]; Stanford Dean’s Fellowship; Hearing Health Foundation [R.C.]; an EMBO long-term fellowship [ALTF 122-2007]; a KWF fellowship from the Dutch Cancer Society [to R.v.A.]; the National Institutes of Health [NIDCD/NIH P30 DC010363 and R01 DC006167 to S.H.; and K08 DC011043 to A.G.C.]; HHMI [R.N.]; the American Otological Society; the Triological Society; a Percy Memorial Award; the Akiko Yamazaki and Jerry Yang Faculty Scholar Fund; the National Organization for Hearing Research Foundation [A.G.C.]; and the Stanford Initiative to Cure Hearing Loss. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.087528/-/DC1
References
- Ahn K. J., Passero F., Jr, Crenshaw E. B., 3rd (2009). Otic mesenchyme expression of Cre recombinase directed by the inner ear enhancer of the Brn4/Pou3f4 gene. Genesis 47, 137–141 [DOI] [PubMed] [Google Scholar]
- Amma L. L., Goodyear R., Faris J. S., Jones I., Ng L., Richardson G., Forrest D. (2003). An emilin family extracellular matrix protein identified in the cochlear basilar membrane. Mol. Cell. Neurosci. 23, 460–472 [DOI] [PubMed] [Google Scholar]
- Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H. J., van Es J. H., Sato T., Stange D. E., Begthel H., van den Born M., et al. (2010). Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 [DOI] [PubMed] [Google Scholar]
- Basch M. L., Ohyama T., Segil N., Groves A. K. (2011). Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J. Neurosci. 31, 8046–8058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Oesterle E. C., Stone J. S., Hume C. R., Huynh H. M., Hayashi T. (2006). Expression of Prox1 during mouse cochlear development. J. Comp. Neurol. 496, 172–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K. A., Liberman M. C., Nadol J. B., Jr (2001). Morphometric analysis of age-related changes in the human basilar membrane. Ann. Otol. Rhinol. Laryngol. 110, 1147–1153 [DOI] [PubMed] [Google Scholar]
- Cabezudo L. M. (1978). The ultrastructure of the basilar membrane in the cat. Acta Otolaryngol. 86, 160–175 [PubMed] [Google Scholar]
- Chai R., Xia A., Wang T., Jan T. A., Hayashi T., Bermingham-McDonogh O., Cheng A. G. (2011). Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 12, 455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R., Kuo B., Wang T., Liaw E. J., Xia A., Jan T. A., Liu Z., Taketo M. M., Oghalai J. S., Nusse R., et al. (2012). Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. USA 109, 8167–8172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudius M. (1856). Bemerkungen uber den Bau der hautigen Spiralleiste der Schnecke. Z. Wiss. Zool. 7, 154–161 [Google Scholar]
- Daudet N., Ripoll C., Molès J. P., Rebillard G. (2002). Expression of members of Wnt and Frizzled gene families in the postnatal rat cochlea. Brain Res. Mol. Brain Res. 105, 98–107 [DOI] [PubMed] [Google Scholar]
- Davies D. (2011). Cell-extracellular matrix versus cell-cell interactions during the development of the cochlear-vestibular ganglion. J. Neurosci. Res. 89, 1375–1387 [DOI] [PubMed] [Google Scholar]
- DeAlmeida V. I., Miao L., Ernst J. A., Koeppen H., Polakis P., Rubinfeld B. (2007). The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 67, 5371–5379 [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A., White P. M., Johnson J. E., Segil N., Groves A. K. (2004). In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Dev. Biol. 272, 432–447 [DOI] [PubMed] [Google Scholar]
- Erkman L., McEvilly R. J., Luo L., Ryan A. K., Hooshmand F., O’Connell S. M., Keithley E. M., Rapaport D. H., Ryan A. F., Rosenfeld M. G. (1996). Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature 381, 603–606 [DOI] [PubMed] [Google Scholar]
- Freyer L., Aggarwal V., Morrow B. E. (2011). Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development 138, 5403–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney C. M., Mahendrasingam S., Penn A., Fettiplace R. (2005). The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J. Neurosci. 25, 7867–7875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T., Walsh J., Cable J., Mooseker M. S., Brown S. D., Steel K. P. (1997). Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil. Cytoskeleton 37, 127–138 [DOI] [PubMed] [Google Scholar]
- Jacques B. E., Puligilla C., Weichert R. M., Ferrer-Vaquer A., Hadjantonakis A. K., Kelley M. W., Dabdoub A. (2012). A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development 139, 4395–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V., Barker N., Kasper M., van Es J. H., Snippert H. J., Clevers H., Toftgård R. (2008). Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40, 1291–1299 [DOI] [PubMed] [Google Scholar]
- Jan T. A., Chai R., Sayyid Z. N., Cheng A. G. (2011). Isolating LacZ-expressing cells from mouse inner ear tissues using flow cytometry. J. Vis. Exp. 58, e3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho E. H., Zhang T., Domon C., Joo C. K., Freund J. N., Costantini F. (2002). Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani M. Y., Cheshier S. H., Cord B. J., Bababeygy S. R., Vogel H., Weissman I. L., Palmer T. D., Nusse R. (2008). Wnt-mediated self-renewal of neural stem/progenitor cells. Proc. Natl. Acad. Sci. USA 105, 16970–16975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach J. A., Falzarano P. R. (1994). Postnatal development of the hamster cochlea. I. Growth of hair cells and the organ of Corti. J. Comp. Neurol. 340, 87–97 [DOI] [PubMed] [Google Scholar]
- Keithley E. M., Tian Q., Robins-Browne R. (1994). Fibronectin-like immunoreactivity of the basilar membrane of celloidin-embedded human temporal bone sections. Acta Otolaryngol. 114, 613–619 [DOI] [PubMed] [Google Scholar]
- Kelley M. W. (2007). Cellular commitment and differentiation in the organ of Corti. Int. J. Dev. Biol. 51, 571–583 [DOI] [PubMed] [Google Scholar]
- Kim K. A., Zhao J., Andarmani S., Kakitani M., Oshima T., Binnerts M. E., Abo A., Tomizuka K., Funk W. D. (2006). R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle 5, 23–26 [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Liu F., Segil N. (2006). A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 133, 2817–2826 [DOI] [PubMed] [Google Scholar]
- Lie D. C., Colamarino S. A., Song H. J., Désiré L., Mira H., Consiglio A., Lein E. S., Jessberger S., Lansford H., Dearie A. R., et al. (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Logan C. Y., Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P. M., Birchmeier W., et al. (2002). Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R., et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgrange B., Belachew S., Thiry M., Nguyen L., Rogister B., Alvarez M. L., Rigo J. M., Van De Water T. R., Moonen G., Lefebvre P. P. (2002). Proliferative generation of mammalian auditory hair cells in culture. Mech. Dev. 112, 79–88 [DOI] [PubMed] [Google Scholar]
- Minear S., Leucht P., Jiang J., Liu B., Zeng A., Fuerer C., Nusse R., Helms J. A. (2010). Wnt proteins promote bone regeneration. Sci. Transl. Med. 2, 29ra30 [DOI] [PubMed] [Google Scholar]
- Mu M. Y., Chardin S., Avan P., Romand R. (1997). Ontogenesis of rat cochlea. A quantitative study of the organ of Corti. Brain Res. Dev. Brain Res. 99, 29–37 [DOI] [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L., Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 [DOI] [PubMed] [Google Scholar]
- Oesterle E. C., Campbell S., Taylor R. R., Forge A., Hume C. R. (2008). Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J. Assoc. Res. Otolaryngol. 9, 65–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T., Groves A. K. (2004). Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis 38, 195–199 [DOI] [PubMed] [Google Scholar]
- Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. (1997). ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 [DOI] [PubMed] [Google Scholar]
- Ootani A., Li X., Sangiorgi E., Ho Q. T., Ueno H., Toda S., Sugihara H., Fujimoto K., Weissman I. L., Capecchi M. R., et al. (2009). Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K., Grimm C. M., Corrales C. E., Senn P., Martinez Monedero R., Géléoc G. S., Edge A., Holt J. R., Heller S. (2007). Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J. Assoc. Res. Otolaryngol. 8, 18–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K., Shin K., Diensthuber M., Peng A. W., Ricci A. J., Heller S. (2010). Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141, 704–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phippard D., Lu L., Lee D., Saunders J. C., Crenshaw E. B., 3rd (1999). Targeted mutagenesis of the POU-domain gene Brn4/Pou3f4 causes developmental defects in the inner ear. J. Neurosci. 19, 5980–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson D. W., Rubel E. W. (1994). Cell division in the gerbil cochlea after acoustic trauma. Am. J. Otol. 15, 28–34 [PubMed] [Google Scholar]
- Ruben R. J. (1967). Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 220 Suppl., 221–244 [PubMed] [Google Scholar]
- Savary E., Sabourin J. C., Santo J., Hugnot J. P., Chabbert C., Van De Water T., Uziel A., Zine A. (2008). Cochlear stem/progenitor cells from a postnatal cochlea respond to Jagged1 and demonstrate that notch signaling promotes sphere formation and sensory potential. Mech. Dev. 125, 674–686 [DOI] [PubMed] [Google Scholar]
- Shah S. M., Kang Y. J., Christensen B. L., Feng A. S., Kollmar R. (2009). Expression of Wnt receptors in adult spiral ganglion neurons: frizzled 9 localization at growth cones of regenerating neurites. Neuroscience 164, 478–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Kempfle J. S., Edge A. S. (2012). Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J. Neurosci. 32, 9639–9648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkkonen S. T., Chai R., Jan T. A., Hartman B. H., Laske R. D., Gahlen F., Sinkkonen W., Cheng A. G., Oshima K., Heller S. (2011). Intrinsic regenerative potential of murine cochlear supporting cells. Sci. Rep. 1, 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz H. M., August B. K., Slapnick S. M. (1992). Epithelial repair following mechanical injury of the developing organ of Corti in culture: an electron microscopic and autoradiographic study. Exp. Neurol. 115, 44–49 [DOI] [PubMed] [Google Scholar]
- van Amerongen R., Bowman A. N., Nusse R. (2012). Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 11, 387–400 [DOI] [PubMed] [Google Scholar]
- Vintersten K., Monetti C., Gertsenstein M., Zhang P., Laszlo L., Biechele S., Nagy A. (2004). Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis 40, 241–246 [DOI] [PubMed] [Google Scholar]
- Warchol M. E. (1999). Immune cytokines and dexamethasone influence sensory regeneration in the avian vestibular periphery. J. Neurocytol. 28, 889–900 [DOI] [PubMed] [Google Scholar]
- White P. M., Doetzlhofer A., Lee Y. S., Groves A. K., Segil N. (2006). Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441, 984–987 [DOI] [PubMed] [Google Scholar]
- Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R., 3rd, Nusse R. (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 [DOI] [PubMed] [Google Scholar]
- Zeng Y. A., Nusse R. (2010). Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6, 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhai S. Q., Shou J., Song W., Sun J. H., Guo W., Zheng G. L., Hu Y. Y., Gao W. Q. (2007). Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. J. Neurosci. Methods 164, 271–279 [DOI] [PubMed] [Google Scholar]
- Zheng L., Sekerková G., Vranich K., Tilney L. G., Mugnaini E., Bartles J. R. (2000). The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 102, 377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H., Espinosa J. S., Su H. H., Muzumdar M. D., Luo L. (2005). Mosaic analysis with double markers in mice. Cell 121, 479–492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.