Abstract
Problem
Numerous lines of evidence implicate Apolipoprotein E (Apo E) in lipid metabolism during pregnancy. Hence, a role for its polymorphism has been envisaged in recurrent pregnancy loss (RPL) considering major structural and functional differences between different Apo E genotypes.
Method of study
A case control study of 81 women with two or more pregnancy losses that did not have any other known risk factors including anatomic anomalies of the reproductive system, infections, immunologic factors, hormonal imbalances, chromosomal abnormalities and environmental factors was carried out. The control group consisted of 81 women with at least two healthy children and no RPL in their reproductive history. DNA was extracted from the peripheral blood following written consent and Apo E genotyping was carried out by amplifying exon 4 of the gene and subjecting it to digestion by HhaI restriction enzyme.
Results
Genotyping was concluded by analyzing different fragment sizes produced, which resulted in finding significantly higher frequency of combined E3/E4 and E4/E4 genotypes in the patients (about 20 %) compared to the normal controls (2.4 %). The genotypes were confirmed by DNA sequencing.
Conclusion
Allelic frequency for E4 was 13.5 % in the patients and only 1 % in the non-RPL group. Our findings confirm and are in line with a number of similar studies carried out on other populations. Therefore, Apo E4 polymorphism seems to be contributing to the thrombophilic risk factors as a background to RPL.
Keywords: Apo E4 polymorphism, Apolipoprotein E, Recurrent pregnancy loss
Introduction
Recurrent pregnancy loss (RPL) is the occurrence of two or more consecutive fetal loss prior to 20 weeks gestation or at the time when the fetus weighs less than 500 g [1]. Known risk factors of RPL are advanced maternal age, unnatural anatomic anomalies, chromosomal abnormalities, hormone imbalances, infections, immunologic problems as well as environmental factors [2,3]. As there is no known cause for 50 % to 75 % of this condition, the involvement of genetic factors is postulated in the etiology of RPL. In recent years numerous studies have been conducted to elucidate the factors responsible for this disorder [4]. These factors include the cytokines [5], xenobiotic enzymes [6], thrombophilic agents [7] and some glycoproteins expressed on the trophoblasts and/or endometrial cell surface [8]. Lately, the study of Apo E polymorphism has attracted attention as a relevant thrombophilic agent [9]. Human Apo E is a 34KD protein composed of 299 amino acids with high expression level in liver and brain [10]. This protein in conjunction with specific LDL receptors has an important role in the metabolism of cholesterol and triglyceride [11]. The Apo E gene is located on chromosome 19q13.2 and has three common alleles E2, E3 and E4 which form six different genotypes. Difference between Apo E alleles lie in amino acids at codons 112 and 158 both within exon 4, forming E2 with Cys112 Cys158, E3 with Cys112 Arg158 and E4 with Arg112 Arg158 composition [12]. The structural differences of these isoforms determine their function with regard to lipid metabolism [13]. Relationships between Apo E polymorphisms and lipid metabolism have been extensively studied in cardiovascular and neurodegenerative disorders such as Alzheimer disease and Parkinson disease and allele E4 has been implicated as risk factor in these conditions [14]. In normal pregnancy the cholesterol level is naturally increased and as a result Apo E could be involved [15]. On this basis, the present study was designed to investigate frequency of various genotypes of Apo E in two groups of women: a group of RPL patients versus normal controls.
Materials & Methods
Patients
162 women including 81 patients who had experienced two or more consecutive RPL (average 6.2 ranging between 2 and 8) as well as 81 women with two healthy children were recruited to this study (Table 1). Due to impracticality and lack of appropriate samples, chromosome analysis for the abortus material or the miscarried fetuses was not feasible. However, their parents (both husband and wife) were studied, demonstrating normal karyotypes. These patients agreed to take part in this study by signing a written consent form. The women patients were evaluated for anatomic abnormalities of their reproductive system either by sonography or hysterosalpingography and all were normal. These women also showed normal for immunologic tests of antiphospholipid antibodies, cardiolipin antibodies, anti-nuclear antibody, double stranded DNA antibody, anti-thyroid antibody, lupus anticoagulant antibody, anti peroxidase antibody and had no familial thrombophilia. All patients had negative TORCH and pap-smear tests results. Therefore, they lacked any of known risk factors in RPL.
Table 1.
Characteristics of RPL patients and non-RPL group
| Samples | Number | Mean age | Mean of pregnancy losses (range) | Mean of gestational week at miscarriage (range) | Mean of live births (range) |
|---|---|---|---|---|---|
| Patients | 81 | 27.7 | 2.6 (2–8) | 8 (4–17) | 0 |
| Non-RPL group | 81 | 30 | 0 | 0 | 3.18(2–12) |
Apo E genotyping
5 ml peripheral blood was taken in EDTA tubes from each patient and DNA was extracted by standard salting out method [16]. A pair of primers with the following sequence encompassing 303 bp within exon 4 was utilized to PCR amplifies a fragment which contained codons 112 and 158:
Apo E-F: 5′-CGGGCACGGCTGTCCAAGGAG-3′
Apo E-R: 5′-CACGCGGCCCTGTTCCACgAG-3′
PCR conditions were: 95 °C for 5′, 95 °C for 50″, 65 °C for 50″, 72 °C for 50″ for 30 cycles 72 °C for 10′. The PCR products were treated whit restriction enzyme HhaI at 37 °C overnight. The sizings of the resulting fragments were determined by electrophoresis on 12 % polyacrylamide gel. The total length of the amplified fragment is 303 bp which contains 10 cutting sites for the enzyme HhaI. Eight of these sites are constant and the other two are polymorphic [17]. The fragments produced for each of the three Apo E alleles are as follows: (Fig. 1)
E2 allele: 112Cys + 158Cys = 91 bp + 83 bp + others (≤33 bp);
E3 allele: 112Cys + 158Arg = 91 bp + 48 bp + 35 bp + others (≤33 bp);
E4 allele: 112Arg + 158Arg = 72 bp + 48 bp + 35 bp + 19 bp + others (≤33 bp)
Fig. 1.
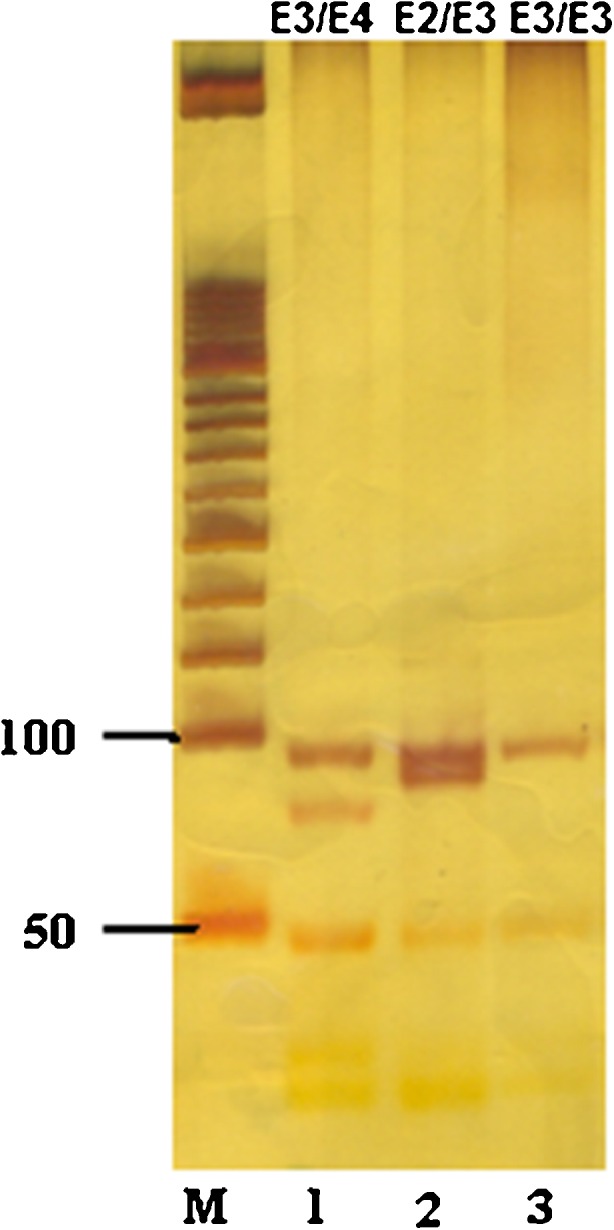
Gel picture of Apo E genotyping: separation of PCR amplified Apo E product digested with enzyme HhaI, on a 12 % polyacrylamide gel. Lane M = 50 bp ladder, Lane 1 = E3⁄E4, Lane 2 = E2⁄ E3, Lane = E3⁄E3
Statistical analysis
The frequency for the three alleles of E2, E3, E4 and resulting genotypes were determined in the patients and non-RPL group. Comparison was made by using chi-square and Fisher Exact test in SPSS version 18 software. The data with less than 0.05 probabilities regarded as significant.
Results
The different genotypic and allelic frequencies of Apo E for patients and non-RPL group are summarized in Table 2. In the patients group 16 individuals had combined E3/E4 and E4/E4 genotypes, whereas only two individuals from control group had such genotypes. This difference is statistically significant. Furthermore E4 allelic frequency in these two groups was also statistically significant: 13.5 % in patients and only 1.25 % in the controls. Odds Ratio (OR) as an indicator of increased risk factor was also calculated in these two groups: at genotype level E3/E4 heterozygosity was observed in patients more than five folds as compared to the control group and the OR was over 12 folds greater for possession of allele E4.
Table 2.
Apo E genotypes and allele frequencies for RPL patients and non-RPL group
| Apo E | Patient, (n %) | Control, (n%) | P-Value | OR (95 % CI) |
|---|---|---|---|---|
| E2/E2 | 0 | 0 | ||
| E2/E3 | 7(8.6) | 11(13.5) | n.s | |
| E2/E4 | 0 | 0 | ||
| E3/E3 | 58(71.6) | 68(83.9) | 0.08 | |
| E3/E4 | 10(12.3) | 2(2.4) | 0.032 | 5.563(1.179–26.255) |
| E4/E4 | 6(7.4) | 0 | 0.030 | |
| APO-E allele frequency | ||||
| E2 | 7 | 11 | n.s | |
| E3 | 133 | 149 | 0.012 | 0.4 (0.2–0.8) |
| E4 | 22 | 2 | 0.00001 | 12.571 (2.904–54.404) |
Discussion
Statistical analysis of the findings in this study indicate the frequency of allele E4 in RPL patients with 13.5 % is significantly higher than the non-RPL group with only 1.2 % (p < 0.05). This reflects in the presence of E4/E4 genotype at 7.4 % frequency in the patients group and its absence in the non-RPL group and the combined frequency of E3/E4 and E4/E4 at 19.7 % in the patients group as against 2.4 % in the non-RPL patients group. This demonstrates a positive association. Therefore, it seems this overwhelming frequency difference of allele E4 and genotypes’ sharing this allele has an impact on the susceptibility to RPL. This may also be demonstrated by calculation of OR. The risk of having RPL in the patients group with combined E3/E4 and E4/E4 genotype was five folds greater than normal controls.
Apo E, a glycoprotein, is expressed in numerous tissues within body [11]. Its different genotypes are related to the lipid concentration level and have already been implicated as association risk factors in cardiovascular disorders, strokes, Alzheimer disease and as susceptibly factor in response to viral, bacterial and parasitic infection [14]. Compared to E3, the most frequent allele, E4 allele of Apo E has a less affinity for LDL receptor which leads to decreased affinity for collection of chylomicrons from blood circulation [18]. Increased level of plasma lipid concentration has adverse effect leading to the formation of venous thrombosis and activation of inflammatory processes in epithelial cells initiated by cytokines [19]. It is also known that placental venous thrombosis causes death of trophoblast cells and consequently fetal death may occur [20]. Pregnant women are prone to blood coagulation and in presence of E4 allele this phenomenon is enhanced due to the lower capacity of this allele to metabolize lipid [7,11]. For a successful pregnancy there should be equilibrium between inflammatory agents and anti-inflammatory cytokine factors [21]. Interleukin 10 (IL10) is the most potent amongst anti-inflammatory factor [14]. It has been shown that in carriers of allele E4, IL10 plasma concentration is reduced [22]. IL10 maintains anti-inflammation capacity and inhibits formation of atherosclerotic plaques which are collectively crucial for a successful pre-implantation and survival of pregnancy [23,24]. Overall, IL10 plays an important role in maintenance of pregnancy, however in Apo E4 carriers the blood level of this cytokine is effectively reduced [20]. Goodman et.al have previously reported positive association of Apo E polymorphism with RPL in a case control study of 69 women [9]. Similar results were followed by Yeniceus et.al who demonstrated that the genotype E3/E4 was more frequent in women with RPL compared non-RPL group [25]. Also Ozornek et.al reported a much higher frequency of allele E4 within their patients with RPL in comparison to non-RPL group [20]. Similar findings were also obtained in a study by Bianca et.al on Italian patients, although these researchers had concluded to the contrary by choosing wrong control groups (thrombophic patients with positive association for Apo E allele E4) [26]. Goodman et.al did reanalysis of their data and compared of these RPL patients with non-RPL group and concluded positive association [27].
The findings of the present investigation show a clear positive association of RPL with genotypes E3/E4 and E4/E4. This is in line with previous studies and adds weight to the concept of considering Apo E polymorphism as a contributing risk factor in RPL.
Acknowledgments
This work was supported by a grant from Tehran Medical Genetics Laboratory. The authors gratefully acknowledge the patients who consented to be involved in the study. The authors wish to acknowledge Dr. Arezou Sayad for her contribution to the statistical analysis and also the personnel of Tehran Medical Genetics Laboratory for their enthusiastic cooperation in this research.
Footnotes
Capsule
This study identified APOE4 polymorphism as one of the significant thrombophilic risk factors predisposing to RPL.
References
- 1.Stirrat GM. Recurrent miscarriage. Lancet. 1990;336:673–5. doi: 10.1016/0140-6736(90)92159-F. [DOI] [PubMed] [Google Scholar]
- 2.Clifford K, Rai R, Regan L. Future pregnancy outcome in unexplained recurrent first trimester miscarriage. Hum Reprod. 1997;12:387–389. doi: 10.1093/humrep/12.2.387. [DOI] [PubMed] [Google Scholar]
- 3.Toth B, Jeschke U, Rogenhofer N, Scholz C, Würfel W, Thaler CJ, Makrigiannakis A. Recurrent miscarriage: current concepts in diagnosis and treatment. J Reprod Immunol. 2010;85:25–32. doi: 10.1016/j.jri.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Suzumori N, Sugiura Ogasawara M. Genetic factors as a causes of miscarriage. Curr Med Chem. 2010;17:3431–3437. doi: 10.2174/092986710793176302. [DOI] [PubMed] [Google Scholar]
- 5.Zammiti W, Mtiraoui N, Khairi H, Gris JC, Almawi WY, Mahjoub T. Associations between tumor necrosis factor-alpha and lymphotoxin-alpha polymorphisms and idiopathic recurrent miscarriage. Reproduction. 2008;135:397–403. doi: 10.1530/REP-07-0322. [DOI] [PubMed] [Google Scholar]
- 6.Parveen F, Faridi RM, Das V, Tripathi G, Agrawal S. Genetic association of phase I and phase II detoxification genes with recurrent miscarriages among North Indian women. Mol Hum Reprod. 2010;16:207–214. doi: 10.1093/molehr/gap096. [DOI] [PubMed] [Google Scholar]
- 7.Pabinger I. Thrombophilia and its impact on pregnancy. Thromb Res. 2009;123:S16–21. doi: 10.1016/S0049-3848(09)70128-8. [DOI] [PubMed] [Google Scholar]
- 8.Stortoni P, Cecati M, Giannubilo SR, Sartini D, Turi A, Emanuelli M, Tranquilli AL. Placental thrombomodulin expression in recurrent miscarriage. Reprod Biol Endocrinol. 2010;8:1–5. doi: 10.1186/1477-7827-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman C, Goodman CS, Hur J, Jeyendran RS, Coulam C. The association of apoprotein E polymorphisms with recurrent pregnancy loss. Am J Reprod Immunol. 2009;61:34–38. doi: 10.1111/j.1600-0897.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 10.Wernette-Hammond ME, Lauer SJ, Corsini A, Walker D, Taylor JM, Rall SC., Jr Glycosylation of human apolipoprotein E. The carbohydrate attachment site is threonine 194. J Biol Chem. 1989;15:9094–9101. [PubMed] [Google Scholar]
- 11.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 12.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects on apolipoproteins E3 and E4 on neuronal growth in Vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 13.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimers disease to AIDS. J Lipid Res. 2009;50:S183–188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Wu LM, Wu J. Cross-talk between apolipoprotein E and cytokines. Mediators Inflamm. 2011;2011:949072. doi: 10.1155/2011/949072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PJ, Broughton Pipkin F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:405–417. doi: 10.1016/j.bpobgyn.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo L, van Dyck CH, Luo X, Kranzler HR, Yang BZ, Gelernter J. Variation at APOE and STH loci and Alzheimer’s disease. Behav Brain Funct. 2006;2:13–23. doi: 10.1186/1744-9081-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 19.Amundsen T, Ueland PM, Waage A. Plasma homocysteine levels in patients with deep venous thrombosis. Arterioscler Thromb Vasc Biol. 1995;15:1321–1323. doi: 10.1161/01.ATV.15.9.1321. [DOI] [PubMed] [Google Scholar]
- 20.Ozornek H, Ergin E, Jeyendran RS, Ozay AT, Pillai D, Coulam C. Is Apolipoprotein E codon 112 polymorphisms associated with recurrent pregnancy loss? Am J Reprod Immunol. 2010;64:87–92. doi: 10.1111/j.1600-0897.2010.00814.x. [DOI] [PubMed] [Google Scholar]
- 21.Vassiliadis S, Ranella A, Papadimitriou L, Makrygiannakis A, Athanassakis I. Serum levels of pro and anti-inflammatory cytokines in non-pregnant women, during pregnancy, labour and abortion. Mediators Inflamm. 1998;7:69–72. doi: 10.1080/09629359891199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jofre-Monseny L, Loboda A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Jozkowicz A, Minihane AM, Dulak J, Rimbach G. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun. 2007;357:319–324. doi: 10.1016/j.bbrc.2007.03.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaouat G, Menu E, de Smedt D, Khrihnan L, Hui L, Assal Meliani A, Martal J, Raghupathy R, Wegmann TG. The emerging role of IL-10 in pregnancy. Am J Reprod Immunol. 1996;35:325–329. doi: 10.1111/j.1600-0897.1996.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 24.Tziakas DN, Chalikias GK, Antonoglou CO, Veletza S, Tentes IK, Kortsaris AX, Hatseras DI, Kaski JC. Apolipoprotein E genotype and circulating interleukin-10 levels in patients with stable and unstable coronary artery disease. J Am Coll Cardiol. 2006;48:2471–2481. doi: 10.1016/j.jacc.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Yenicesu GI, Cetin M, Ozdemir O, Cetin A, Ozen F, Yenicesu C, Yildiz C, Kocak N. A Prospective Case–Control Study analyzes 12 thrombophilic gene mutations in turkish couples with recurrent pregnancy loss. Am J Reprod Immunol. 2010;63:126–136. doi: 10.1111/j.1600-0897.2009.00770.x. [DOI] [PubMed] [Google Scholar]
- 26.Bianca S, Barrano B, Cutuli N, Indaco L, Cataliotti A, Milana G, Barone C, Ettore G. No association between apolipoprotein E polymorphisms and recurrent pregnancy loss. Fertil Steril. 2010;93:276. doi: 10.1016/j.fertnstert.2009.07.971. [DOI] [PubMed] [Google Scholar]
- 27.Goodman C, Coulam C, Jeyendran RS. Association of apolipoprotein E polymorphisms and recurrent pregnancy loss. Fertil Steril. 2010;93:e19. doi: 10.1016/j.fertnstert.2009.12.016. [DOI] [PubMed] [Google Scholar]


