Abstract
Purpose
To compare the frequency of chromosomal heteromorphisms in reproductive failure and fertile control individuals in Northeast China, and investigate the impact on reproductive failure
Methods
1751 males and 1424 couples with reproductive failure (n = 4599) and 777 fertile control individuals in Northeast China were enrolled. Chromosome karyotype analysis was performed on peripheral blood lymphocytes with standard G-banding. Additionally, C-banding was performed with heterochromatin heteromorphisms, and NORs-banding with satellites/stalks variations. Multiplex polymerase chain reaction (PCR) adopted for the amplification using nine specific sequence tagged sites (STS) were used to detect Y-chromosome microdeletions with Y chromosome variations (Yqh±). At the same time, 38 heteromorphic probands’ family members were recalled for performing karyotype analysis and to be surveyed for their detailed reproductive history.
Results
The frequency of chromosomal heteromorphisms in reproductive failure patients (2.74 %, 126/4599) was of no statistically significant difference as compared with fertile control individuals (2.06 %, 16/777) (P > 0.05). Eight cases of Y variation (Yqh±) probands with Y-chromosomal microdeletions were detected among 44 reproductive failure patients and 6 fertile control men. In the 38 recalled families, the probands of fathers or mothers, even some of their brothers or sisters, had the same heteromorphic karyotypes as probands’ despite that they didn’t have any adverse reproductive history.
Conclusions
There was no statistically significant difference in frequency of chromosomal heteromorphisms between reproductive failure and fertile control individuals in Northeast China. Males with Y variations (Yqh±) should be ordered Y-chromosomal microdeletions detection. Through the analysis of 38 recalled families, we can also conclude that chromosomal heteromorphisms were not the impact factors for reproductive failure.
Keywords: Chromosomal heteromorphisms, Infertility, Malformed childbearing history, Recurrent spontaneous abortions, Reproductive failure, Stillbirth, Pedigree analysis, Y-chromosomal microdeletions
Introduction
Chromosomal heteromorphisms, as known as chromosomal polymorphisms, include varying sizes of heterochromatin blocks, satellites, repeat sequence regions and inversions [1]. However, surveys showed higher frequency variants in reproductive failure individuals compared with normal people [2]. Reproductive failure, also defined as abnormal reproductive outcomes, such as infertility, RSA (recurrent spontaneous abortions) or stillbirth and malformed childbearing history. At present, the relationship between chromosome heteromophisms and reproductive failure is still controversial. A number of authors reported that chromosome polymorphisms were related to infertility and recurrent abortions [3–6]. A review of literature showed screening prospective gamete donors for chromosome variants may help enhance the success of in vitro fertilization IVF [7]. However, it seems have no adverse effects on the outcome of IVF–embryo transfer treatment with chromosomal heteromorphisms [8].
In order to add more evidence whether the chromosomal heteromorphisms influences reproduction failure, we analyzed the frequency of heteromorphisms with abnormal reproductive outcome in Northeast China and compared with fertile control individuals. More importantly, 38 heteromorphic patients and their family members without any adverse reproductive history were recalled to perform chromosomal karyotype analyses.
Materials and methods
Patients
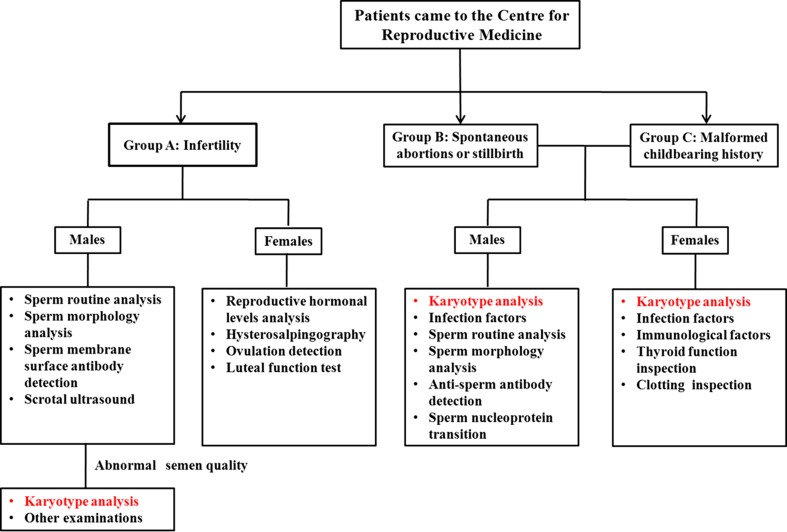
From March 2009 to August 2012, a retrospective cohort of 4599 patients was performed karyotype analysis (including 1424 couples and 1751 males) with reproductive failure from the Centre for Reproductive Medicine in Northeast China. The mean age of the 4599 cases was 30.5 years (23~49 years). A flow chart of patients was in Fig. 1. These patients were divided into three groups (Group A-C): Group A infertility, male patients who had abnormal semen quality were underwent karyotype analyses (n = 1751), Group B recurrent spontaneous abortions or stillbirth, the 1283 couples (1283 males and 1283 females) who had two or more recurrent spontaneous abortions at less than 28 weeks of gestation or fetal death occurs after about 20 week of pregnancy were ordered karyotype analysis (n = 2566); Group C malformed childbearing history, the 141 couples had at least one child with congenital malformation, genetic syndromes, or mental retardation were performed karyotype analysis (n = 282). The fertile control group (n = 777) that consisted of 486 men and 291 women, of a mean age of 27.3 years (24–40 years), had no adverse reproductive history who had born at least one phenotypically normal child as volunteers from obstetrical department. Consequent to their noted chromosomal heteromorphic probands, 38 patients’ family members, including their parents, brothers or sisters were recalled for further investigations and surveillance of their detailed reproductive history. Paternity tests were performed in 38 probands and their parents, and the results showed they were biological parent child relations. Appropriate written voluntary consent was obtained from all the individuals and the study was approved by the Chinese Association of Humanitarianism and Ethics.
Fig. 1.
A flow chart of patients with reproductive failure in our Centre. 1751 males with abnormal semen quality in Group A, 2566 individuals in Group B and 282 patients in Group C who were ordered karyotype analysis composed the individuals of this present study(n = 4599)
Semen analysis
Samples were obtained after a 3–7 day period of ejaculatory abstinence, and semen analysis was performed three times within an interval of 3 months according to the World Health Organization guidelines [9]. Semen quality inferior to that of the men from the general population was considered abnormal. Absence of spermatozoa in the semen ejaculate, if detected in the three times, the case was considered as azoospermia (A). Oligozoospermia was diagnosed with a sperm concentration <15 × 106/ml.
Chromosomal analysis
Karyotype analysis of 4599 reproductive failure patients and 777 fertile control individuals was performed. Briefly, peripheral blood lymphocytes were cultured in lymphocyte culture medium (Yishengjun; BaiDi Bio-Technology, Guangzhou, China) at 37 °C for 72 h, followed with 50 μg/ml colchicine (Yishengjun; BaiDi Bio-Technology, Guangzhou, China) arrest of mitosis for 1 h before culture termination. Harvesting of the peripheral blood lymphocytes was performed by hypotension, fixation, trypsinization and Giemsa banding (GTG-banding) (350–400 bands level). At least 15 metaphases were analyzed for each individual case and heteromorphisms were reported according to the International System for Human Cytogenetic Nomenclature (ISCN, 2009) [10]. Selective banding studies, such as C-banding was performed for heterochromatin polymorphism detection, and NOR-banding for satellites/stalks variations.
Classification of chromosomal heteromorphisms
Increase in lengths of the stalks on the short arm of chromosome of the acrocentric chromosomes (D/G-genome) was recorded as 13/14/15/21/22pstk+ [10]. Double satellites on the short arm of 13/14/15/21/22 can also be observed and were designated as pss [10]. The pericentric inversions of chromosomes 9, were also considered a heteromorphism [8]. Increase in length of the heterochromatin on the long arm of chromosome 1/9/16 were designated as 1/9/16/Yqh+. Heterochromatin can also be detected in these chromosomes, such as 1/9/16/Yqh- [10]. Multiple variations were consisted of more than one kind of variant. All karyotypes were examined independently, under light microscope, by three laboratory technicians, at different times in the laboratory to avoid uncertainty and variable results.
Y-chromosome microdeletion analysis
All the 44 cases of reproductive failure patients and 6 cases of fertile control men with Y variation (Yqh±) were checked for Y-chromosome microdeletions detections. Genomic DNA was isolated from peripheral blood using the Tiangen blood DNA extraction mini kit (Beijing Tiangen Biotech Co., Ltd, China). The screening for Y-chromosome microdeletions was performed by multiplex polymerase chain reaction (PCR) using a series of 9 specific sequence-tagged sites (STSs), including SY84 and SY86 for the AZFa region, SY27, SY134 and SY143 for AZFb region, SY152, SY157, SY254 and SY255 for AZFc region. Detailed experimental procedures were the same as Wang et.al described [11].
Statistical analysis
Statistical analysis was performed with SPSS® version 17.0 statistical package (SPSS Inc., Chicago, IL, USA) for Windows®. The Chi-square test and Fisher’s exact test was used to compare statistical significance between chromosomal heteromorphisms of the reproductive failure individuals and fertile control group. A P-value > 0.05 was considered to be no statistically significant and all P-values were two-sided.
Results
In the 4599 cases (including 1751 males and 1424 couples) with adverse reproductive outcome, there were 126 patients with chromosomal heteromorphisms, including 97 males and 29 females. The incidence of total variants in reproductive failure individuals was 2.74 % (126/4599). There was no significant difference as compared with fertile control group 2.06 % (16/777) (P = 0.333).
The most common variant observed were inv(9) (0.70 %), followed by Yqh- (0.65 %) and 1/9/16qh+ (0.43 %) in 126 cases of reproductive failure individuals. Of the 16 heteromorphisms in fertile control group, inv(9) and Yqh- showed the highest frequency (0.51 %) (Table 1). Multiple variations were consisted of many kinds of heteromorphisms, such as 46, XY, 13pstk+, 22pstk+; 46,XX, inv(9) (p11q12), 16qh+ (Table 2).
Table 1.
The frequency of chromosomal heteromorphisms in reproductive failure individuals compared with fertile controls
| Group | No.of heteromorphisms | 13/14/15 pstk± | 21/22 pstk± | 21/22 pss | inv(9) | 1/9/16 qh± | Multiple variation | Yqh+ | Yqh- |
|---|---|---|---|---|---|---|---|---|---|
| Reproductive failure individuals (n = 4599) | 126 (2.74 %) | 10 (0.22 %) | 15 (0.33 %) | 2 (0.04 %) | 32 (0.70 %) | 20 (0.43 %) | 3 (0.07 %) | 14 (0.30 %) | 30 (0.65 %) |
| Fertile control individuals (n = 777) | 16 (2.06 %) | 2 (0.26 %) | 2 (0.26 %) | 0 (0 %) | 4 (0.51 %) | 2 (0.26 %) | 0 (0 %) | 2 (0.26 %) | 4 (0.51 %) |
Figures in parenthesis () are percentages in individuals not couples
P > 0.05, the frequency of chromosomal heteromorphisms in reproductive failure individuals was no significantly difference compared with fertile control individuals; Fisher exact test
Table 2.
Karyotypes of 126 patients with chromosomal heteromorphisms and their clinical manifestations
| Types of heteromorphisms | Karyotypes | Group A | Group B | Group C | Total |
|---|---|---|---|---|---|
| 13/14/15pstk± | 46,XX/XY,13pstk+ | 1 | 3 | 0 | 4 |
| 46,XY,14pstk+ | 1 | 0 | 0 | 1 | |
| 46,XX/XY,15pstk+ | 0 | 4 | 0 | 4 | |
| 46,XY,15pstk- | 0 | 1 | 0 | 1 | |
| 21/22pstk± | 46,XX/XY,21pstk+ | 1 | 3 | 1 | 5 |
| 46,XX/XY,21pstk- | 2 | 1 | 0 | 3 | |
| 46,XX/XY,22pstk+ | 6 | 1 | 0 | 7 | |
| 21/22pss | 46,XY,21pss | 1 | 0 | 0 | 1 |
| 46,XY,22pss | 0 | 1 | 0 | 1 | |
| inv(9) | 46,XX/XY, inv(9)(p11q12) | 15 | 13 | 0 | 28 |
| 46,XX/XY, inv(9)(p11q13) | 1 | 3 | 0 | 4 | |
| 1/9/16qh± | 46,XX/XY,1qh+ | 1 | 7 | 1 | 9 |
| 46,XX/XY,9qh+ | 1 | 6 | 0 | 7 | |
| 46,XX,9qh- | 0 | 2 | 0 | 2 | |
| 46,XX/XY,16qh+ | 1 | 1 | 0 | 2 | |
| Multiple variation | 46,XY,13pstk+,22pstk+ | 0 | 1 | 0 | 1 |
| 46,XX,inv(9)(p11q12),16qh+ | 0 | 2 | 0 | 2 | |
| Y variation | 46,XY,Yqh+ | 8 | 5 | 1 | 14 |
| 46,XY,Yqh- | 28 | 2 | 0 | 30 | |
| Total | 67 | 56 | 3 | 126 |
The karyotypes of 126 patients of chromosomal heteromorphisms and their clinical manifestations were showed in Table 2. In Group A of the 1751 infertility males, there was 67 individuals with chromosomal heteromorphisms, and the frequency is the highest (3.83 %, 67/1751). In Group B, the frequency of heteromorphisms is 2.18 % (56/2566), and 1.06 % (3/282) in Group C. All the 44 cases of reproductive failure patients and 6 cases of fertile control men with Y variation (Yqh±) were subjected to Y-chromosome microdeletions detections; 8 of these cases had a detectable microdeletions (Table 3).
Table 3.
Y-chromosome microdeletions in eight cases of Y variations

Distribution of Y-chromosome microdeletions in the three regions. Symbol ( ) indicates presence of STS marker, (-) indicates absence of STS marker.
) indicates presence of STS marker, (-) indicates absence of STS marker.
A azoospermia O oligozoospermia
Chromosomal karyotype analysis of 38 probands and their family members was performed and the probands’ clinical manifestation also showed in Table 4. All the probands’ fathers or mothers, brothers or sisters that had the same heteromorphic karyotypes, did not have the same reproductive failure but normal reproductive history.
Table 4.
Pedigree analyses of 38 families with chromosomal heteromorphisms
| Family No. | Karyotypes | Proband’s clinical manifestation | Family No. | Karyotypes | Proband’s clinical manifestation | ||
|---|---|---|---|---|---|---|---|
| 1 | P | 46,XX,13pstk+ | Group B | 2 | P | 46,XX,21pstk+ | Group B |
| F | 46,XY | F | 46,XY | ||||
| M | 46,XX,13pstk+ | M | 46,XX,21pstk+ | ||||
| B | 46,XY,13pstk+ | B | 46,XY,21pstk+ | ||||
| H | 46,XY | H | 46,XY | ||||
| 3 | P | 46,XX,15pstk+ | Group B | 4 | P | 46,XY,22pstk+ | Group A |
| F | 46,XY | F | 46,XY,22pstk+ | ||||
| M | 46,XX,15pstk+ | M | 46,XX | ||||
| H | 46,XY | W | 46,XX | ||||
| 5 | P | 46,XY,21pstk- | Group A | 6 | P | 46,XX,21pstk+ | Group B |
| F | 46,XY,21pstk- | F | ND | ||||
| M | 46,XX | M | 46,XX,21pstk+ | ||||
| B | 46,XY,21pstk- | H | 46,XY | ||||
| W | 46,XX | 8–12 | P | 46,XY,inv(9)(p11q12) | 8–10: Group B | ||
| 7 | P | 46,XY,inv(9)(p11q12) | Group A | F | 46,XY | 11–12: Group A | |
| F | 46,XY,inv(9)(p11q12) | M | 46,XX,inv(9)(p11q12) | ||||
| M | 46,XX | W | 46,XX | ||||
| B | 46,XY,inv(9)(p11q12) | 15 | P | 46,XY,inv(9)(p11q12) | Group A | ||
| W | 46,XX | F | 46,XY | ||||
| 13–14 | P | 46,XY,inv(9)(p11q12) | 13: Group B 14: Group A | M | 46,XX,inv(9)(p11q12) | ||
| F | 46,XY,inv(9)(p11q12) | S | 46,XX,inv(9)(p11q12) | ||||
| M | 46,XX | W | 46,XX | ||||
| S | 46,XX,inv(9)(p11q12) | 17 | P | 46,XX,1qh+ | Group B | ||
| W | 46,XX | F | 46,XY | ||||
| 16 | P | 46,XX,1qh+ | Group A | M | 46,XX,1qh+ | ||
| F | ND | B | 46,XY,1qh+ | ||||
| M | 46,XX,1qh+ | H | 46,XY | ||||
| H | 46,XY | 19 | P | 46,XY,9qh+ | Group B | ||
| 18 | P | 46,XY,9qh+ | Group A | F | 46,XY | ||
| F | 46,XY,9qh+ | M | 46,XX,9qh+ | ||||
| M | 46,XX | W | 46,XX | ||||
| W | 46,XX | 21 | P | 46,XY,16qh+ | Group A | ||
| 20 | P | 46,XX,16qh+ | Group B | F | 46,XY,22pstk+ | ||
| F | 46,XY | M | 46,XX,16qh+ | ||||
| M | 46,XX,16qh+ | S | 46,XX,16qh+ | ||||
| B | 46,XY | W | 46,XX | ||||
| S1 | 46,XX | 23–24 | P | 46,XY,Yqh+ | 23: Group A | ||
| S2 | 46,XX,16qh+ | F | 46,XY,Yqh+ | 24: Group C | |||
| H | 46,XY | M | ND | ||||
| 22 | P | 46,XY,Yqh+ | Group C | W | 46,XX | ||
| F | 46,XY,Yqh+ | 26–36 | P | 46,XY,Yqh- | 26–28: Group A | ||
| M | ND | F | 46,XY,Yqh- | 29–30: Group B | |||
| W | 46,XX,inv(2)(q21q32) | M | ND | 31–36*: Group A | |||
| W | 46,XX | ||||||
| 25 | P | 46,XY,Yqh+ | Group A | 37–38 | P | 46,XY,Yqh- | Group A |
| F | 46,XY,Yqh+ | F | 46,XY,Yqh- | ||||
| M | ND | M | ND | ||||
| B | 46,XY,Yqh+ | B | 46,XY,Yqh- | ||||
| W | 46,XX | W | 46,XX |
Discussion
Recently, increasingly studies reported an increased incidence of heteromorphisms in infertile couples that may suggest some impact on reproductive failure [4, 8, 12, 13]. Brothman et al. concluded that common cytogenetic variants were considered to be heteromorphisms without clinical significance [14]. Chromosomal heterochromatic regions, that were the last to enter synapse, changing the timing of the whole division and leading first to probable meiotic defects, were found to alter synapsis of homologous chromosomes during meiosis, and eventually be involved into infertility induction [15]. However, Feride et al. showed an undefined relationship between chromosome heteromorphisms and infertility [16].
In the present study, inv (9) was the highest frequency of morphological variations. Some previous reports on the mechanisms of reproductive failure couples with an inv (9) carrier suggest that crossing over in an inversion loop during meiosis leads to an unbalanced genetic composition of each chromosome [17]. However, Madon et al. considered polymorphisms of heterochromatic regions as normal variants in humans [14].
Y chromosome variations were other kinds of prevalent chromosomal heteromorphisms in the study, including increase or decrease in the length of the heterochromatin on the long arm (Yqh+/Yqh-). However, the impact of Y variations on reproductive capacity was uncertain. Antonelli et al. found that too many DNA repeats at specific regions of the Y chromosome may have an impact on the pairing and synapsis of X and Y chromosomes during meiosis and that may decrease the reproductive capacity [18]. Kalantari et al. concluded that Y chromosome heteromorphisms did not directly affect the sperm count [19].
However, very few data have described simultaneous Y chromosome variations that had Y-chromosomal microdeletions. In the present study of all the 44 cases of reproductive failure males and 6 cases of fertile control individuals with Yqh± were subjected to Y-chromosome microdeletions detections where 8 cases of microdeletions were detected (Table 2). The genes in AZF regions were considered critical for spermatogenesis and Y-chromosome microdeletions had been associated with the severity of spermatogenic defects [20]. These microdeletions may explain the 8 cases of probands’ reproductive incapacity noted in this study. Thus, males with Y chromosomal variations should be ordered Y-chormosomal microdeletions detection. However, as regard to other patients of Y chromosome variations (Yqh±) without mircodeletions, and taking into consideration that Y chromosome are inherited by their fathers and passed to their sons, the present study did not reveal any link between heteromorphism and reproductive failure since heteromorphism is noted upon examination of patients’ fathers and brothers who were fertile.
The increase in the length of the secondary constriction in the long arm of chromosomes 1, 9 and 16 is also common in chromosome variations. The repeat segments may cause clinical symptoms because of increased highly repetitive DNA sequences [21]. Heterochromatin in chromosomal polymorphism variations can regulate gene expression by reversible transformation between heterochromatin (non-coding DNA sequences) and euchromatin (expressed DNA sequences) [22, 23].
D/G-genome chromosomes heteromorphisms show increased heterochromatin at the chromosome telomere, the short arm, and the nucleolar organizing region (NOR). When chromatin variation occurs in these regions, it causes defects in centromere function and kinetochore assembly, difficulty in homologous chromosome pairing, and impacts on cell division, thus affects gamete formation.
In the present study, although heteromorphisms could affect gametogenesis and lead to infertility, the frequency of chromosomal heteromorphisms in reproductive failure patients (2.74 %) was of no statistically significant difference compared with fertile control individuals (2.06 %) (P > 0.05) in Northeast China (Table 1), suggesting that heteromorphisms could not be associated with having infertility, recurrent spontaneous abortions or stillbirth and malformed childbearing history.
In addition, to our knowledge, this is the first time to analyze the relationship between reproductive failure and chromosomal heteromorphisms through pedigrees. We recalled family members, including parents, brother and sisters of the 38 probands with chromosomal heteromorphisms, surveyed their detailed reproductive history and did chromosomal karyotype analysis. In all the recalled family members of all probands, there was a similarity in the karyotypes of the respective proband family members that was not reflected in a similar adverse reproductive history in them. Taking into consideration that other factors that known to lead to adverse effect in fertilitity were excluded in this study, we can conclude that chromosomal heteromorphisms are not the sole impact factors for reproductive failure.
In summary, we believe that chromosomal heteromorphisms do not play a role in reproductive problems. However, our report was limited by only using cytogenetic detection methods, without confirmation by genetic testing, except through Y- chromosome microdeletions detections. Analysis at the molecular level, may be needed to unveil any relation between heteromorphisms and reproductive failure taking into consideration, the heterochromatin have been regarded to have more crucial cellular roles than previously thought [14, 24].
Acknowledgements
We thank all the patients and donors of blood samples. We thank all staff of the Andrology Laboratory for their excellent work. We also thank Elfateh Fadlalla for his English-language assistance and critical review. This work was kindly supported by funds from the National Population and Family Planning Commission of P.R. China (NO. 2011-GJKJS-07).
Footnotes
Capsule Chromosomal heteromorphisms are not the sole impact factors for individuals with reproductive failure in Northeast China. Males with Y variations (Yqh±) should be ordered Y-chromosomal microdeletions detection. It is important to do pedigree analysis to verify the effect of chromosomal heteromorphisms on reproductive failure.
Contributor Information
Yuan Dong, Email: dongyuancg@163.com.
Rui-Zhi Liu, Email: lrz410@126.com.
References
- 1.Denise Mari C, Fernanda AM, Rubens PN, et al. Correlation between chromosomal variants and male infertility in a population of Brazilian infertile men. Reproductive System & Sexual Disorders. 2012;1(1):1–6. [Google Scholar]
- 2.Bhasin MK. Human population cytogenetics: a review. Int J Hum Genet. 2005;5(2):83–152. [Google Scholar]
- 3.Zhu YJ, Liu SY, Wang H, et al. The prevalence of azoospermia factor microdeletion on the Y chromosome of Chinese infertile men detected by muti-analyte suspension array technology. Asian J Androl. 2008;10(6):873–881. doi: 10.1111/j.1745-7262.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 4.Madon PF, Athalye AS, Parikh FR. Polymorphic variants on chromosomes probably play a significant role in infertility. Reprod BioMed Online. 2005;11(6):726–732. doi: 10.1016/S1472-6483(10)61691-4. [DOI] [PubMed] [Google Scholar]
- 5.Tsenghi C, Metaxotou-Stavridaki C, Strataki-Benetou M, et al. Chromosome studies in couples with repeated spontaneous abortions. Obset Gynecol. 1976;47(4):463–468. [PubMed] [Google Scholar]
- 6.Minocherhomji S, Athalye AS, Madon PF, et al. A case–control study identifying chromosomal polymorphic variations as forms of epigenetic alterations associated with the infertility phenotype. Fertil Steril. 2009;92(1):88–95. doi: 10.1016/j.fertnstert.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 7.Prochi FM, Arundhati SA, Firuza RP. Polymorphic variants on chromosomes probably play a significant role in infertility. Reproductive BioMedicine Online. 2005;11(6):726–732. doi: 10.1016/S1472-6483(10)61691-4. [DOI] [PubMed] [Google Scholar]
- 8.Hong Y, Zhou YW, Tao J, et al. Do polymorphic variants of chromosomes affect the outcome of in vitro fertilization and embryo transfer treatment? Hum Reprod. 2011;26:933–940. doi: 10.1093/humrep/deq333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Switzerland: WHO; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer LG, Slovak ML (eds): International Software Consulting Network (ISCN). An International System for Human Cytogenetic Nomenclature. Basel: S Karger, 2009; pp 53–4.
- 11.Wang RX, Fu X, Yang YP, et al. Male infertility in China: laboratory finding for AZF microdeletions and chromosomal abnormalities in infertile men from Northeastern China. J Assist Reprod Genet. 2010;27:391–396. doi: 10.1007/s10815-010-9420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura Y, Kitamura M, Nishimura K, et al. Chromosomal variants among 1790 infertile men. Int J Urol. 2001;8:49–52. doi: 10.1046/j.1442-2042.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 13.Yakin K, Balaban B, Urman B. Is there a possible correlation between chromosomal variants and spermatogenesis? Int J Urol. 2005;12:984–989. doi: 10.1111/j.1442-2042.2005.01185.x. [DOI] [PubMed] [Google Scholar]
- 14.Brothman AR, Schneider NR, Saikevych I, Cytogenetics Resource Committee, College of American Pathologists/American College of Medical Genetics et al. Cytogenetic heteromorphisms: Survey results and reporting practices of Giemsa-band regions that we have pondered for years. Arch Pathol Lab Med. 2006;130:947–949. doi: 10.5858/2006-130-947-CHSRAR. [DOI] [PubMed] [Google Scholar]
- 15.Codina-Pascual M, Navarro J, Oliver-Bonet M, et al. Behaviour of human heterochromatic regions during the synapsis of homologous chromosomes. Hum Reprod. 2006;21(6):1490–1497. doi: 10.1093/humrep/del028. [DOI] [PubMed] [Google Scholar]
- 16.Feride IS, Zerrin Y, Ozge OY, et al. Chromosome heteromorphisms: an impact on infertility. J Assist Reprod Genet. 2008;25:191–195. doi: 10.1007/s10815-008-9216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boue J, Taillemite JL, Hazael-Massieux P, et al. Association of pericentric inversion of chromosome 9 and reproductive failure in ten unrelated families. Humangenetik. 1975;30:217–224. doi: 10.1007/BF00279187. [DOI] [PubMed] [Google Scholar]
- 18.Antonelli A, Gandini L, Petrinelli P, et al. Chromosomal alterations and male infertility. J Endocrinol Invest. 2000;23:677–683. doi: 10.1007/BF03343793. [DOI] [PubMed] [Google Scholar]
- 19.Kalantari P, Sepehri H, Behjati F, et al. Chromosomal studies in infertile men. Tsitol Genet. 2001;35:50–54. [PubMed] [Google Scholar]
- 20.Kato H, Komori S, Nakata Y, et al. Screening for deletions in interval D16-22 of the Y chromosome in azoospermic and oligozoospermic Japanese men. J Hum Genet. 2001;46:110–114. doi: 10.1007/s100380170097. [DOI] [PubMed] [Google Scholar]
- 21.Broccoli D. Function, replication and structure of the mammalian telomere. Cytotechnology. 2004;45:3–12. doi: 10.1007/s10616-004-5120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frenster JH, Herstein PR. Gene de-repression. N Engl J Med. 1973;288:1224–1229. doi: 10.1056/NEJM197306072882310. [DOI] [PubMed] [Google Scholar]
- 23.Nakatsu SL, Masek MA, Landrum S, et al. Activity of DNA templatesduring cell division and cell differentiation. Nature. 1974;248:334–335. doi: 10.1038/248334a0. [DOI] [PubMed] [Google Scholar]
- 24.Lissitsina J, Mikelsaar R, Punab M. Cytogenetic analyses in infertile men. Arch Androl. 2006;52(2):91–95. doi: 10.1080/01485010500316030. [DOI] [PubMed] [Google Scholar]