Abstract
Purpose
To determine expression of G-protein estrogen receptor (GPER) in mouse oocyte membrane during maturation.
Methods
The expression of GPER from different maturation stages of oocytes, in vivo and in vitro matured oocytes as well as aging oocytes was examined by immune-fluorescence GPR30 antibody and the images were analyzed by laser scanning confocal microscope. Further confirmation was performed by Western blots for cell fractionation.
Results
Significant fluorescent signal was observed on the surface of mouse oocytes. The image expression was lower in germinal vesicle (GV) stage than mature metaphase-II (M-II) stage oocytes. There was high expression in in-vivo matured oocytes compared to in vitro matured oocytes. The highest expression was observed in aging oocytes compared with other oocytes.
Conclusions
The changes of expression of GPER on mouse oocytes plasma membrane confirm oocyte membrane maturation, suggesting that those changes of GPER may be related to the functional role of oocyte maturation.
Keywords: Mouse, Oocyte, Membrane, Maturation, GPER
Introduction
Estrogens play a critical role in regulating many physiological processes in reproductive and non-reproductive tissues and mediating intracellular signaling pathways through binding to receptors. In addition to these classic genomic actions by activation of nuclear estrogen receptor (nER) and resulting in changes of transcription rates of a large number of estrogen-responsive genes, some estrogen receptors associated with the cell surface membrane and can be rapidly activated by exposure of cells to estrogen through non-genomic estrogen actions [1, 2]. Several cell and tissue models have been found in hypothalamic neurons, pancreatic islets, marophages, and human breast cancer cells, are not involved in nER but mediated by novel membrane estrogen receptor (mER) [3–6]. One purported membrane or endoplasmic reticulum ER is the G protein coupled receptor 30 (GPR30) [7–9].
Recent studies are suggesting GPR30 is one of mER that evoked comprehensive interests. G-protein estrogen receptor-1, known as GPER, mediates 17β-estradiol (E2) activation of signal transduction pathway in a variety of cells and displays as a typical of E2 binding membrane estrogen receptor. Estrogen treatment in GPER transfected cells causes rapid increasing in cAMP and calcium [10, 11], and further studies have shown that estrogen-mediated GPER-dependent activation of the MAP kinase Erk1/2 via EGFR [8]. Adenylyl cyclase activation by GPER has also been demonstrated [6, 12]. It has also been reported that GPER is the receptor mediating PI3K activation in response to estrogen via EGFR transactivation in ER-negative breast cancer cells such as SKBr3 [13]. Those findings suggest that GPER acts as the intermediary in non-classical rapid estrogen actions [14, 15].
It has been proposed that the process of oocyte membrane changes can be referred as oocyte membrane maturation [16]. Interestingly, Atlantic croaker GPER has been characterized as a membrane estrogen receptor coupled to a stimulatory G protein and expressed on the plasma membranes of the oocytes [1]. Estrogens have been found to act through GPER to maintain meiotic arrest via transactivation of EGFR and phosphorylation of MAPK3/1 signaling pathways in Zebrafish oocytes [17], indicating that GPER also plays a role in controlling oocyte maturation in this fish species. However, it is unclear that whether or not there is GPER on membrane of mammalian oocytes.
The objective of the present study is to determine the location and expression of GPER on the membrane of mouse oocytes and to compare the changes of expression of GPER during oocyte maturation as well as in vivo and in vitro matured oocytes.
Materials and methods
Animals
Mice (CD1, female: 8–10 weeks-old) were employed in this study. Mice were housed in a temperature-and light-controlled room, and free access to the food and water under a photoperiod 12 h-light and 12 h-dark. The experimental protocols and animal handling procedures were reviewed and approved by Animal Ethics Committee of McGill University.
Mature oocytes from ovaries stimulated by gonadotropins
Mature oocytes are defined here as the oocytes extruded first polar body (1 PB) into its pelliviteline space (PVS). Female mice were injected intraperitoneally with 5.0 IU of pregnant mare stimulating gonadotropin (PMSG; Sigma Chemical Co., USA). For mature oocyte collection, the female mice were further injected intraperitoneally with 5 IU of human chorionic gonadotropin (HCG; Sigma Chemical Co., USA) after 48 h of PMSG injection. Post 14 h of HCG injection, the mice were killed and the oviducts were dissected and placed into a Petri-dish containing modified human tubal fluid -HEPES buffered medium [18] (mHTF-HEPES) supplemented with 1.0 mg/ml bovine serum albumin (BSA; Sigma Chemical Co., USA). By tearing the ampullae, cumulus-oocyte complexes (COCs) were released and collected for immune-fluorescence or other experiments.
Immature oocytes from natural cycling ovaries
For immature oocyte collection, the natural cycling mice (without any stimulation with gonadotropins) were killed, and the ovaries and oviducts were dissected and placed into a Petri dish containing mHTF-HEPES. Before puncturing the visible follicles, the oviducts were checked to ensure that there were no COCs ovulated into the ampullae from the ovaries under a stereomicroscope. The visible follicles were punctured using 25-gauge needle. The fully growing immature oocytes (GV) surrounded with cumulus cells from antral follicles were selectively collected for in vitro maturation (IVM) of culture.
In-vitro maturation (IVM) of immature oocytes
After collection of immature COCs, they were rapidly washed three times in mHTF-HEPES containing 1.0 mg/ml BSA and then for maturation in culture. Oocyte maturation involved placing the COCs in Oocyte Maturation Medium (Cooper Surgical/SAGE, USA) supplemented with 10 % FBS, and 75 mIU/ml recombinant human FSH and LH (Serono, Canada). Briefly, following washing, 30–40 selected COCs were cultured for 17–18 h in an Organ Tissue Culture Dish (60 × 15 mm; Falcon) containing 1.0 ml of Oocyte Maturation Medium in an incubator at 37.0 °C with an atmosphere of 5 % CO2, 95 % air and high humidity. Following maturation in culture, COCs were denuded from cumulus cells by mechanically pipetting with a fine diameter pipette in mHTF-HEPES supplemented with 85 unit/ml hyaluronidase (Sigma Chemical Co., USA) for assessment of maturity. The mature oocytes were determined by the presence of a first polar body extrusion under a stereomicroscope, and then the mature oocytes were prepared for experiments.
Immuno-fluorescence and imaging
For GPER immune-staining experiments, oocytes collected with different stages were fixed with 3 % paraformaldehyde, and labeled with antibody to GPR30 (Abcam, USA) and followed by Alexa Fluor® 568 Goat Anti-Rabbit IgG (H+L) (Invitrogen, USA) and the nuclear stain DAPI. Images were obtained using a 63× (NA 1.40) oil Plan-Apochromat objective and detected with GaSaP detector by Zeiss LSM780 laser scanning confocal microscope (Carl Zeiss, Inc, Germany). DAPI was excited with 405 Diode laser, and GPER was excited with 561 nm DPASS laser. The Hi-Low intensity mode was used and Gain (master) and offset were kept the same during acquisition. Surface labeling was quantified using ImagePro analysis software (Olympus, Japan). Nuclei were selected by tracing and measured. An area outside the nuclear was similarly traced and measured, the value subtracted from the nuclear fluorescence values was measured as membrane fluorescence. For background subtraction, a parallel set of samples was similarly fixed and stained, except there was no primary antibody incubation. For 3D acquisition, Z-stacks with a 0.5 μm interval were captured. 3D Movie files were created using Image J software (Carl Zeiss, Inc, Germany).
Cell fractionation and Western blots
Cell fractionation was performed using the Plasma Membrane Protein Extraction Kit (Abcam 65400, USA). Briefly, plasma membrane proteins were purified from post-nuclear supernatants of oocytes. Cytosolic and plasma membrane fractions were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes for Western blot analysis of GPR30. For western blots, equal amounts of cell fraction lysates were separated on 10 % SDS-PAGE gels, transferred to nitrocellulose membranes (Millipore, USA) and immune-blotted with antibodies to GRP30, GAPDH, Na+/K+-ATPase α1 subunit, followed by appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Abcam, USA). Chemiluminescence was revealed using ECL (GE Healthcare Bio-Sciences, USA) and densitometry performed using Quantity One V4.62 software (Bio-Rad Laboratories, Hercules, CA, USA).
Experimental design
At the beginning, in vivo matured oocytes (n = 68) were collected to confirm whether or not expression of GPER on the membrane of mouse oocytes. Subsequently, quantitative expression of GPER in the different stages of oocytes (GV and M-II; n = 97 and n = 102, respectively), in vivo (n = 95) and in vitro matured (n = 98) oocytes as well as aged oocytes (mature oocytes further culture 5 h in vitro; n = 105) were compared.
Statistical analysis
Statistical analyses were performed in SPSS 16.0 software. The intensity of scanned images was compared. P value of <0.05 was considered to be statistically significant differences.
Results
Expression of GPER on oocytes plasma membrane
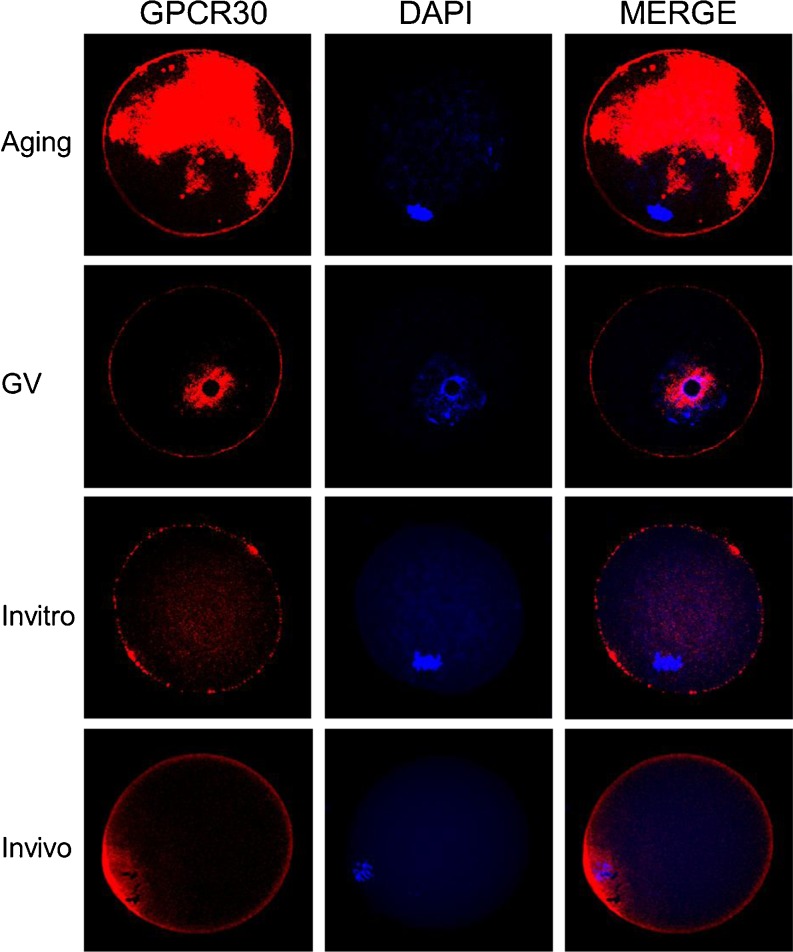
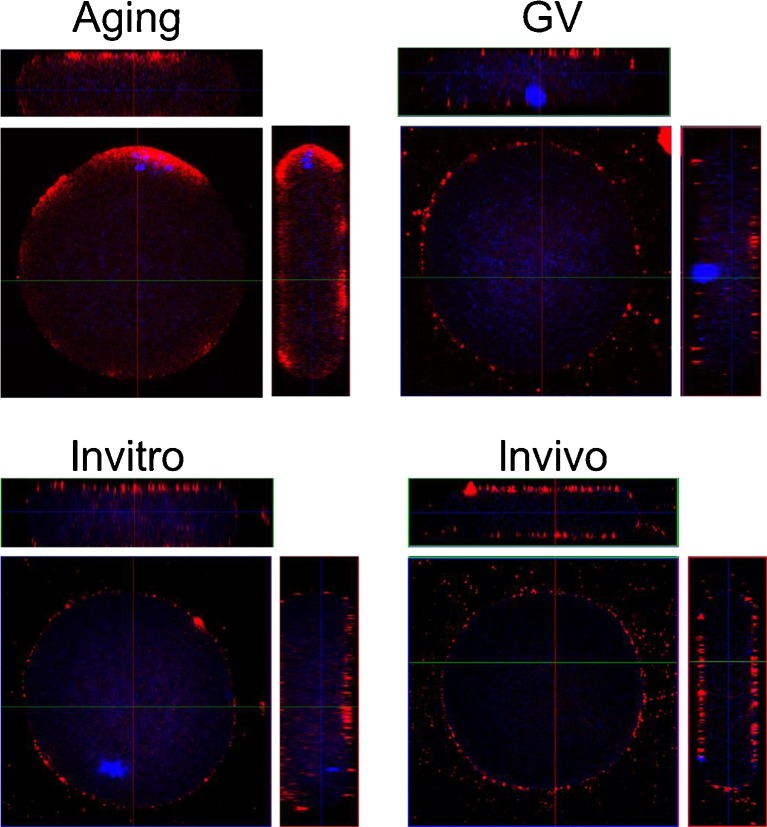
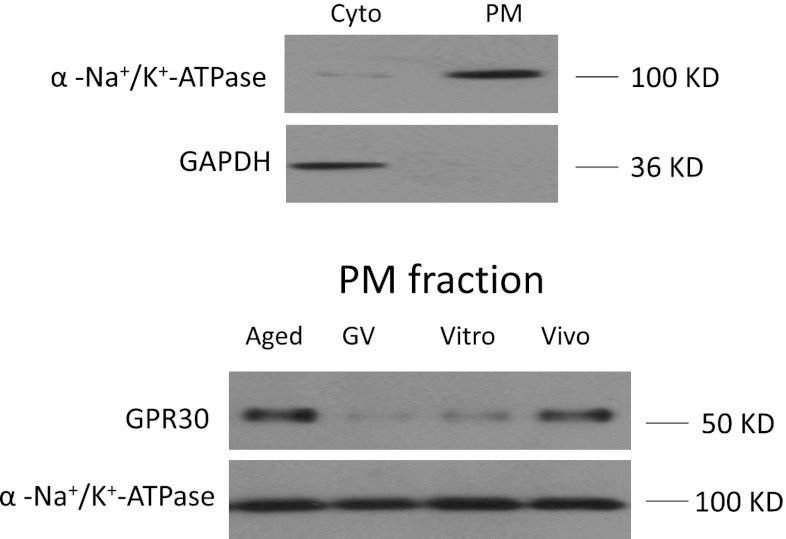
As shown in Fig. 1 (In vivo), there was GPER expression on the surface of mouse oocytes labeled by the specific GPER antibody and Alexa-568 nm conjugated second antibody. Z-stacks and 3D reconstruction also illustrated that GPER was observed on the plasma membrane of mouse oocytes (Fig. 2, In vivo). GPER were detected dominantly in membrane fraction (Fig. 4).
Fig. 1.
The images of GPR30 expression on mouse oocyte membrane during different maturation stages
Fig. 2.
The images from 3D acquisition of GPR30 expression on mouse oocyte membrane during different maturation stages
Fig. 4.
The confirmation of GPR30 expression on mouse oocyte membrane during different maturation stages by Western blots. Cyto cytosolic fraction; PM plasma membrane
The changes of GPER expression in different stages of oocyte maturation
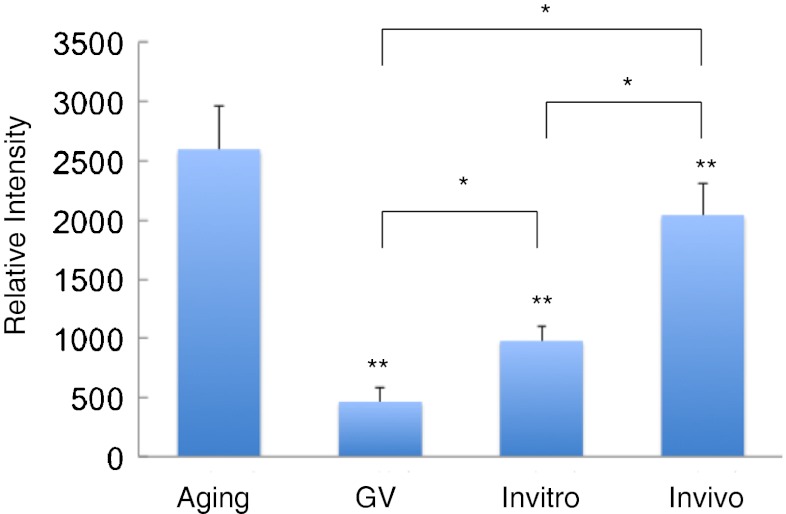
As shown in Figs. 2 and 3, the intensity of GPCR30 immuno-staining was significantly increased (P < 0.05) by over four-fold of initial values and reached the maximal level in aging oocytes compared with GV stage oocyte. Also western blot analysis showed a similar expression pattern of GPER protein expression as that was observed in GPER immune- fluorescence (Fig. 4). The expression level of GPER was lower in GV stage while reached the highest level at aging stage in the plasma membrane fraction.
Fig. 3.
Comparison of relative intensities of GPR30 expression on mouse oocyte membrane during different maturation stages. * indicates significant differences (P < 0.05) between groups; ** indicates significant differences (P < 0.01) compared with the aging group
The level of GPER expression from in vivo and in vitro matured oocytes
As shown in Fig. 2 (In vivo and In vitro) and 3 (In vivo and In vitro), the intensity of immune-staining of plasma membrane was approximately 2 times higher in the oocytes maturated in vivo than the oocytes matured in vitro. Furthermore, Western blots confirmed that the level of GPER expression on plasma membrane was significantly higher in oocytes matured in vivo compared to the oocytes matured in vitro (Fig. 4).
Discussions
In this study, we demonstrated first time that GPER is expressed on the plasma membrane of mouse oocytes by the methods of immune-fluorescence and western blots. We also demonstrated that expression of GPER on oocytes plasma membrane is increased following oocyte maturation and that there are different expressions of GPER from in vivo and in vitro matured oocytes and that the aging oocytes expressed the highest level of GPER.
The levels of cAMP [19] and cAMP-dependent protein kinases: protein kinase A (PKA), protein kinase C (PKC) and protein phosphatases (PPase) [17, 20] mediates the oocytes maturation. A major intracellular signaling cascade family implicated in the mitogen-activated protein kinase (MAPK) pathways [20, 21] plays important roles during the meiotic maturation of oocytes. Inhibition of the G-protein alpha S subunit stimulates xenopus oocyte maturation, suggesting that G-proteins also involved in oocyte maturation [22]. From the results of the presence of GPER on the oocyte membrane indicate that GPER may relate to the oocyte maturation process.
It has been shown that estrogen plays a key role in oocytes maturation in mammalian models by inducing a series of cellular signaling including inositol triphosphate (IP3) and ryanodine (Ry)-mediated intracellular free calcium concentration ([Ca2+]i) [23–26] and calcium signaling [27]. The development of the ability of oocytes to release Ca2+ in response to the fertilizing spermatozoa is an essential step during oocyte maturation [28]. In addition, GPER acts as one of the estrogen membrane receptors [17], and it mediates estrogen action [29]. Estrogen-mediated GPER-dependent activation of the MAPK Erk1/2 via EGFR transactivation [14, 15]. GPER might regulate calcium flux, cAMP and MAPK, leading to oocytes maturation [26, 30, 31]. The results of the present study showed that the level of GPER expression on oocyte membrane is increased gradually during oocyte maturation, indicating that GPER may act as an oocyte plasma membrane receptor binding to estrogen to influence directly the quality of oocytes maturation. Although the functional role of GPER in the oocytes has not been clarified yet, the evidence from present study implies a possible linkage between GPER and oocytes membrane maturation.
It is common believe that the embryonic developmental potential of in vitro matured oocytes is lower or poor than in vivo matured oocytes [32–35]. It has been reported that the expressions of growth differentiation factor-9 (GDF-9) and insulin-like growth factor II (IGF-II) are higher in vivo matured oocytes than in vitro matured oocytes [36]. Our results showed that not only GPER was expressed on oocyte membrane in both of in vivo and in vitro maturated oocytes, but also expressed significantly higher in in-vivo matured than in vitro matured oocytes. The correlation of GPER on oocyte plasma membrane with the quality of oocytes is not clear. However, the results of the present study showed that GPER on oocyte plasma membrane may play an important role during oocyte maturation.
It has been known that oocyte activation by sperm penetration or parthenogenetic activation is associated with the development of Ca2+ release system during oocyte maturation [28, 37]. The events of oocyte activation at fertilization are mediated by a sperm-induced increase in the concentration of intracellular free Ca2+ [38, 39]. Oocytes need to be primed with estradiol to develop Ca2+ oscillations during maturation [37, 40]. In addition, it has been reported that the parthenogenetic activation by chemicals is relatively easier in aging oocytes compared to ‘fresh’ mature oocytes [41]. Interestingly, we found that the significant expression of GPER on oocyte membrane was revealed in the aging oocytes compared with other oocytes, suggesting that the development of GPER on oocyte membrane during maturation may be involved in subsequent oocyte activation process.
In conclusion, the results from present study indicate that there is expression of GPER on mouse oocytes plasma membrane and that the level of GPER expression may be related to the functional role of oocyte maturation. Further study is required to investigate the potential role of GPER during oocyte maturation.
Acknowledgments
This research was supported by MUHC Reproductive Center and a grant from the China Natural Science Foundation (81270746) to RCC.
Footnotes
Capsule
The changes of expression of GPER on mouse oocytes plasma membrane confirm “oocyte membrane maturation”
Contributor Information
Ji-Chun Li, Email: lijichun2010@gmail.com.
Ri-Cheng Chian, Email: ri-cheng.chian@mcgill.ca.
References
- 1.Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–26. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas P, Alyea R, Pang Y, Peyton C, Dong J, Berg AH. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids. 2010;75:595–602. doi: 10.1016/j.steroids.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–91. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, et al. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58:2292–302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rettew JA, McCall SHT, Marriott I. GPR30/GPER-1 mediates rapid decreases in TLR4 expression on murine macrophages. Mol Cell Endocrinol. 2010;328:87–92. doi: 10.1016/j.mce.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–8. doi: 10.1016/S0960-0760(01)00190-X. [DOI] [PubMed] [Google Scholar]
- 7.Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–82. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/me.14.10.1649. [DOI] [PubMed] [Google Scholar]
- 9.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 10.Thomas D, Kim HY, Hanley MR. Regulation of inositol trisphosphate-induced membrane currents in Xenopus oocytes by a Jurkat cell calcium influx factor. Biochem J. 1996;318(Pt 2):649–56. doi: 10.1042/bj3180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas D, Kim HY, Morgan R, Hanley MR. Double-stranded-RNA-activated protein kinase (PKR) regulates Ca2+ stores in Xenopus oocytes. Biochem J. 1998;330(Pt 2):599–603. doi: 10.1042/bj3300599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 13.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;265–266:138–42. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pace MC, Thomas P. Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod. 2005;73:988–96. doi: 10.1095/biolreprod.105.041400. [DOI] [PubMed] [Google Scholar]
- 15.Ge C, Yu M, Zhang C. G protein-coupled receptor 30 mediates estrogen-induced proliferation of primordial germ cells via EGFR/Akt/beta-catenin signaling pathway. Endocrinology. 2012;153:3504–16. doi: 10.1210/en.2012-1200. [DOI] [PubMed] [Google Scholar]
- 16.Chian RC, Buckett WM, Tan SL. In-vitro maturation of human oocytes. Reprod Biomed Online. 2004;8:148–66. doi: 10.1016/S1472-6483(10)60511-1. [DOI] [PubMed] [Google Scholar]
- 17.Peyton C, Thomas P. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio) Biol Reprod. 2011;85:42–50. doi: 10.1095/biolreprod.110.088765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn PJ, Takahashi H, Hatta I. Characterization of complexes formed in fully hydrated dispersions of dipalmitoyl derivatives of phosphatidylcholine and diacylglycerol. Biophys J. 1995;68:1374–82. doi: 10.1016/S0006-3495(95)80310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50(Suppl 1):S195–219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 20.Mishra A, Joy KP. Involvement of mitogen-activated protein kinase in 2-hydroxyestradiol-17beta-induced oocyte maturation in the catfish Heteropneustes fossilis and a note on possible interaction with protein phosphatases. Gen Comp Endocrinol. 2006;147:329–35. doi: 10.1016/j.ygcen.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Fan HY, Sun QY. Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod. 2004;70:535–47. doi: 10.1095/biolreprod.103.022830. [DOI] [PubMed] [Google Scholar]
- 22.Gallo CJ, Hand AR, Jones TL, Jaffe LA. Stimulation of Xenopus oocyte maturation by inhibition of the G-protein alpha S subunit, a component of the plasma membrane and yolk platelet membranes. J Cell Biol. 1995;130:275–84. doi: 10.1083/jcb.130.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moody WJ, Lansman JB. Developmental regulation of Ca2+ and K+ currents during hormone-induced maturation of starfish oocytes. Proc Natl Acad Sci U S A. 1983;80:3096–100. doi: 10.1073/pnas.80.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tesarik J, Sousa M. Comparison of Ca2+ responses in human oocytes fertilized by subzonal insemination and by intracytoplasmic sperm injection. Fertil Steril. 1994;62:1197–204. doi: 10.1016/s0015-0282(16)57185-4. [DOI] [PubMed] [Google Scholar]
- 25.Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, et al. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133:4355–65. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toranzo GS, Buhler MC, Buhler MI. Participation of IP3R, RyR and L-type Ca2+ channel in the nuclear maturation of Rhinella arenarum oocytes. Zygote 2012:1–14. [DOI] [PubMed]
- 27.Tosti E. Calcium ion currents mediating oocyte maturation events. Reprod Biol Endocrinol. 2006;4:26. doi: 10.1186/1477-7827-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll J, Jones KT, Whittingham DG. Ca2+ release and the development of Ca2+ release mechanisms during oocyte maturation: a prelude to fertilization. Rev Reprod. 1996;1:137–43. doi: 10.1530/ror.0.0010137. [DOI] [PubMed] [Google Scholar]
- 29.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/me.16.1.70. [DOI] [PubMed] [Google Scholar]
- 30.Kostellow AB, Ziegler D, Morrill GA. Regulation of Ca2+ and cyclic AMP during the first meiotic division in amphibian oocytes by progesterone. J Cyclic Nucleotide Res. 1980;6:347–58. [PubMed] [Google Scholar]
- 31.Silvestre F, Boni R, Fissore RA, Tosti E. Ca2+ signaling during maturation of cumulus-oocyte complex in mammals. Mol Reprod Dev. 2011;78:744–56. doi: 10.1002/mrd.21332. [DOI] [PubMed] [Google Scholar]
- 32.Buckett WM, Chian RC, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007;110:885–91. doi: 10.1097/01.AOG.0000284627.38540.80. [DOI] [PubMed] [Google Scholar]
- 33.Huang JY, Chen HY, Park JY, Tan SL, Chian RC. Comparison of spindle and chromosome configuration in in vitro- and in vivo-matured mouse oocytes after vitrification. Fertil Steril. 2008;90:1424–32. doi: 10.1016/j.fertnstert.2007.07.1335. [DOI] [PubMed] [Google Scholar]
- 34.Son WY, Chung JT, Demirtas E, Holzer H, Sylvestre C, Buckett W, et al. Comparison of in-vitro maturation cycles with and without in-vivo matured oocytes retrieved. Reprod Biomed Online. 2008;17:59–67. doi: 10.1016/S1472-6483(10)60294-5. [DOI] [PubMed] [Google Scholar]
- 35.Cao YX, Chian RC. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med. 2009;27:456–64. doi: 10.1055/s-0029-1241055. [DOI] [PubMed] [Google Scholar]
- 36.Kim DH, Ko DS, Lee HC, Lee HJ, Park WI, Kim SS, et al. Comparison of maturation, fertilization, development, and gene expression of mouse oocytes grown in vitro and in vivo. J Assist Reprod Genet. 2004;21:233–40. doi: 10.1023/B:JARG.0000042008.83699.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51:1088–98. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- 38.Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–30. [PubMed] [Google Scholar]
- 39.Whitaker MJ, Swann K. Lighting the fuse at fertilization. Development. 1993;117:1–12. [Google Scholar]
- 40.Herbert M, Gillespie JI, Murdoch AP. Development of calcium signalling mechanisms during maturation of human oocytes. Mol Hum Reprod. 1997;3:965–73. doi: 10.1093/molehr/3.11.965. [DOI] [PubMed] [Google Scholar]
- 41.Ma SF, Liu XY, Miao DQ, Han ZB, Zhang X, Miao YL, et al. Parthenogenetic activation of mouse oocytes by strontium chloride: a search for the best conditions. Theriogenology. 2005;64:1142–57. doi: 10.1016/j.theriogenology.2005.03.002. [DOI] [PubMed] [Google Scholar]