Abstract
Purpose
To compare the number of oocytes per follicles in ovulation induction with 10,000 IU urinary hCG (uhCG) and two different doses of recombinant hCG (rhCG) in women undergoing intracytoplasmic sperm injection (ICSI) cycles.
Methods
This study was a prospective, randomized controlled trial which was performed on 180 primary infertile women undergoing ICSI cycles. All eligible patients underwent a standard GnRH-a long protocol. When at least two follicles reached a diameter of 18 mm, all patients were randomized to receive 10,000 IU urinary hCG or 250 μg recombinant hCG or 500 μg recombinant hCG for ovulation induction. Primary outcome measure included the number of oocytes retrieved per aspirated follicles. Secondary outcome measures were the number of oocytes retrieved, the number of mature oocytes, the number and quality of generated embryos, fertilization rate, implantation rate, chemical and clinical pregnancy rates and OHSS occurrence rate.
Results
The mean number of retrieved oocytes per follicles were 71.82 ± 15.09, 69.84 ± 17.44 and 77.16 ± 17.61 in 10,000 IU uhCG, 250 μg rhCG and 500 μg rhCG, respectively which was significantly higher with 500 μg rhCG than the lower dose(P = .04). Other cycles and clinical outcomes were comparable between groups.
Conclusion
Recombinant hCG shows equivalent efficacy to urinary hCG in terms of the number of oocytes per aspirated follicles in selected patients undergoing ICSI; however, 500 μg rhCG seems to be more advantageous than the lower dose in this indication. Larger randomized trials are needed to generalize this strategy.
ClinicalTrials.gov identifier: NCT01507376.
Keywords: Intracytoplasmic sperm injection, Oocyte per follicle, Ovulation induction, Recombinant hCG, Urinary hCG
Introduction
The importance of LH surge in the final maturation of the oocyte and in oocyte retrieval has been well characterized [1]. Since hCG and LH bind and function through a common hCG/LH receptor, uhCG can mimic the endogenous LH surge in assisted reproductive techniques and due to higher receptor affinity acts as a more potent alternative [2].
There have been widespread uses of urinary hCG in several decades. However, as a urinary derived preparation, it comprises some disadvantages such as vast amount of urine required for getting the highest purity, the possible contamination with other proteins and batch to batch inconsistency [3] which leads to variations in clinical results between patients and also within the same patients in different cycles [4]. Subcutaneous rhCG has been recently introduced for final follicular maturation and ovulation induction in infertile women undergoing ART. rhCG preparations are derived from genetically engineered Chinese hamster ovary cells through recombinant DNA technology [5]. This product is purified by repeated chromatographic steps to produce a high specific activity outcome which makes the drug free from urinary contaminations and suitable for subcutaneous injection and self administration with lower local reactions and higher tolerability [6–8].
The pharmacokinetics and pharmacodynamics of rhCG are equal to uhCG which makes it a suitable alternative for uhCG in clinical applications [9]. In clinical practice, several trials have been performed to compare safety and efficacy of urinary and recombinant hCG preparations with different points of views. Some randomized trials have found equal efficiency with these two preparations [8, 10, 11] whereas some others have observed better outcomes in women who received rhCG [12, 13]. On the other hand, based on a systematic review on 11 studies, uhCG has been stated as the best option for ovulation induction in ART [14]. Moreover, the optimal dose of rhCG preparations for ovulation induction has not still been determined [7, 15].
In many IVF cycles, in spite of numerous developed follicles visualized at ultrasound, scant number of oocytes is retrieved which leads to poor pregnancy outcomes. Even though, the exact reason of this phenomenon is still ambiguous, many reports in the literature claim that rhCG can improve the number of oocytes per follicles in patients that have been encountered with lack of retrieved oocytes from follicles in previous cycles [4, 16].
Therefore, this study aimed to compare oocyte per follicle ratio in ovulation induction with 10,000 IU uhCG and two different doses of rhCG in infertile women undergoing ICSI.
Materials and methods
Study design
This single center, open-label, parallel, randomized controlled trial was performed in Reproductive Biomedicine Research Center, Royan Institute, Tehran, Iran between October 2010 and February 2012.
This study was reviewed and approved by the institutional review board and Ethics Committee of royan institute and registered in the Clinical Trial Website (www.clinicaltrials.gov, number NCT01507376). All volunteers for participation in this study were informed regarding the purpose and method of the study and signed the written informed consent.
Patients
A total of 180 primary infertile women who were eligible for the ICSI program were entered into this study.
Inclusion criteria were listed as per here under:
Indication for ICSI procedures and long protocol
Age: 20–37 years old
Body Mass Index (BMI) ≤30 Kg/m2
Normal ovarian reserve based on antral follicle count >5 and basal FSH < 12 IU/L
Regular spontaneous menstrual cycles (25–35 days)
Male and/or tubal factor
The presence of two functional ovaries and no previous ovarian surgery
The presence of normal uterine cavity based on recent hysterosalpingographic or hystroscopic evaluation
No clinically current serious systemic disease
No more than two previous ART attempts
No ovarian stimulation treatment in the preceding 2 months
Exclusion criteria were:
Contraindications to any type of gonadotropin agents
Polycystic ovarian syndrome
Poor ovarian response to Controlled Ovarian Hyperstimulation (COH) in recent cycle
Previous history of poor ovarian response to COH
In this study, all patients underwent ICSI cycle that the cumulus oophorus is removed for maturity assessment. Therefore, patients underwent conventional IVF cycles were excluded.
Randomization and stimulation regimens
In this study, to minimize bias due to different regimens, all eligible patients underwent a standard long protocol using GnRH-a (Superfact,Aventis,Frankfurt,Germany) at a subcutaneous daily dose of 0.5 mg commencing on the day 17–19 of the natural menstrual cycle as a pre-treatment. Once pituitary desensitization was confirmed (endometrial thickness <5 mm and serum estradiol level <50 pg/ml), the GnRH-a dose was reduced to half and ovarian stimulation was initiated.
In all study patients, ovarian stimulation started with a dose of 150–225 IU r-FSH (gonal-F, Merck Serono, Switzerland) with regard to the patients’ age and continued until the day of ovulatory hCG administration according to the ovarian response. When at least two follicles were greater than 18 mm, all patients were randomly allocated to three following groups for ovulation induction:
10,000 IU urinary hCG (Choriomon,IBSA,Lugano,Switzerland) intramuscularly was administered,
received a subcutaneous injection of 250 μg recombinant hCG (Ovitrelle, Merck Serono, Geneva, Switzerland),
received a subcutaneous injection of 500 μg recombinant hCG (Ovitrelle; Merck Serono, Geneva, Switzerland)
A permuted block method was used for randomization which was generated by the statistician and applied by a midwife in clinic. The block size was considered equal to six. Oocyte retrieval was performed 34–36 h later.
Immediately, before ICSI procedure, the cumulus corona cells were removed and each oocyte was examined under an inverted high-resolution microscope for assessment of maturity. The oocyte maturity assessed according to Veeck criteria [17].
ICSI was performed and embryos were scored after 44–72 h according to the previous stated quality criteria [18]. Embryo transfer was done routinely on 2nd or 3rd day after oocyte retrieval and up to 3 embryos per patient were transferred at most. Luteal phase was supported by 400 mg twice a day of progesterone (Cyclogest;Actavis,United Kingdom) vaginally from the day after oocyte retrieval.
Outcome measures
Primary outcome measure included the number of oocytes retrieved per number of aspirated follicles. Secondary outcome measures were the number of oocytes retrieved, the number of mature oocytes, the number and quality of generated embryos, fertilization rate, implantation rate, chemical and clinical pregnancy rates and OHSS occurrence rate.
The implantation rate was considered as the number of gestational sacs with fetal heart rate, divided by the number of embryos transferred. Clinical pregnancy was defined as the presence of a gestational sac with fetal heart rate on ultrasound.
Statistical analysis
All data analysis was performed by using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 18.0. The comparison of quantitative variables between three groups was done by one way analysis of variance (ANOVA). Also, two ways analysis of variance with interaction between BMI and group (250 μg rhCG and 500 μg rhCG) was performed to analyze number of retrieved oocytes per follicles. Test of homogeneity of variance and normality of data were done using Levene and Kolmogorov-Smirnov tests respectively. Qualitative variables were analyzed by Chi-square test. P < .05 was considered statistically significant. Values are expressed as mean ± SD or number and percentage in tables.
In a one-way ANOVA study, sample sizes of 59 obtained for each of the 3 groups. A total sample of 177 subjects achieve 80 % power to detect differences among the means versus the alternative of equal means using a F test with a 0.05000 significance level. The size of the variation in the means is represented by their standard deviation which is 1.41. The common standard deviation within a group is assumed to be 6.00. Considering the 10% loss in the sample, the sample size obtained 65 in each group.
Results
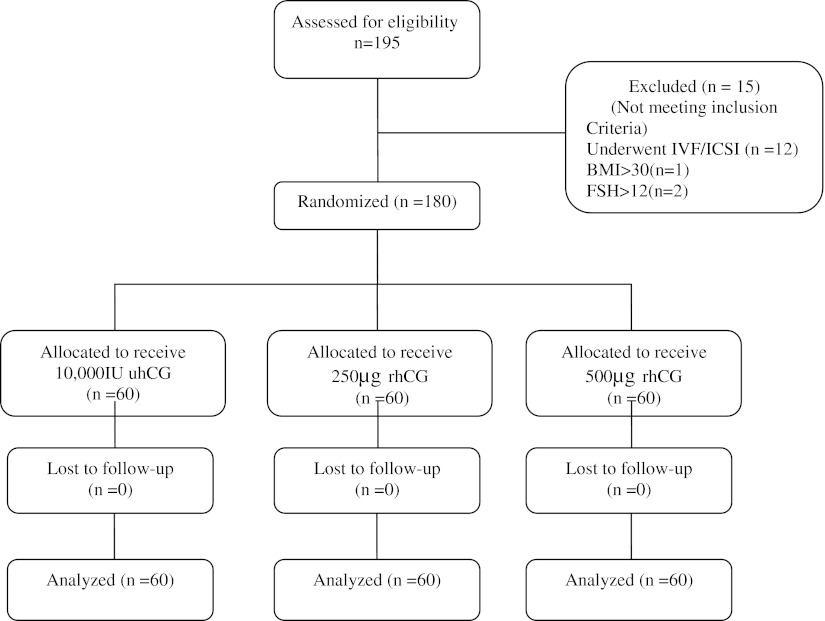
195 primary infertile women were assessed for eligibility. Overall, 180 infertile women which were eligible for participation, were entered to this study, of whom 60 patients were randomly allocated to receive 10,000 IU uhCG, 60 patients were allocated to receive 250 μg rhCG and 60 patients were allocated to receive 500 μg rhCG (Fig. 1).
Fig. 1.
Flow diagram of the clinical trial according to the CONSORT guideline
Baseline characteristics of patients treated with recombinant or urinary hCG has been shown in Table 1. There were no differences in age, BMI, duration of infertility, causes of infertility, previous IVF attempts and basal FSH between groups.
Table 1.
Baseline characteristics of patients treated with uhCG or rhCG
| Variable | Group A uhCG n = 60 | Group B rhCG (250 μg) n = 60 | Group C rhCG (500 μg) n = 60 | p-value |
|---|---|---|---|---|
| Age(years) | 29.02 ± 4.00 | 29.23 ± 3.46 | 28.12 ± 3.71 | 0.22 |
| BMI(kg/m2) | 24.85 ± 2.08 | 24.44 ± 2.50 | 24.06 ± 2.31 | 0.17 |
| Duration of infertility (years) | 7.22 ± 3.84 | 7.23 ± 3.70 | 7.17 ± 3.76 | 0.99 |
| Causes of infertility | ||||
| Tubal | 1(1.7) | 0(0) | 2(3.3) | 0.22 |
| Male | 56(93.3) | 60(100) | 57(95) | |
| Tubal and male | 3(5) | 0(0) | 1(1.7) | |
| Number of previous IVF attempts | ||||
| 0 | 55(91.7) | 57(95) | 57(95) | 0.17 |
| 1 | 2(3.3) | 3(5) | 3(5) | |
| 2 | 3(5) | 0(0) | 0(0) | |
| Basal FSH(mIU/mL) | 6.71 ± 1.77 | 6.71 ± 2.30 | 6.56 ± 2.57 | 0.91 |
Values are expressed as mean ± standard deviation or number (percentage)
Cycle outcomes of patients treated with uhCG or rhCG have been presented in Table 2.
Table 2.
Cycle outcomes of patients treated with uhCG or rhCG
| Variable | Group A uhCG n = 60 | Group B rhCG 250 μg n = 60 | Group C rhCG 500 μg n = 60 | p-value |
|---|---|---|---|---|
| Duration of stimulation (day) | 10.13 ± 1.50 | 10.37 ± 1.54 | 10.37 ± 1.40 | 0.60 |
| Total dose of FSH required (IU) | 1626 ± 385.77 | 1647 ± 614.74 | 1586 ± 321.42 | 0.75 |
| Endometrial thickness(mm) | 9.7 ± 1.38 | 9.6 ± 1.74 | 10.01 ± 1.58 | 0.35 |
| Number of aspirated Follicles | 16.27 ± 7.80 | 17.65 ± 7.73 | 16.08 ± 6.81 | 0.45 |
| Number of retrieved oocytes | 11.37 ± 5.3 | 12.40 ± 6.44 | 12.25 ± 5.30 | 0.56 |
| Number of retrieved oocytes per aspirated follicles | 71.82 ± 15.09 | 69.84 ± 17.44 | 77.16 ± 17.61 a | 0.04 |
| Number of metaphase II oocytes (MII) | 9.62 ± 4.50 | 10.67 ± 5.88 | 10.75 ± 5.07 | 0.41 |
| Number of immature oocytes (MI&GV) | 1.41 ± 2.64 | 1.11 ± 1.53 | 1.15 ± 1.60 | 0.66 |
| Fertilization rate | 61.2(364/595) | 64.1(408/637) | 59.8(383/640) | 0.28 |
| Number of produced embryos | 6.18 ± 3.78 | 6.98 ± 5.02 | 6.62 ± 3.67 | 0.58 |
| Number of excellent quality embryos | 1.60 ± 2.14 | 2.40 ± 3.04 | 1.85 ± 2.00 | 0.18 |
| Number of good quality embryos | 2.30 ± 1.90 | 2.20 ± 1.92 | 2.12 ± 1.60 | 0.85 |
| Number of transferred embryos | 2.07 ± .95 | 1.72 ± 1.13 | 2.15 ± .84 | 0.06 |
| Number of embryos frozen | 2.05 ± 2.82 | 3.12 ± 3.94 | 2.40 ± 3.02 | 0.19 |
Values are expressed as mean ± standard deviation or number (percentage)
aGroup C versus group B (P = .04)
Duration of stimulation, total dose of FSH required, endometrial thickness, number of aspirated follicles, number of retrieved oocytes, number of metaphase II oocytes and immature oocytes, fertilization rate, number of produced embryos, excellent and good quality embryos, number of transferred embryos were not significantly different between groups.
Mean number of retrieved oocytes per follicles were 71.82 ± 15.09, 69.84 ± 17.44 and 77.16 ± 17.61 in 10,000 IU uhCG, 250 μg rhCG and 500 μg rhCG, respectively which was significantly higher with 500 μg rhCG than the lower dose(P = .04). Overall, 27 patients didn’t undergo embryo transfer including: 7 patients (11.7 %) in group A, 15 patients (25 %) in group B and 5 patients (8.3 %) in group C. In 26 patients, all embryos were frozen and in one patient no embryo was formed. No cycle cancellation was observed in three groups during the study.
No significant differences have been shown between three groups in terms of implantation rate and chemical and clinical pregnancy rates (Table 3). OHSS were occurred in 13 patients. All of the cases were mild and only one moderate case was observed. The occurrence rate of OHSS was comparable between groups (Table 3). The interaction between BMI and group (250 μg rhCG and 500 μg rhCG) was not significant (p = 0.13).
Table 3.
clinical outcomes of patients treated with uhCG or rhCG
| Variable | Group A uhCG n = 60 | Group B rhCG 250 μg n = 60 | Group C rhCG 500 μg n = 60 | p-value |
|---|---|---|---|---|
| Implantation rate (%) | 24.2(30/124) | 25.2(26/103) | 20.2(26/129) | 0.61 |
| Chemical pregnancy rate (%) | 43.4(23/53) | 46.7(21/45) | 43.6(24/55) | 0.93 |
| Clinical pregnancy rate (%) | 43.4(23/53) | 42.2(19/45) | 34.5(19/55) | 0.60 |
| Occurrence of OHSS (%) | 3(5) | 4(6.7) | 6(10) | 0.56 |
Values are expressed as number (percentage)
Discussion
The initiation of LH surge in spontaneous menstrual cycles which occur abruptly in midcycle [19] leads to periovulatory events such as resumption of oocytes meiotic maturation, follicle rupture, oocyte expelling and corpus luteum formation [1].
hCG can act as a surrogate for LH surge to induce final oocyte maturation and ovulation in COH protocols and develop similar periovulatory events such as softening of the connective tissue of follicle which makes easy detachment of oocyte cumulus complex from the follicles wall, hence ease of aspiration [20]. On the other hand, 4–8 h after hCG injection, the cumulus cells become dispersed which leads to cumulus oocyte contact disruption [21].
With considering the aforementioned substantial role of the hCG in intrafollicular events and ovum release in IVF cycles, the number of oocytes per follicle would be a proper and reasonable measure for assessing the efficiency of the hCG. Generally, IVF cycles are rarely encountered with empty follicle syndrome. However, the occurrence of obtaining few numbers of oocytes from great number of growing follicles is a common phenomenon.
It has been reported that the inconsistency and low bioavailability of urinary preparations of hCG might result to lack of retrieved oocytes or empty follicle syndrome [4, 22] which is due to some difficulties through production, packaging and storage process of the drug and also rapid clearance of the drug by liver [23]. Recently, the high purity and batch to batch consistency of recombinant preparations provides an effective and well tolerated alternative for urinary derived agents in IVF cycles.
In our study, we found that the number of retrieved oocytes per follicles was comparable between rhCG and uhCG. However, 500 μg rhCG was better than the lower dose in this indication.
Littman and Milki found that addition of 250 μg recombinant hCG to 5000 IU urinary hCG for ovulation induction increase oocyte/follicle ratio in patients with scant oocyte yield in previous cycles [16]. Conversely, in a retrospective analysis on 744 patients, significant higher percentage of oocytes were retrieved per number of follicles in uhCG compared to rhCG group (86 % versus 80 %) [24]. On the other hand, Driscoll et al didn’t find any significant differences in the number of retrieved oocytes/follicles between two groups triggered with urinary and recombinant hCG [10].
This study demonstrated that the number of retrieved oocytes and mature oocytes were not significantly different between groups. Our findings confirm the data from a previous Open-label RCT which the number of retrieved oocytes was comparable between 10,000 IU uhCG, 250 μg rhCG and 500 μg rhCG [7]. Several other studies showed similar results in comparison of urinary and recombinant hCG [6, 8, 10, 11, 25]. Moreover, Al-Inany et al in a systematic review concluded that in selecting the type of hCG for trigger of ovulation, additional factors such as safety, cost and drug availability should be considered [26].
Contrary to aforementioned studies, in some previous randomized trials, the oocytes quality was improved by administration of 250 μg rHCG rather than 5000 or 10,000 IU uhCG [12, 27].
In contrast to two previous studies [13, 28], we didn’t find any superiority in administration of rhCG to uhCG in fertilization, implantation and pregnancy rates.
In our study, the number of retrieved oocytes per follicles was significantly higher with administration of 500 μg rhCG than the lower dose. Whereas, some studies believe that 250 μg rhCG is the optimal dose for ovulation induction and no advantage could be attributed to the higher dose of this treatment [29]. We also found that higher dose of rhCG had no more beneficial effect in women with BMI > 26 than women with lower BMI. Kahraman et al, reported the similar result [30]. Chang et al, found that the numbers of 2PN fertilized oocytes and cleaved embryos were significantly higher with 500 μg rhCG than the lower dose without enhancement of OHSS incidence [7].
In the current study, the incidence of OHSS as a serious complication of this treatment was comparable between groups. Surprisingly, in the study of Chan et al, there was a trend toward higher incidence of OHSS with 250 μg rhCG compared to 500 μg rhCG, although it didn’t reach statistically significant [15]. Therefore, there is still no general consensus regarding the proper dosage of rhCG with consideration of the safety and efficacy and further studies would be required.
In this study, the main outcome depends on the experience of the infertility specialist to a certain degree that means oocyte retrieval performed with different specialist would be one of the limitations of the current study.
To sum up, recombinant hCG shows equivalent efficacy to urinary hCG in terms of the number of oocytes per aspirated follicles in selected patients undergoing ICSI; however, 500 μg rhCG seems to be more advantageous than the lower dose in this indication. Larger randomized trials are needed to generalize this strategy.
Acknowledgments
We would like to extend our special thanks to Shabnam Khodabakhshi and Azam Sanati for their help in data collection and data entry.
Footnotes
Capsule
Recombinant hCG shows equivalent efficacy to urinary hCG in terms of the number of oocytes per aspirated follicles in selected patients undergoing ICSI; however, 500 μg rhCG seems to be more advantageous than the lower dose in this indication.
References
- 1.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–55. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 2.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–95. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 3.The International Recombinant Human Chorionic Gonadotropin Study Group Induction of ovulation in World Health Organization group II anovulatory women undergoing follicular stimulation with recombinant human follicle-stimulating hormone: a comparison of recombinant human chorionic gonadotropin (rhCG) and urinary hCG. Fertil Steril. 2001;75(6):1111–8. doi: 10.1016/S0015-0282(01)01803-9. [DOI] [PubMed] [Google Scholar]
- 4.Penarrubia J, Balasch J, Fabregues F, Creus M, Civico S, Vanrell JA. Recurrent empty follicle syndrome successfully treated with recombinant human chorionic gonadotrophin. Hum Reprod. 1999;14(7):1703–6. doi: 10.1093/humrep/14.7.1703. [DOI] [PubMed] [Google Scholar]
- 5.Loumaye E, Martineau I, Piazzi A, et al. Clinical assessment of human gonadotrophins produced by recombinant DNA technology. Hum Reprod. 1996;11(Suppl 1):95–107. doi: 10.1093/humrep/11.suppl_5.95. [DOI] [PubMed] [Google Scholar]
- 6.Abdelmassih V, Oliveira FG, Goncalves SP, Varella AD, Diamond MP, Abdelmassih R. A prospective, randomized and blinded comparison between 10,000 IU urinary and 250 microg recombinant human chorionic gonadotropin for oocyte maturation in in vitro fertilization cycles. J Assist Reprod Genet. 2005;22(4):149–53. doi: 10.1007/s10815-005-4911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang P, Kenley S, Burns T, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: results of a clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (Profasi) for induction of final follicular maturation in in vitro fertilization-embryo transfer. Fertil Steril. 2001;76(1):67–74. doi: 10.1016/S0015-0282(01)01851-9. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs P, Kovats T, Bernard A, Zadori J, Szmatona G, Kaali SG. Comparison of serum and follicular fluid hormone levels with recombinant and urinary human chorionic gonadotropin during in vitro fertilization. Fertil Steril. 2008;90(6):2133–7. doi: 10.1016/j.fertnstert.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Trinchard-Lugan I, Khan A, Porchet HC, Munafo A. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotrophin in healthy male and female volunteers. Reprod Biomed Online. 2002;4(2):106–15. doi: 10.1016/S1472-6483(10)61927-X. [DOI] [PubMed] [Google Scholar]
- 10.Driscoll GL, Tyler JP, Hangan JT, Fisher PR, Birdsall MA, Knight DC. A prospective, randomized, controlled, double-blind, double-dummy comparison of recombinant and urinary HCG for inducing oocyte maturation and follicular luteinization in ovarian stimulation. Hum Reprod. 2000;15(6):1305–10. doi: 10.1093/humrep/15.6.1305. [DOI] [PubMed] [Google Scholar]
- 11.Sakhel K, Khedr M, Schwark S, Ashraf M, Fakih MH, Abuzeid M. Comparison of urinary and recombinant human chorionic gonadotropin during ovulation induction in intrauterine insemination cycles: a prospective randomized clinical trial. Fertil Steril. 2007;87(6):1357–62. doi: 10.1016/j.fertnstert.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 12.The European Recombinant Human Chorionic Gonadotrophin Study Group Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction treatment--recombinant HCG versus urinary HCG. The European Recombinant Human Chorionic Gonadotrophin Study Group. Hum Reprod. 2000;15(7):1446–51. doi: 10.1093/humrep/15.7.1446. [DOI] [PubMed] [Google Scholar]
- 13.Papanikolaou EG, Fatemi H, Camus M, et al. Higher birth rate after recombinant hCG triggering compared with urinary-derived hCG in single-blastocyst IVF antagonist cycles: a randomized controlled trial. Fertil Steril. 2010;94(7):2902–4. doi: 10.1016/j.fertnstert.2010.04.077. [DOI] [PubMed] [Google Scholar]
- 14.Youssef MA, Al-Inany HG, Aboulghar M, Mansour R, Abou-Setta AM. Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database Syst Rev. 2011;4:CD003719. doi: 10.1002/14651858.CD003719.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Chan CC, Ng EH, Tang OS, Yeung WS, Lau EY, Ho PC. A prospective, randomized, double-blind study to compare two doses of recombinant human chorionic gonadotropin in inducing final oocyte maturity and the hormonal profile during the luteal phase. J Clin Endocrinol Metab. 2005;90(7):3933–8. doi: 10.1210/jc.2004-2169. [DOI] [PubMed] [Google Scholar]
- 16.Littman ED, Milki AA. The combination of urinary and recombinant HCG improves outcome in patients with decreased oocyte/follicle ratio in previous cycles. Eur J Obstet Gynecol Reprod Biol. 2003;109(1):60–2. doi: 10.1016/S0301-2115(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 17.Veeck LL. Oocyte assessment and biological performance. Ann N Y Acad Sci. 1988;541:259–74. doi: 10.1111/j.1749-6632.1988.tb22263.x. [DOI] [PubMed] [Google Scholar]
- 18.Rezazadeh Valojerdi M, Eftekhari-Yazdi P, Karimian L, Hassani F, Movaghar B. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet. 2009;26(6):347–54. doi: 10.1007/s10815-009-9318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoff JD, Quigley ME, Yen SS. Hormonal dynamics at midcycle: a reevaluation. J Clin Endocrinol Metab. 1983;57(4):792–6. doi: 10.1210/jcem-57-4-792. [DOI] [PubMed] [Google Scholar]
- 20.Meniru GI, Craft IL. Evidence from a salvaged treatment cycle supports an aetiology for the empty follicle syndrome that is related to terminal follicular developmental events. Hum Reprod. 1997;12(11):2385–7. doi: 10.1093/humrep/12.11.2385. [DOI] [PubMed] [Google Scholar]
- 21.Phillips DM, Dekel N. Maturation of the rat cumulus-oocyte complex: structure and function. Mol Reprod Dev. 1991;28(3):297–306. doi: 10.1002/mrd.1080280313. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig M, Doody KJ, Doody KM. Use of recombinant human chorionic gonadotropin in ovulation induction. Fertil Steril. 2003;79(5):1051–9. doi: 10.1016/S0015-0282(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 23.Zegers-Hochschild F, Fernandez E, Mackenna A, Fabres C, Altieri E, Lopez T. The empty follicle syndrome: a pharmaceutical industry syndrome. Hum Reprod. 1995;10(9):2262–5. doi: 10.1093/oxfordjournals.humrep.a136281. [DOI] [PubMed] [Google Scholar]
- 24.Krotz S, Bhagavath B, Hackett R, Pagidas K, Carson S, Robins J. Comparison of IVF retrieval and clinical outcomes in 744 patients using recombinant versus urinary human chorionic gonadotropins to trigger ovulation. Fertil Steril. 2008;90:S224–S5. doi: 10.1016/j.fertnstert.2008.07.543. [DOI] [Google Scholar]
- 25.Uhler ML, Beltsos AN, Grotjan HE, Lederer KJ, Lifchez AS. Age-matched comparison of recombinant and urinary HCG for final follicular maturation. Reprod Biomed Online. 2006;13(3):315–20. doi: 10.1016/S1472-6483(10)61433-2. [DOI] [PubMed] [Google Scholar]
- 26.Al-Inany HG, Aboulghar M, Mansour R, Proctor M. Recombinant versus urinary human chorionic gonadotrophin for ovulation induction in assisted conception. Cochrane Database Syst Rev. 2005;2:CD003719. doi: 10.1002/14651858.CD003719.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Farrag A, Costantini A, Manna C, Grimaldi G. Recombinant HCG for triggering ovulation increases the rate of mature oocytes in women treated for ICSI. J Assist Reprod Genet. 2008;25(9–10):461–6. doi: 10.1007/s10815-008-9262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeke J, Kanyo K, Zeke H, et al. Pregnancy rates with recombinant versus urinary human chorionic gonadotropin in in vitro fertilization: an observational study. Sci World J. 2011;11:1781–7. doi: 10.1100/2011/409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clua E, Martinez F, Tur R, Sanmartin P, Chueca A, Barri PN. Triggering ovulation with 250 mug or 500 mug of r-hCG in oocyte donors treated with antagonist protocol has no effect on the number of mature oocytes retrieved: a randomized clinical trial. Gynecol Endocrinol. 2012;28(9):678–81. [DOI] [PubMed]
- 30.Kahraman S, Karlikaya G, Kavrut M, Karagozoglu H. A prospective, randomized, controlled study to compare two doses of recombinant human chorionic gonadotropin in serum and follicular fluid in woman with high body mass index. Fertil Steril. 2010;93(6):2084–7. doi: 10.1016/j.fertnstert.2009.08.026. [DOI] [PubMed] [Google Scholar]