Abstract
Despite being a histologically dynamic organ, mechanisms coordinating uterine regeneration during the menstrual/estrous cycle and following parturition are poorly understood. In the current study, we hypothesized that endometrial epithelial tissue regeneration is accomplished, in part, by mesenchymal-to-epithelial transition (MET). To test this hypothesis, fate mapping studies were completed using a double transgenic (Tg) reporter strain, Amhr2-Cre; Rosa26-Stopfl/fl-EYFP (i.e., flox-stop EYFP reporter). EYFP expression was observed in Müllerian duct mesenchyme-derived stroma and myometrium, but not epithelia in young and peripubertal double Tg female mice. However, mosaic EYFP expression was observed in epithelia of double Tg mice after parturition. To ensure the observed epithelial EYFP expression was not due to leaky Amhr2 promoter activity, resulting in aberrant Cre expression, transgenic mice expressing LacZ under the control of the Amhr2 promoter (Amhr2-LacZ) were used to monitor β-galactosidase (β-Gal) activity within the uterus. β-Gal activity was not detected in luminal or glandular epithelia regardless of age, reproductive status, or degree of damage incurred within the uterus. Lastly, a unique population of transitional cells was identified that expressed the epithelial cell marker, pan-cytokeratin, and the stromal cell marker, vimentin. These cells localized predominantly to the regeneration zone in the mesometrial region of the endometrium. These findings suggest a previously unappreciated role for MET in endometrial regeneration and have important implications for proliferative diseases of the endometrium such as endometriosis.
Introduction
Approximately 11% of women in the United States of reproductive age (15–44) are infertile [1]. Endometrial cancer and endometriosis are two hyperproliferative and debilitating diseases of the endometrium that often result in infertility. In 2012, it is estimated that more than 47,000 new cases of endometrial cancer will be diagnosed in the United States [2], and an additional 5.5 million women in North America suffer from endometriosis [3]. In addition, many women using in vitro fertilization experience pregnancy failure due to inadequate thickening of the endometrium for implantation [4]. Despite the extensive regenerative capacity of the uterus and the prevalence of proliferative diseases of the endometrium, our current understanding of the mechanisms of uterine regeneration and how deregulation of these processes may contribute to endometrial disease is limited.
The uterus of most hemochorial implanting species is a remarkably resilient organ that undergoes two postnatal developmental processes which involve dramatic tissue remodeling. The first occurs early postnatally as the incompletely formed Müllerian ducts mature to form the majority of the adult female reproductive tract, including the uterus [reviewed in: 5,6]. In mice, maturation of the uterus begins at birth and involves region-specific luminal epithelial (LE) differentiation, mesenchymal differentiation to form the stromal and myometrial layers, and formation of epithelial glands. By postnatal day (PND) 15, the basic uterine architecture consisting of LE, glandular epithelium (GE), stroma, and multi-layered myometrium is established. The second process, which can be broadly classified as involution, occurs during the menstrual cycle in humans and old world primates as well as after parturition in all placental mammals. Throughout a woman's reproductive years, the uterus undergoes 300–400 cycles of tissue remodeling, including cellular proliferation, differentiation, degeneration (menses), and regeneration. During menses, endometrial tissue comprising the functionalis layer degenerates and is shed from the body. This, in turn, results in the need for substantial endometrial regeneration. Extensive endometrial regeneration also occurs after parturition and expulsion of the placenta, in which only a small portion of the endometrium remains. It is presumed that this residual tissue serves as the seed for endometrial regeneration. Proper endometrial regeneration after menses or parturition is required for preparation of the uterus for ensuing reproductive cycles and pregnancies.
Most adult organs exhibit some degree of plasticity, and advances in stem cell biology have established a role for adult stem cells in tissue renewal. Recently, it has been proposed that bone marrow-derived cells contribute to uterine regeneration [7–9]; however, the functional contribution of these cells to the endometrium has not yet been established. Cervelló et al. demonstrated that although bone marrow-derived cells engraft in the endometrium of transplant recipients, they do not contribute to the side population [10]. An alternative or additive mechanism of regeneration to stem cell theory is cellular transdifferentiation, an example of which is mesenchymal-to-epithelial transition (MET). During MET, mesenchymal cells are reprogrammed, thereby gradually losing mesenchymal cell characteristics while gaining epithelial cell traits [11]. MET and its counterpart, epithelial-to-mesenchymal transition (EMT), are fundamental processes that occur during embryo development and are also implicated in tumor metastasis [reviewed in: 12].
The occurrence of cellular transdifferentiation in uterine biology has previously been reported in the context of prenatal developmental. The Müllerian ducts, which give rise to the oviducts, uterus, and anterior portion of the vagina, are formed via MET and EMT [13]. The Müllerian ducts develop from the coelomic epithelium, which is initially derived from the intermediate mesoderm. Cells of the intermediate mesoderm undergo partial MET to form the mesoepithelial cells of the coelomic epithelium, which then either complete MET to form the Müllerian duct epithelium or undergo EMT to form the Müllerian duct mesenchyme (MDM) [13–15]. Although transformation of coelomic epithelium into MDM was observed more than 50 years earlier [16], the possibility that cellular transformation also serves as a mechanism to regenerate adult endometrium has not been evaluated. In this study we used a lineage tracing technique to map the fate of MDM-derived cells in the adult uterus to test the hypothesis that epithelial tissue regeneration in the mouse endometrium is accomplished in part by MET.
Materials and Methods
Animals
All protocols involving animal experiments were approved by the Institutional Animal Care and Use Committee at Washington State University or Massachusetts General Hospital. B6;129S7-Amhr2tm3(cre)Bhr/Mmnc (Amhr2-Cre) [17] mice were obtained from the Mutant Mouse Regional Resource Centers, B6.129×1-Gt(ROSA)26Sortm1(EYFP)Cos/J (Rosa26-Stopfl/fl-EYFP) [18] mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and Amhr2-LacZ mice were kindly provided by Dr. Richard Behringer [19]. CD1 mice were purchased from Charles River Laboratories (Wilmington, MA).
Menses-like mouse model
Female mice were placed with vasectomized male CD1 mice and designated day of pseudopregnancy (DOPP) 0.5 on observation of a vaginal plug. Sesame oil (20 μL) was then injected into the uterine lumen on DOPP 4 to artificially induce endometrial decidualization. At 48 or 72 h postoil induced decidualization, progesterone (P4) stimulus was removed by ovariectomy (ovex) to allow the deciduoma to degenerate. Coincident with degeneration of the deciduoma was the regeneration of the endometrium, which was complete by 72 h after the removal of P4 stimulus. Mice were euthanized, and uteri were collected at 0, 24, 36, or 48-h postovex during endometrial regeneration.
Fate mapping studies
Amhr2-Cre mice were crossed to Rosa26-Stopfl/fl-EYFP reporter mice to generate double transgenic females (Amhr2-Cre; Rosa26-EYFP) that constitutively express EYFP after Cre-mediated excision of a loxP floxed stop codon from the Rosa-26 promoter in cells with Amhr2 promoter activity (i.e., MDM-derived cells). Uteri were collected from Amhr2-Cre; Rosa26-EYFP females at PND 14 (n=3), PND 25 (n=3), and after 2–3 pregnancies (n=6). Uteri from control (Rosa26-Stopfl/fl-EYFP; lacking Cre) mice were collected for each time point. All tissues from Amhr2-Cre; Rosa26-EYFP and control mice were fixed in 4% paraformaldehyde (PFA) for 5–15 min on ice and were processed for gelatin embedding and freezing and direct visualization of fluorescence.
Assessment of Amhr2 promoter activity
For Amhr2 promoter activity experiments, uteri were obtained from Amhr2-LacZ mice at PND 14 and 25, nulliparous mice at 6 weeks and 6 months of age, 3 days postpartum (DPP; implantation sites), and 48 h-post ovex using the menses-like mouse model described earlier (n=3 per time point). Uteri from control mice that lacked the Amhr2-LacZ transgene were collected from wild-type littermates at PND 25, 6 weeks and 3 months of age, DPP 3 and 48 h postovex.
β-galactosidase staining
Tissues collected from Amhr2-LacZ and control mice were fixed for 15–60 min in 4% PFA on ice followed by 3 washes in rinse buffer (40 mM NaH2PO4, 0.16 mM Na2HPO4·7H2O, 2 mM MgCl2, 0.2 mM sodium deoxycholate, and 0.02% Nonidet-P40). Tissues were then incubated in X-Gal (5-bromo-4-chloro-3-indolyl β-D-galactopyronoside; Sigma Chemical Co., St. Louis, MO) staining solution (1 mg/mL X-Gal, 5 mM K3Fe(CN)6, 4 mM K4Fe(CN)6·3H2O in rinse buffer) for 24 h at 37°C with agitation followed by postfixation in 10% neutral-buffered formalin overnight at room temperature (RT). The next day, tissues were processed for gelatin embedding and freezing in preparation for thin sectioning.
Gelatin embedding and frozen tissue preparation
After β-galactosidase staining of LacZ expressing and control uteri or afer PFA fixation of all other uteri (e.g., EYFP and CD1), the tissues were washed thrice in phosphate-buffered saline (PBS) and then incubated overnight at 4°C in 15% sucrose buffered in PBS. Samples were then incubated at 37°C for 1 h in gelatin (15% sucrose, 7.5% gelatin in PBS), embedded in gelatin, frozen at −50°C to −65°C in isopentane cooled by liquid nitrogen, and stored at −80°C until sectioning. Tissues were cryo-sectioned at 5–8 μm and thaw mounted. After removal of gelatin in 37°C PBS, LacZ expressing tissues were counterstained with hematoxylin (Fisher Scientific, Pittsburgh, PA) and cover slipped with aqueous mounting medium (Aqua Mount, Lerner Laboratories, Pittsburgh, PA). EYFP expressing samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) mounting medium and viewed directly using fluorescence microscopy. CD1 tissues were used for vimentin and pan-cytokeratin dual-immunofluorescence.
Vimentin and pan-cytokeratin dual-immunofluorescence
Co-localization of vimentin and pan-cytokeratin was performed on tissues obtained from CD1 females during endometrial regeneration (0, 24, 36, and 48 h postovex; n=3 per time point). Uteri were collected, fixed in 4% PFA for 20 min on ice, and processed for gelatin embedding and freezing. 5 μm sections were thaw mounted, gelatin was removed in 37°C PBS and dual-immunofluorescence for vimentin (mouse monoclonal 1:50; Cell Signaling Technology, Danvers, MA; cat # 5741S), and pan-cytokeratin (rabbit monoclonal 1:250; Sigma-Aldrich, St. Louis, MO; cat # C2931) was performed using a modified protocol for the Vector Mouse on Mouse (M.O.M.) Kit (Vector Laboratories, Inc., Burlingame, CA). Briefly, tissues were incubated for 1 h at RT in blocking solution (0.1% triton ×100, 0.1% BSA, 10% normal donkey serum, 10% normal goat serum, and 3.6% M.O.M. kit blocking reagent in PBS), followed by duplicate 2-min washes in PBS. After incubation in diluent (0.1% triton ×100, 0.1% BSA, 10% normal donkey serum, 10% normal goat serum, and 8% M.O.M. kit protein concentrate in PBS) for 5 min at RT, primary antibodies were applied for 90 min at RT. Tissues were washed twice for 2 min in PBS and incubated with secondary antibodies (Alexa Fluor 546 donkey anti-rabbit IgG, 1:500 and Alexa Fluor 488 goat anti-mouse IgG, 1:500; Life Technologies, Grand Island, NY) for 45 min at RT followed by 2 final 2-min PBS washes before being counter stained with DAPI mounting medium and cover slipped. Omission of primary antibodies served as a negative control.
For cell counts, images of the mesometrial endometrium were taken at 630× magnification on either side of the presumptive luminal space in the regeneration zone. Images of vimentin expression, pan-cytokeratin expression, and DAPI staining were taken from 2 tissue sections from each animal at each time point (0, 24, 36 and 48 h postovex; n=3 per time point). The images were analyzed using NIH Image J software for total number of cells, total number of pan-cytokeratin positive cells, and the total number of cells that co-localized for vimentin and pan-cytokeratin. Statistical analyses were performed with GraphPad Prism 5 software (La Jolla, CA) using the one-way ANOVA with Tukey's Multiple Comparison Test on the acquired cell counts, resulting in significance at P<0.05.
Results
MET during endometrial regeneration
To study MET in the uterus, the Cre-loxP system was used to label MDM-derived cells (i.e., stroma and myometrium) in the uterus in an effort to map potential changes in cell fate. Anti-Müllerian hormone type II receptor (Amhr2) gene promoter activity has been shown to be restricted, within the uterus, to mesenchymal cell types [19]. By crossing Amhr2-Cre mice, which express Cre recombinase under the control of the Amhr2 promoter, to Rosa26-Stopf/f-EYFP mice, we generated double transgenic offspring (Amhr2-Cre; Rosa-EYFP) with indelibly labeled mesenchymal cells beginning at embryonic day 12.5 (E12.5) when Amhr2 promoter activity, and thus Cre expression, is first detected [17]. We first looked at EYFP expression in the uteri of double transgenic female mice on PND 14 (Fig. 1A–C) and PND 25 (Fig. 1D–F) in which minimal tissue turnover and homeostasis occur. While constitutive stromal and myometrial EYFP expression was observed, EYFP was not expressed in the GE or LE (Fig. 1). EYFP expression was then assessed in the uteri of double transgenic mice after 2 or 3 pregnancies in which substantially greater endometrial remodeling and regeneration occur compared with nulliparous female mice. In support of our hypothesis, EYFP expression was not only observed in uterine mesenchymal tissue, but was also observed in the GE and LE (Fig. 2A–F), suggesting the occurrence of MET. EYFP expression was not observed in Rosa26-Stopf/f-EYFP singularly transgenic control mice lacking cre recombinase expression (data not shown). These results suggest that MET serves as a mechanism of endometrial re-epithelialization after natural decidualization.
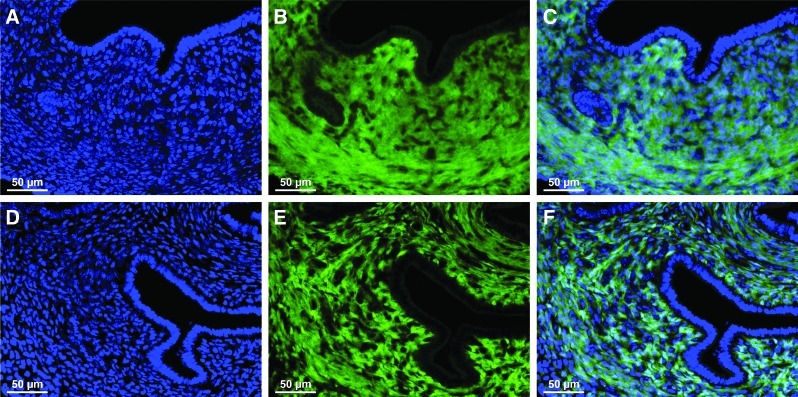
FIG. 1.
Localization of EYFP expression in uteri from young Amhr2-Cre; Rosa-EYFP double transgenic mice. Representative uterine cross-sections from postnatal day (PND) 14 (A–C) and PND 25 (D–F) mice. (B, E) EYFP expression was restricted to the stroma and myometrium and was not present in the luminal epithelium (LE) and glandular epithelium (GE). (A, D) Nuclear DAPI staining. (C) Merged image of A and B. (F) Merged image of D and E. Color images available online at www.liebertpub.com/scd
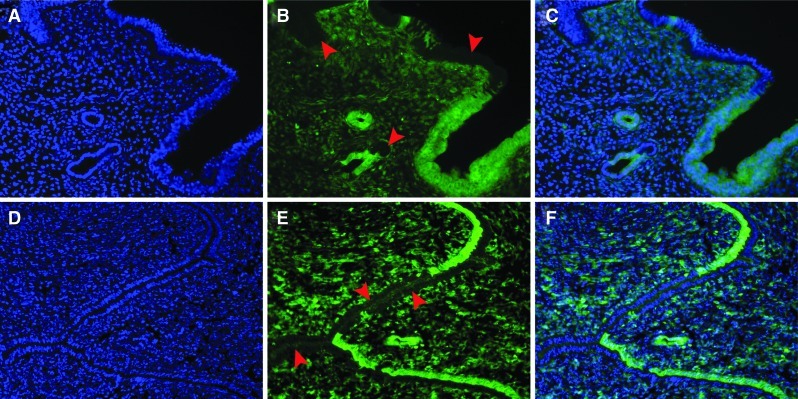
FIG. 2.
Mesenchymal-to-epithelial transition during endometrial regeneration. Representative cross-sections of fully regenerated endometrium in uteri from 2 Amhr2-Cre; Rosa-EYFP double transgenic female mice after 2–3 pregnancies each (A–F). After pregnancy and parturition re-epithelialization occurred and EYFP positive cells were found interspersed with EYFP negative cells (arrowheads) in the LE and GE (B, C, E, F). (A, D) Nuclear DAPI staining. (B, E) EYFP expression. (C, F) Merged images of A and B, and D and E respectively. Color images available online at www.liebertpub.com/scd
Assessment of Amhr2 promoter activity
To ensure that the epithelial EYFP expression was not due to leaky Amhr2 promoter activity in epithelial cells, we conducted promoter activity experiments using transgenic mice that express LacZ directly under the control of the endogenous Amhr2 promoter (Amhr2-LacZ). Thus, Amhr2-LacZ mice only express LacZ when the Amhr2 promoter is active. Both Amhr2-Cre and Amhr2-LacZ mice were created using similar knock-in strategies at the same insertion site [17,19]. In prepubertal Amhr2-LacZ mice, LacZ was expressed throughout the stroma and myometrium (Fig. 3A–D) and in adult nulliparous mice, LacZ expression was restricted to the myometrium (Fig. 3E–H). Although mesenchymal LacZ expression patterns vary slightly with the age of the mouse, what remains consistent across all ages is that LacZ was not expressed in either the GE or LE. LacZ expression was not observed in WT littermate controls at PND 25 and 3 months of age (Fig. 3I, J). We then looked at Amhr2 promoter activity during endometrial regeneration after a normal pregnancy and using the menses-like model. Both natural and mechanical decidualization (i.e., menses-like model) result in endometrial repair; however, the mechanical model results in more profound decidualization throughout the entire uterus, yielding more tissue for experiments in a shorter amount of time. LacZ expression was observed in the stroma and myometrium in the uteri of Amhr2-LacZ mice as expected, but not in the epithelium 3 days postpartum after a normal pregnancy (Fig. 4A, B). Likewise, LacZ expression was detected in the myometrium 48 h postovex after induced decidualization; however, there was no LacZ expression in the epithelium (Fig. 4C, D). As previously reported, these findings confirm that Amhr2 promoter activity is restricted to the uterine stroma and myometrium regardless of the age, reproductive status, or the degree of damage incurred within the uterus.
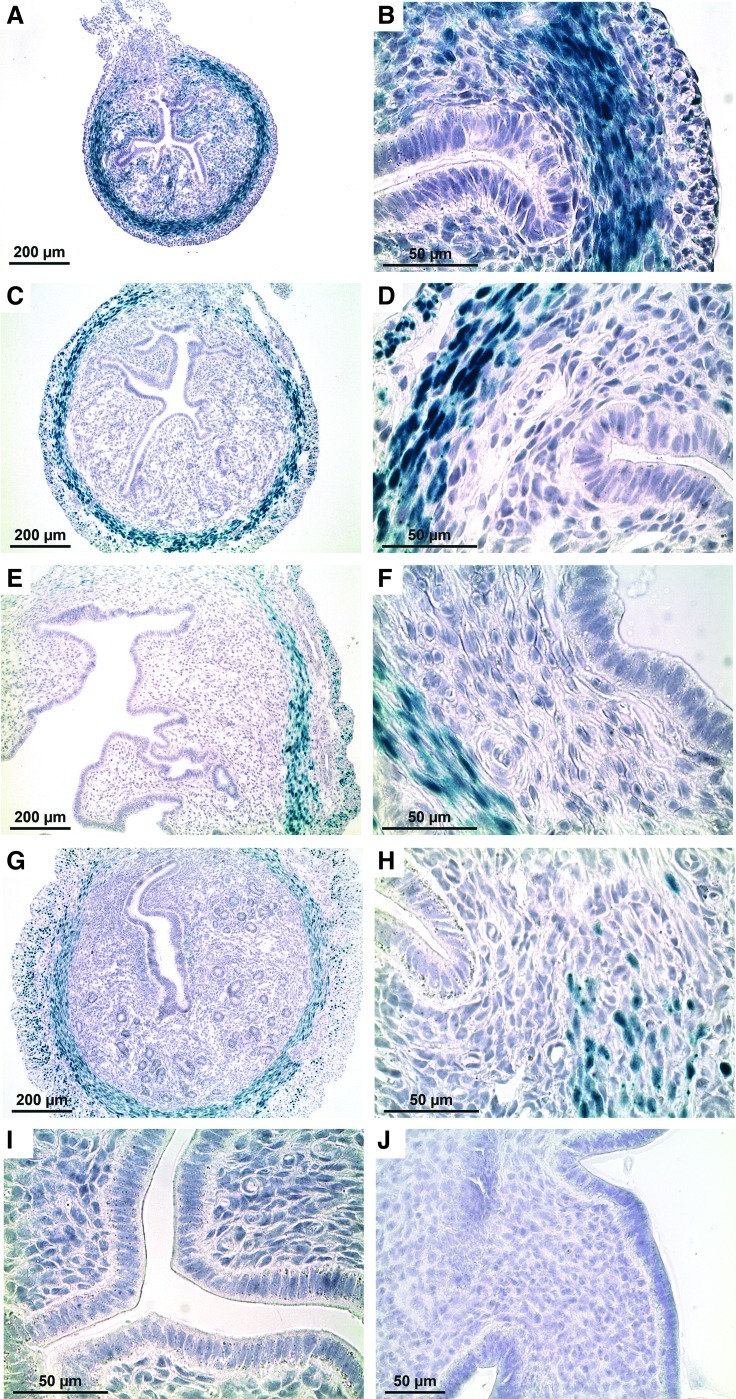
FIG. 3.
Amhr2 promoter activity in uteri from prepubertal and adult nulliparous mice. LacZ expression was abundant in the myometrium and stroma of the PND 14 mouse uterus (A, B). LacZ continued to be expressed at 25 days (prepubertal) (C, D), 6 weeks of age (sexually mature; E, F), and in the adult (6 months) nulliparous mouse (G, H), although expression became more restricted to the myometrium as the age of the mouse increased. There was no LacZ expression in the epithelium, luminal or glandular, of uteri from Amhr2-LacZ mice, regardless of age (A–H). Uterine cross-sections from WT control mice at PND 25 (I) and adult (3 months) nulliparous (J) showed no LacZ expression. Color images available online at www.liebertpub.com/scd
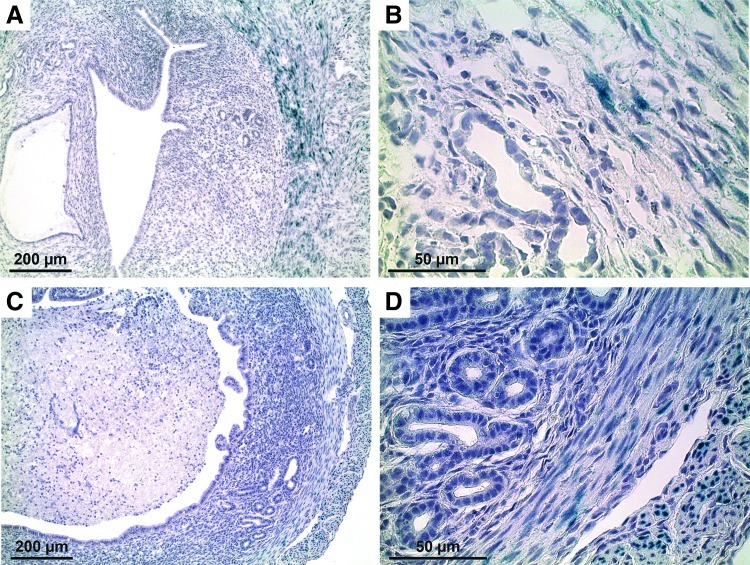
FIG. 4.
Amhr2 promoter activity in uteri during endometrial regeneration 3 days postpartum or 2 days postovariectomy (ovex) using the menses-like model. In the uterus 3 days postpartum (A, B), LacZ was expressed in the myometrium and very minimally in the sub-luminal epithelium (A) but not in the epithelium (B). In the uteri of mice 48-h post-ovex (C, D), there was very slight LacZ expression in the outermost myometrium (C) but not in the epithelium (D). Color images available online at www.liebertpub.com/scd
Identifying transitional cells during endometrial regeneration
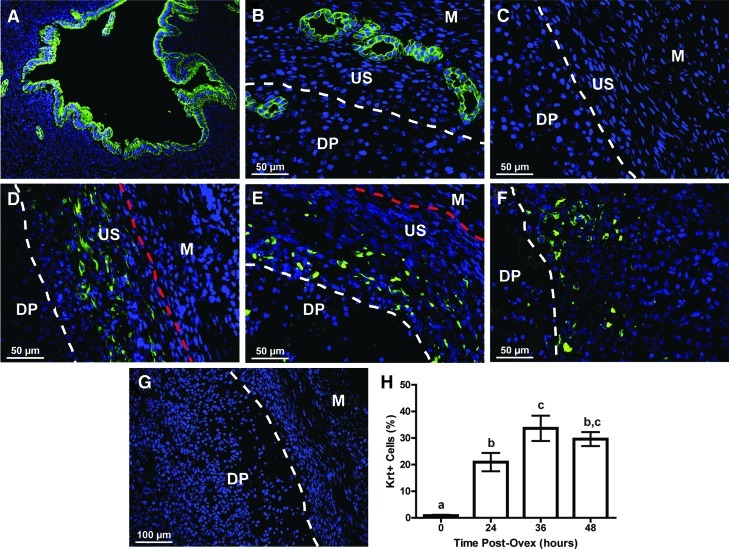
The epithelial cell marker, pan-cytokeratin, was used to identify epithelial-like cells in the endometrium during regeneration after endometrial breakdown. In the adult nulliparous mouse uterus, pan-cytokeratin expression was restricted to the GE and LE (Fig. 5A). Likewise, at 0 h postovex, before removal of P4 stimulus and subsequent regeneration, pan-cytokeratin was expressed only in the minimal GE that localizes exclusively to the anti-mesometrial pole (Fig. 5B). However, pan-cytokeratin was not expressed in the myometrium or undecidualized stroma in either the mesometrial (Fig. 5C) or anti-mesometrial poles (Fig. 5B). Once endometrial regeneration began, approximately 21% of the cells in the regenerating stroma expressed pan-cytokeratin at 24 h postovex (Fig. 5H). By 36 and 48 h postovex, approximately 34% and 30% of the cells in the regeneration zone expressed pan-cytokeratin, respectively (Fig. 5H). In addition to an increase in pan-cytokeratin positive cells within the stroma, the location of these cells within the undecidualized/regenerating stroma changed over the first 48 h after removal of P4. At 24 h postovex, pan-cytokeratin expressing “stromal” cells were located at the stromal-myometrial border (Fig. 5D, dotted line between US and M), whereas at 36 and 48 h postovex, the cells transitioned to the presumptive luminal surface where re-epithelialization ensued (Fig. 5E, F; dotted line between DP and US). This occurred primarily in the mesometrial zone of regeneration. These unique cytokeratin positive stromal cells do not appear to originate from glands, given that GE localize to the anti-mesometrial pole.
FIG. 5.
Pan-cytokeratin expression in the regenerating mouse endometrium over time using the menses-like model. In the adult nulliparous uterus, pan-cytokeratin expression was exclusively localized to LE and GE (A). Likewise, at 0 h postovex before the initiation of regeneration, pan-cytokeratin expression was restricted to the GE located in the anti-mesometrial pole (B) of the uterus and to any residual LE located within the decidual plug (DP, data not shown). However, pan-cytokeratin was not expressed in the undecidualized stroma (US) of the endometrium or the myometrium (M) in the mesometrial (C) or anti-mesometrial (B) poles. At 24 h postovex, pan-cytokeratin began to be expressed (H, ∼21%) in the regenerating endometrium, specifically in the US at the stromal-myometrial border (dotted line between US and M) in the mesometrial pole (D). As time progressed, stromal pan-cytokeratin expression in the mesometrial zone of regeneration peaked at 36 h postovex (H, ∼34%) and continued to be expressed at comparable levels at 48 h postovex (H, ∼30%). The location of pan-cytokeratin expressing cells moved from the stromal-myometrial border (dotted line between US and M) at 24 h postovex (D), in toward the presumptive luminal space (dotted line between DP and US) at 36 h (E) and 48 h (F) postovex where the LE will re-form. Dotted line between DP and US delineates the regenerating endometrium from the degenerating DP. Dotted line between US and M indicates the stromal-myometrial border. (G) Representative uterine cross-section at 24 h postovex with primary antibody omitted from immunofluorescence protocol. (H) Pan-cytokeratin (Krt) expression as a percentage of total cells counted on either side of the presumptive lumen in the mesometrial pole at the indicated times postovex. Different letters indicate significance at P<0.05. Color images available online at www.liebertpub.com/scd
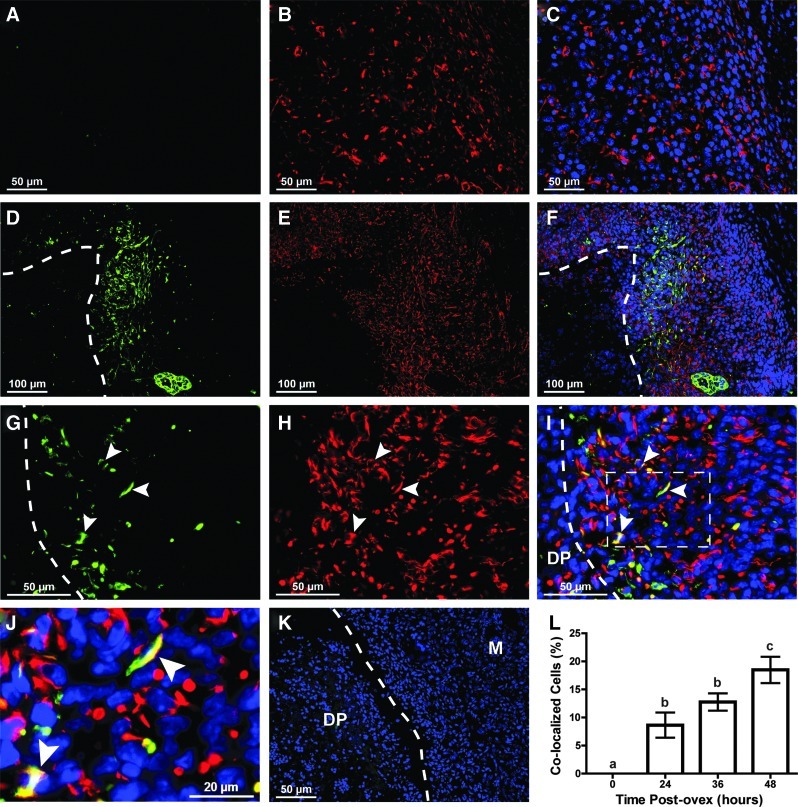
Due to the unique location of the pan-cytokeratin expressing cells (i.e., located within the stroma) and since these cells morphologically resemble stromal cells more so than epithelial cells, we further characterized these cells using the stromal cell marker vimentin. Pan-cytokeratin and vimentin co-localized cells were not present in the endometrium at the time of ovariectomy (Fig. 6A–C, H). However, co-localization began at 24 h and peaked at 48 h postovex with 18.5% of the total cells in the mesometrial regeneration zone co-localizing for pan-cytokeratin and vimentin (Fig. 6H). The co-localized cells were primarily located in the mesometrial pole in the regeneration zone near the stromal-myometrial border at 24 h postovex (data not shown) and just adjacent to the degenerating deciduoma at 36 (data not shown) and 48 h postovex (Fig. 6D–F, white dotted line). The co-localization of pan-cytokeratin (green) and vimentin (red) in cells at the regeneration zone at 48 h postovex can clearly be seen at high magnification (Fig. 6G–J, arrowheads). These data suggest that during endometrial regeneration, cells transition between mesenchymal and epithelial states and that they progress from the stromal-myometrial border to the presumptive luminal interface as a mechanism to facilitate endometrial epithelial repair.
FIG. 6.
Co-localization of vimentin and pan-cytokeratin in the stroma of regenerating endometrium using the menses-like model. (A–J) pan-cytokeratin (green; epithelial cell marker) and vimentin (red; stromal cell marker) expression in the regenerating endometrium at 0 h (A–C) and 48 h (D–J) postovex. (A–C) Presumptive mesometrial regeneration zone at 0 h postovex (before removal of progesterone stimulus) showed no expression of pan-cytokeratin and subsequently, no co-localization of pan-cytokeratin and vimentin (L, 0%). At 48 h postovex, low magnification (D–F) showed that the majority of pan-cytokeratin expressing cells as well as pan-cytokeratin and vimentin co-localized “stromal” cells were located in the mesometrial pole in the regeneration zone, adjacent to the degenerating DP. At high magnification (G–J), individual cells located in the regenerating stroma at the border of the presumptive lumen and the degenerating DP (dotted line) were seen to co-localize for vimentin and pan-cytokeratin (arrowheads, representative co-localized cells) (A, D, G) pan-cytokeratin expression, (B, E, H) vimentin expression, (C, F, I, J) merged images with DAPI counterstain (blue). (J) Digital magnification of area inside the dotted square in (I) showing co-localization of pan-cytokeratin and vimentin (arrowheads). Dotted line demarcates the DP (inside) from the regenerating endometrium (outside). M; myometrium (K) Representative uterine cross-section at 48 h postovex with primary antibody omitted from immunofluorescence protocol. (L) Percentage of cells counted in the mesometrial regeneration zone that co-localized for vimentin and pan-cytokeratin at the designated time points. Different letters indicate significance at P<0.05. Color images available online at www.liebertpub.com/scd
Discussion
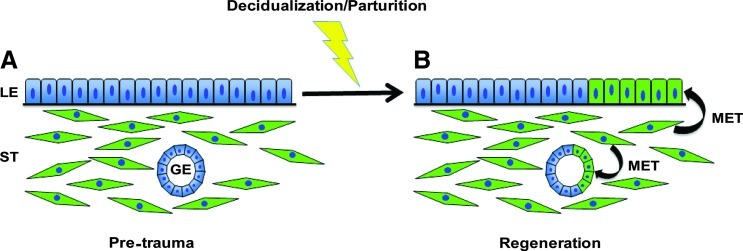
Using fate-mapping techniques, we identified the presence of MDM-derived cells within the glandular and luminal epithelium during endometrial regeneration, suggesting the occurrence of MET during this process (Fig. 7). MET appears to be reserved as a mechanism of re-epithelialization for cases of extensive endometrial regeneration (e.g., after parturition) but not for adenogenesis (gland formation) or postnatal expansion of the epithelium. This is evidenced by the appearance of EYFP-positive cells in the epithelium only after regeneration but not in young or peripubertal mice. Huang et al. recently demonstrated that MET does not occur in nonpregnant females during the estrous cycle [20]. While cellular transdifferentiation has been suggested to occur in other reproductive tissues such as the ovary [21,22], our study demonstrates cellular transdifferentiation in the adult uterus under normal physiological conditions. Since large continuous regions of epithelial cells that express EYFP are found in the endometrium, it is unlikely that fusion of stromal cells with overlying epithelial cells is responsible. Thus, however unlikely, further research is needed to definitively rule out this possibility.
FIG. 7.
Working model of MET during endometrial regeneration. (A) Pretrauma endometrium. (B) Endometrium after normal physiological trauma (i.e., decidualization/parturition) and regeneration. During regeneration, stromal (ST) cells undergo mesenchymal-to-epithelial transition (MET) to replace regions of epithelium that are lost/damaged during decidualization and parturition. Color images available online at www.liebertpub.com/scd
Based on the results of our study, we propose that prenatal developmental programming of the uterus remains intact in the adult uterus as a mechanism of tissue regeneration/repair. There is strong developmental precedence for MET and EMT during the formation of the Müllerian duct and the subsequent embryonic uterus [23,24]. Two studies conducted simultaneously employed various lineage tracing experiments to track the fate of coelomic epithelial cells, and each concluded that the Müllerian ducts, including mesenchymal and epithelial cell types, develop from the coelomic epithelium via cell proliferation [23,24]. The studies revealed the mesoepithelial nature of the developing Müllerian duct cells as indicated by positive staining for both pan-cytokeratin and vimentin [23]. Interestingly, as the Müllerian ducts develop from E12.5 to E13.5, vimentin expression decreases, pan-cytokeratin expression is maintained, and E-cadherin, another epithelial cell marker, begins to be expressed [23]. The change in expression of mesenchymal to epithelial cell markers is likely indicative of cellular transformation (i.e., MET) during Müllerian duct development.
We have likewise shown that during endometrial regeneration in the adult, a unique population of pan-cytokeratin expressing cells exists within the stroma, exclusively localized to the regeneration zone. These cells appear only during endometrial regeneration. As regeneration progresses from 24 to 48 h postovex, the pan-cytokeratin-positive cells move from the stromal-myometrial border to the presumptive luminal interface where the LE will re-form. In addition, the pan-cytokeratin expressing cells present in the stroma do not appear to originate from GE as evidenced by their lack of physical connection to glands at any time point during regeneration, and because the majority of the cells are located in the mesometrial pole that is devoid of glands in the mouse. On further investigation of these unique cells, we were able to show that 18.5% of the cells in the mesometrial regeneration zone at 48 h postovex co-express pan-cytokeratin and vimentin. Co-localization of vimentin and pan-cytokeratin suggests that these cells are in a mesenchymal-epithelial transitional state and may have originated by MET. However, it is unknown whether the pan-cytokeratin and vimentin expressing “stromal” cells derive from a mesenchymal stem cell pool or whether dedifferentiation-redifferentiation of mature stromal cells occurs. Due to the location of the co-localized cells at 24 h postovex at the stromal-myometrial border, we speculate that they originate from mesenchymal stem cells which are purported to reside in the same vicinity [25–27]. However, further investigation is needed to clearly determine the origin of these cells. Furthermore, additional experiments are needed to determine the contribution of EMT (the counterpart of MET) as a source of pan-cytokeratin and vimentin co-localized cells.
It is interesting to note that mechanisms coordinating formation of the kidney, which shares developmental origins in the urogenital ridge with the Müllerian duct, involve MET. The uteric bud, which branches from the Wolffian duct, contacts the intermediate mesoderm-derived metanephros, inducing condensation of the mesenchymal cells, which then undergo MET, ultimately resulting in the formation of an S-shaped epithelial tube [28]. EMT is known to be causally related to kidney fibrosis, and the process of MET helps repair fibrotic kidney in the adult [29,30]. Since developmental programming remains intact in the adult kidney to facilitate repair, it is reasoned here that the processes of MET and EMT also serve as mechanisms of endometrial regeneration after normal physiological damage that occurs after parturition. Similar mechanisms may occur after parturition in women, as well as after menses where cyclical tissue remodeling is more extensive than in estrous cycling animals such as rodents.
EMT and MET are exploited during the progression of diseases such as cancer [reviewed by: 31,32–34]. Approximately 80% of all cancers are epithelial in origin, and it is thought that the ability of the tumor to metastasize is accomplished by the transition of sessile epithelial cells into migratory mesenchymal cells. Once the tumor reaches the secondary site, the cells undergo MET, allowing the tumor to set up residence in the new location. MET and EMT could also play a role in endometriosis, where ectopic endometrial tissue (stroma and epithelium) implants within the peritoneal cavity [35]. A definitive cause of endometriosis is not known; however, the strongest theory of pathogenesis is retrograde menstruation (i.e., menstruation into the abdominal cavity). Based on our data, and due to the apparent plasticity of uterine mesenchymal cells, we speculate that MET may be a mechanism of endometriotic lesion formation and maintenance through retrograde menstruation of endometrial mesenchymal tissue.
Although EMT and MET are known to occur during embryogenesis and are implicated in cancer pathogenesis, our study provides evidence that these processes occur under normal physiological circumstances in the adult. However, as in other tissues, there seems to be multiple mechanisms that facilitate endometrial regeneration. Our data show mosaic EYFP epithelial expression after parturition. Theoretically, if MET were the sole mechanism of re-epithelialization, we would expect to see complete epithelial expression of EYFP in our fate mapping studies. However, it should be noted that an alternate explanation for the mosaic EYFP expression is the transformation of EYFP-negative stromal cells (i.e., stromal cells without Amhr2 promoter activity) into epithelial cells, resulting in EYFP-negative epithelial cells. Furthermore, it is unclear at this time whether the stromal-derived epithelial cells are functionally capable of contributing to the establishment of subsequent pregnancies. In other tissues, such as skin, rapidly formed epithelial tissue has been shown to serve only a transient role in the repair process, and these cells are eventually replaced by epithelial cells derived from the bulge region [36]. Huang et al. suggest that MET-derived epithelial cells are retained for at least 2 months after endometrial repair [20]. However, although retained long term, further studies are needed to confirm whether the stromal-derived epithelial cells are capable of contributing to embryo implantation and proper stromal-epithelial crosstalk.
Several lines of investigation have already established the differentiation potential of the endometrium. By example, Wolff et al., recently reported the transdifferentiation of human endometrial-derived stem-like cells into dopamine producing neurons [37]. Transplanted dopamine producing cells were shown to survive in the transplant location as well as migrate to sites of damage and spontaneously differentiate in vivo [37]. Likewise, endometrial stem cells can be pushed down a pathway toward insulin production in culture and transplantation experiments using the modified insulin producing cells continued to produce insulin in vivo and regulated blood glucose levels in diabetic mice [38]. In similar studies, Li et al. [39] and Hida et al. [40] reported transdifferentiation of human endometrial mesenchymal stem-like cells into insulin-producing cells and human menstrual blood-derived mesenchymal cells into cardiac precursor-like cells, respectively, which, when xenotransplanted into diseased mouse models, helped restore tissue function. These results along with our own demonstrate the plasticity of endometrial mesenchymal cells, as well as their potential use in regenerative medicine.
Uterine regeneration, including re-epithelialization, is a highly dynamic process in which the mechanisms directing this event are not well understood. Several hypothesized mechanisms of regeneration have been proposed, and these include (1) re-epithelialization of the luminal epithelium by proliferation of residual glandular stumps [25,41,42]; (2) uterine mesenchymal and epithelial stem cells within the endometrium that give rise to lineage differentiating cells; and (3) endothelial, stromal, and epithelial cells that undergo cellular transdifferentiation to repopulate the endometrium [41]. More recent efforts indicate the existence of highly clonogenic epithelial and stromal cells obtained from human endometrium [43,44] as well as the presence of human endometrial side population cells that are characteristic of somatic stem cells [45]. Furthermore, label-retaining techniques have been used to identify slow-cycling, quiescent epithelial, and stromal cells in the female reproductive tract as potential stem cells [26,27,46]. Pericytes in both murine [47] and human [48] endometrium are proposed as a source of mesenchymal stem/progenitor cells. Several of the aforementioned studies indicate the likelihood that stem/progenitor cells exist at the stromal-myometrial border. Our data take this theory a step further by suggesting that stem cells at the stromal-myometrial border may be the source of mesenchymal-epithelial transitional cells which help facilitate endometrial regeneration. These data in combination with our own demonstrate the complexity of uterine regeneration and the likelihood that multiple mechanisms play a pertinent role in this process. Delineating the normal mechanisms of endometrial regeneration could lead to a better understanding of how these processes, when gone awry, contribute to hyper- and hypo-proliferative diseases of the reproductive tract, such as cancer, endometriosis, and Asherman's syndrome.
Acknowledgments
This work was supported in part by Vincent Memorial Research Funds and NIH HD066297. Submitted material will be presented at the 45th annual Society for the Study of Reproduction conference.
Author Disclosure Statement
The authors have no relevant financial or nonfinancial relationships to disclose.
References
- 1.Martinez G. Daniels K. Chandra A. National Health Statistics Reports no. 51. National Center for Health Statistics; Hyattsville, MD: 2012. Fertility of men and women aged 15–44 years in the United States: national survey of family growth, 2006–2010. [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Review 1975-2009 (Vintage 2009 Populations) In: Howlader N, editor; Noone AM, editor; Krapcho M, editor; Neyman N, editor; Aminou R, editor; Altekruse SF, editor; Kosary CL, editor; Ruhl J, editor; Tatalovich Z, editor; Cho H, editor; Mariotto A, editor; Eisner MP, editor; Lewis DR, editor; Chen HS, editor; Feuer EJ, editor; Cronin KA, editor. National Cancer Institute; Bethesda, MD: 2012. [Google Scholar]
- 3.National Institute of Child Health, Human Development. Endometriosis. 2012. www.nichd.nih.gov/health/topics/endometri/conditioninfo/pages/at-risk.aspx www.nichd.nih.gov/health/topics/endometri/conditioninfo/pages/at-risk.aspx
- 4.Al-Ghamdi A. Coskun S. Al-Hassan S. Al-Rejjal R. Awartani K. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol. 2008;6:37. doi: 10.1186/1477-7827-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer TE. Hayashi K. Hu J. Carpenter KD. Gerald PS. Comparative developmental biology of the mammalian uterus. In: Gerald P. Schatten., editor. Current Topics in Developmental Biology. Academic Press; 2005. pp. 85–122. [DOI] [PubMed] [Google Scholar]
- 6.Massé J. Watrin T. Laurent A. Deschamps S. Guerrier D. Pellerin I. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol. 2009;53:411–424. doi: 10.1387/ijdb.082680jm. [DOI] [PubMed] [Google Scholar]
- 7.Du H. Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25:2082–2086. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 8.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Ikoma T. Kyo S. Maida Y. Ozaki S. Takakura M. Nakao S. Inoue M. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201:608.e1–e8. doi: 10.1016/j.ajog.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Cervelló I. Gil-Sanchis C. Mas A. Faus A. Sanz J. Moscardó F. Higueras G. Sanz MA. Pellicer A. Simón C. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One. 2012;7:e30260. doi: 10.1371/journal.pone.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugo H. Ackland ML. Blick T. Lawrence MG. Clements JA. Williams ED. Thompson EW. Epithelial—mesenchymal and mesenchymal—epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi A. Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- 14.Dressler GR. Tubulogenesis in the developing mammalian kidney. Trends Cell Biol. 2002;12:390–395. doi: 10.1016/s0962-8924(02)02334-6. [DOI] [PubMed] [Google Scholar]
- 15.Klattig J. Rnglert C. The Müllerian duct: recent insights into its development and regression. Sex Dev. 2007;1:271–278. doi: 10.1159/000108929. [DOI] [PubMed] [Google Scholar]
- 16.Gruenwald P. Growth and Development of the uterus: the relationship of epithelium to mesenchyme. Ann N Y Acad Sci. 1959;75:436–440. doi: 10.1111/j.1749-6632.1959.tb44566.x. [DOI] [PubMed] [Google Scholar]
- 17.Jamin SP. Arango NA. Mishina Y. Hanks MC. Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 18.Srinivas S. Watanabe T. Lin C-S. William C. Tanabe Y. Jessell T. Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arango NA. Kobayashi A. Wang Y. Jamin SP. Lee H-H. Orvis GD. Behringer RR. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 20.Huang CC. Orvis GD. Wang Y. Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7:e44285. doi: 10.1371/journal.pone.0044285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora JM. Fenwick MA. Castle L. Baithun M. Ryder TA. Mobberley M. Carzaniga R. Franks S. Hardy K. Characterization and significance of adhesion and junction-related proteins in mouse ovarian follicles. Biol Reprod. 2012;86(153):1–14. doi: 10.1095/biolreprod.111.096156. [DOI] [PubMed] [Google Scholar]
- 22.Irving-Rodgers HF. Harland ML. Rodgers RJ. A novel basal lamina matrix of the stratified epithelium of the ovarian follicle. Matrix Biol. 2004;23:207–217. doi: 10.1016/j.matbio.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Orvis GD. Behringer RR. Cellular mechanisms of Müllerian duct formation in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guioli S. Sekido R. Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 25.Klattig J. Sierig R. Kruspe D. Makki MS. Englert C. WT1-mediated gene regulation in early urogenital ridge development. Sex Dev. 2007;1:238–254. doi: 10.1159/000104774. [DOI] [PubMed] [Google Scholar]
- 26.Chan RWS. Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 27.Murphy LJ. Ghahary A. Uterine insulin-like growth factor-1: regulation of expression and its role in estrogen-induced uterine proliferation. Endocr Rev. 1990;11:443–453. doi: 10.1210/edrv-11-3-443. [DOI] [PubMed] [Google Scholar]
- 28.Horster MF. Braun GS. Huber SM. Embryonic renal epithelia: induction, nephrogenesis, and cell differentiation. Physiol Rev. 1999;79:1157–1191. doi: 10.1152/physrev.1999.79.4.1157. [DOI] [PubMed] [Google Scholar]
- 29.Zeisberg M. Hanai J-I. Sugimoto H. Mammoto T. Charytan D. Strutz F. Kalluri R. BMP-7 counteracts TGF-[beta]1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 30.Zeisberg M. Shah AA. Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094–8100. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 31.Moustakas A. Heldin CH. Signaling networks guiding epithelial–mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiery JP. Acloque H. Huang RYJ. Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Polyak K. Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 34.Chaffer CL. Thompson EW. Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 35.Matsuzaki S. Darcha C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum Reprod. 2012;27:712–721. doi: 10.1093/humrep/der442. [DOI] [PubMed] [Google Scholar]
- 36.Diaugustine RP. Petrusz P. Bell GI. Brown CF. Korach KS. Mclachlan JA. Teng CT. Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology. 1988;122:2355–2363. doi: 10.1210/endo-122-6-2355. [DOI] [PubMed] [Google Scholar]
- 37.Wolff EF. Gao X-B. Yao KV. Andrews ZB. Du H. Elsworth JD. Taylor HS. Endometrial stem cell transplantation restores dopamine production in a parkinson's disease model. J Cell Mol Med. 2011;15:747–755. doi: 10.1111/j.1582-4934.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santamaria X. Massasa EE. Feng Y. Wolff E. Taylor HS. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther. 2011;19:2065–2071. doi: 10.1038/mt.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H-Y. Chen Y-J. Chen S-J. Kao C-L. Tseng L-M. Lo W-L. Chang C-M. Yang D-M. Ku H-H, et al. Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells. J Pharmacol Exp Ther. 2010;335:817–829. doi: 10.1124/jpet.110.169284. [DOI] [PubMed] [Google Scholar]
- 40.Hida N. Nishiyama N. Miyoshi S. Kira S. Segawa K. Uyama T. Mori T. Miyado K. Ikegami Y, et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 41.Fujino A. Arango NA. Zhan Y. Manganaro TF. Li X. Maclaughlin DT. Donahoe PK. Cell migration and activated PI3K/AKT-directed elongation in the developing rat Mullerian duct. Dev Biol. 2009;325:351–362. doi: 10.1016/j.ydbio.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaitu'u-Lino TJ. Ye L. Gargett CE. Reepithelialization of the uterine surface arises from endometrial glands: evidence from a functional mouse model of breakdown and repair. Endocrinology. 2010;151:3386–3395. doi: 10.1210/en.2009-1334. [DOI] [PubMed] [Google Scholar]
- 43.Gargett CE. Schwab KE. Zillwood RM. Nguyen HPT. Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan RWS. Schwab KE. Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 45.Cervelló I. Gil-Sanchis C. Mas A. Delgado-Rosas F. Martínez-Conejero JA. Galán A. Martínez-Romero A. Martínez S. Navarro I, et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5:e10964. doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilhelm D. Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16:1839–1851. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaitu'u-Lino TUJ. Ye L. Salamonsen LA. Girling JE. Gargett CE. Identification of label-retaining perivascular cells in a mouse model of endometrial decidualization, breakdown, and repair. Biol Reprod. 2012;86:184. doi: 10.1095/biolreprod.112.099309. [DOI] [PubMed] [Google Scholar]
- 48.Spitzer TLB. Rojas A. Zelenko Z. Aghajanova L. Erikson DW. Barragan F. Meyer M. Tamaresis JS. Hamilton AE. Irwin JC. Giudice LC. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]