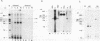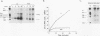Abstract
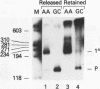
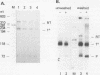
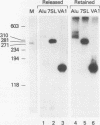
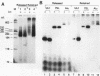
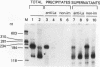
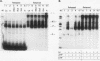
Ample evidence indicates that Alu family interspersed elements retrotranspose via primary transcripts synthesized by RNA polymerase III (pol III) and that this transposition sometimes results in genetic disorders in humans. However, Alu primary transcripts can be processed posttranscriptionally, diverting them away from the transposition pathway. The pol III termination signal of a well-characterized murine B1 (Alu-equivalent) element inhibits RNA 3' processing, thereby stabilizing the putative transposition intermediary. We used an immobilized template-based assay to examine transcription termination by VA1, 7SL, and Alu class III templates and the role of transcript release in the pol III terminator-dependent inhibition of processing of B1-Alu transcripts. We found that the RNA-binding protein La confers this terminator-dependent 3' processing inhibition on transcripts released from the B1-Alu template. Using pure recombinant La protein and affinity-purified transcription complexes, we also demonstrate that La facilitates multiple rounds of transcription reinitiation by pol III. These results illustrate an important role for La in RNA production by demonstrating its ability to clear the termination sites of class III templates, thereby promoting efficient use of transcription complexes by pol III. The role of La as a potential regulatory factor in transcript maturation and how this might apply to Alu interspersed elements is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeniyi-Jones S., Zasloff M. Transcription, processing and nuclear transport of a B1 Alu RNA species complementary to an intron of the murine alpha-fetoprotein gene. Nature. 1985 Sep 5;317(6032):81–84. doi: 10.1038/317081a0. [DOI] [PubMed] [Google Scholar]
- Arias J. A., Dynan W. S. Promoter-dependent transcription by RNA polymerase II using immobilized enzyme complexes. J Biol Chem. 1989 Feb 25;264(6):3223–3229. [PubMed] [Google Scholar]
- Bachmann M., Pfeifer K., Schröder H. C., Müller W. E. Characterization of the autoantigen La as a nucleic acid-dependent ATPase/dATPase with melting properties. Cell. 1990 Jan 12;60(1):85–93. doi: 10.1016/0092-8674(90)90718-t. [DOI] [PubMed] [Google Scholar]
- Bieker J. J., Martin P. L., Roeder R. G. Formation of a rate-limiting intermediate in 5S RNA gene transcription. Cell. 1985 Jan;40(1):119–127. doi: 10.1016/0092-8674(85)90315-0. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Brown D. D. Nucleotide sequences in Xenopus 5S DNA required for transcription termination. Cell. 1981 Apr;24(1):261–270. doi: 10.1016/0092-8674(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Wormington W. M., Brown D. D. Stable transcription complexes of Xenopus 5S RNA genes: a means to maintain the differentiated state. Cell. 1982 Feb;28(2):413–421. doi: 10.1016/0092-8674(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Brow D. A. In vitro transcripts of a yeast variant 5 S rRNA gene exhibit alterations in 3'-end processing and protein binding. J Biol Chem. 1987 Oct 15;262(29):13959–13965. [PubMed] [Google Scholar]
- Campbell F. E., Jr, Setzer D. R. Transcription termination by RNA polymerase III: uncoupling of polymerase release from termination signal recognition. Mol Cell Biol. 1992 May;12(5):2260–2272. doi: 10.1128/mcb.12.5.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. C., Keene J. D. Isolation and analysis of cDNA clones expressing human lupus La antigen. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2115–2119. doi: 10.1073/pnas.82.7.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. C., Kenan D., Martin B. J., Keene J. D. Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem. 1988 Dec 5;263(34):18043–18051. [PubMed] [Google Scholar]
- Chan E. K., Sullivan K. F., Fox R. I., Tan E. M. Sjögren's syndrome nuclear antigen B (La): cDNA cloning, structural domains, and autoepitopes. J Autoimmun. 1989 Aug;2(4):321–327. doi: 10.1016/0896-8411(89)90159-5. [DOI] [PubMed] [Google Scholar]
- Chang D. Y., Maraia R. J. A cellular protein binds B1 and Alu small cytoplasmic RNAs in vitro. J Biol Chem. 1993 Mar 25;268(9):6423–6428. [PubMed] [Google Scholar]
- Daniels G. R., Deininger P. L. Integration site preferences of the Alu family and similar repetitive DNA sequences. Nucleic Acids Res. 1985 Dec 20;13(24):8939–8954. doi: 10.1093/nar/13.24.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieci G., Duimio L., Coda-Zabetta F., Sprague K. U., Ottonello S. A novel RNA polymerase III transcription factor fraction that is not required for template commitment. J Biol Chem. 1993 May 25;268(15):11199–11207. [PubMed] [Google Scholar]
- Englander E. W., Wolffe A. P., Howard B. H. Nucleosome interactions with a human Alu element. Transcriptional repression and effects of template methylation. J Biol Chem. 1993 Sep 15;268(26):19565–19573. [PubMed] [Google Scholar]
- Francoeur A. M., Chan E. K., Garrels J. I., Mathews M. B. Characterization and purification of lupus antigen La, and RNA-binding protein. Mol Cell Biol. 1985 Mar;5(3):586–590. doi: 10.1128/mcb.5.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Goldberg Y. P., Rommens J. M., Andrew S. E., Hutchinson G. B., Lin B., Theilmann J., Graham R., Glaves M. L., Starr E., McDonald H. Identification of an Alu retrotransposition event in close proximity to a strong candidate gene for Huntington's disease. Nature. 1993 Mar 25;362(6418):370–373. doi: 10.1038/362370a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 1989 Mar;8(3):841–850. doi: 10.1002/j.1460-2075.1989.tb03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Chubb A., Maroney P. A., Hannon G., Altman S., Nilsen T. W. Multiple cis-acting elements are required for RNA polymerase III transcription of the gene encoding H1 RNA, the RNA component of human RNase P. J Biol Chem. 1991 Dec 5;266(34):22796–22799. [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N., Weiner A. M. Formation of the 3' end of U1 snRNA requires compatible snRNA promoter elements. Cell. 1986 Oct 24;47(2):249–258. doi: 10.1016/0092-8674(86)90447-2. [DOI] [PubMed] [Google Scholar]
- Hoffman D. W., Query C. C., Golden B. L., White S. W., Keene J. D. RNA-binding domain of the A protein component of the U1 small nuclear ribonucleoprotein analyzed by NMR spectroscopy is structurally similar to ribosomal proteins. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2495–2499. doi: 10.1073/pnas.88.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., Sakamoto K. Alu interspersed repeats: selfish DNA or a functional gene family? New Biol. 1990 Sep;2(9):759–770. [PubMed] [Google Scholar]
- Jagadeeswaran P., Forget B. G., Weissman S. M. Short interspersed repetitive DNA elements in eucaryotes: transposable DNA elements generated by reverse transcription of RNA pol III transcripts? Cell. 1981 Oct;26(2 Pt 2):141–142. doi: 10.1016/0092-8674(81)90296-8. [DOI] [PubMed] [Google Scholar]
- Jahn D., Wingender E., Seifart K. H. Transcription complexes for various class III genes differ in parameters of formation and stability towards salt. J Mol Biol. 1987 Jan 20;193(2):303–313. doi: 10.1016/0022-2836(87)90221-x. [DOI] [PubMed] [Google Scholar]
- James P., Whelen S., Hall B. D. The RET1 gene of yeast encodes the second-largest subunit of RNA polymerase III. Structural analysis of the wild-type and ret1-1 mutant alleles. J Biol Chem. 1991 Mar 25;266(9):5616–5624. [PubMed] [Google Scholar]
- Jelinek W. R., Schmid C. W. Repetitive sequences in eukaryotic DNA and their expression. Annu Rev Biochem. 1982;51:813–844. doi: 10.1146/annurev.bi.51.070182.004121. [DOI] [PubMed] [Google Scholar]
- Jüttermann R., Hosokawa K., Kochanek S., Doerfler W. Adenovirus type 2 VAI RNA transcription by polymerase III is blocked by sequence-specific methylation. J Virol. 1991 Apr;65(4):1735–1742. doi: 10.1128/jvi.65.4.1735-1742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Riggs D. L., Negri R., Nguyen L. H., Geiduschek E. P. Transcription factor IIIB generates extended DNA interactions in RNA polymerase III transcription complexes on tRNA genes. Mol Cell Biol. 1989 Jun;9(6):2551–2566. doi: 10.1128/mcb.9.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek S., Renz D., Doerfler W. DNA methylation in the Alu sequences of diploid and haploid primary human cells. EMBO J. 1993 Mar;12(3):1141–1151. doi: 10.1002/j.1460-2075.1993.tb05755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuda D., Sinnett D., Richer C., Deragon J. M., Striker G. Evolution of mouse B1 repeats: 7SL RNA folding pattern conserved. J Mol Evol. 1991 May;32(5):405–414. doi: 10.1007/BF02101280. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Martin P. L., Roeder R. G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983 Nov 18;222(4625):740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- Liu W. M., Schmid C. W. Proposed roles for DNA methylation in Alu transcriptional repression and mutational inactivation. Nucleic Acids Res. 1993 Mar 25;21(6):1351–1359. doi: 10.1093/nar/21.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
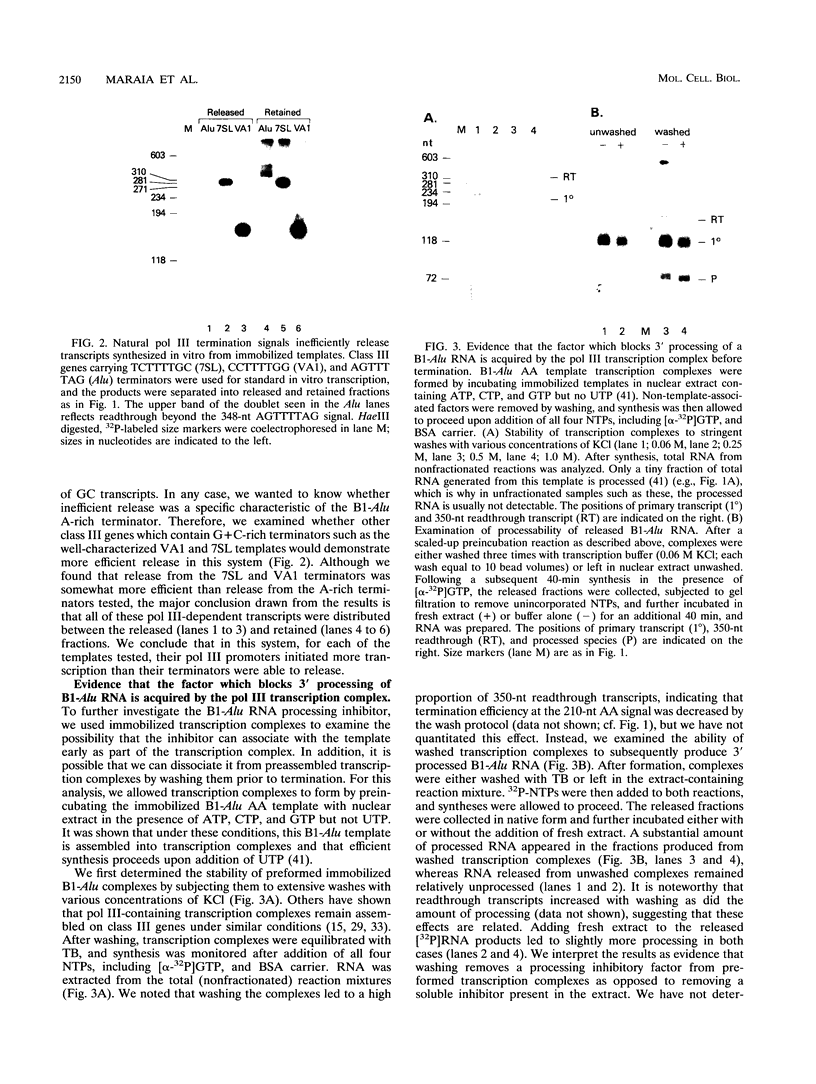
- Maraia R. J., Chang D. Y., Wolffe A. P., Vorce R. L., Hsu K. The RNA polymerase III terminator used by a B1-Alu element can modulate 3' processing of the intermediate RNA product. Mol Cell Biol. 1992 Apr;12(4):1500–1506. doi: 10.1128/mcb.12.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
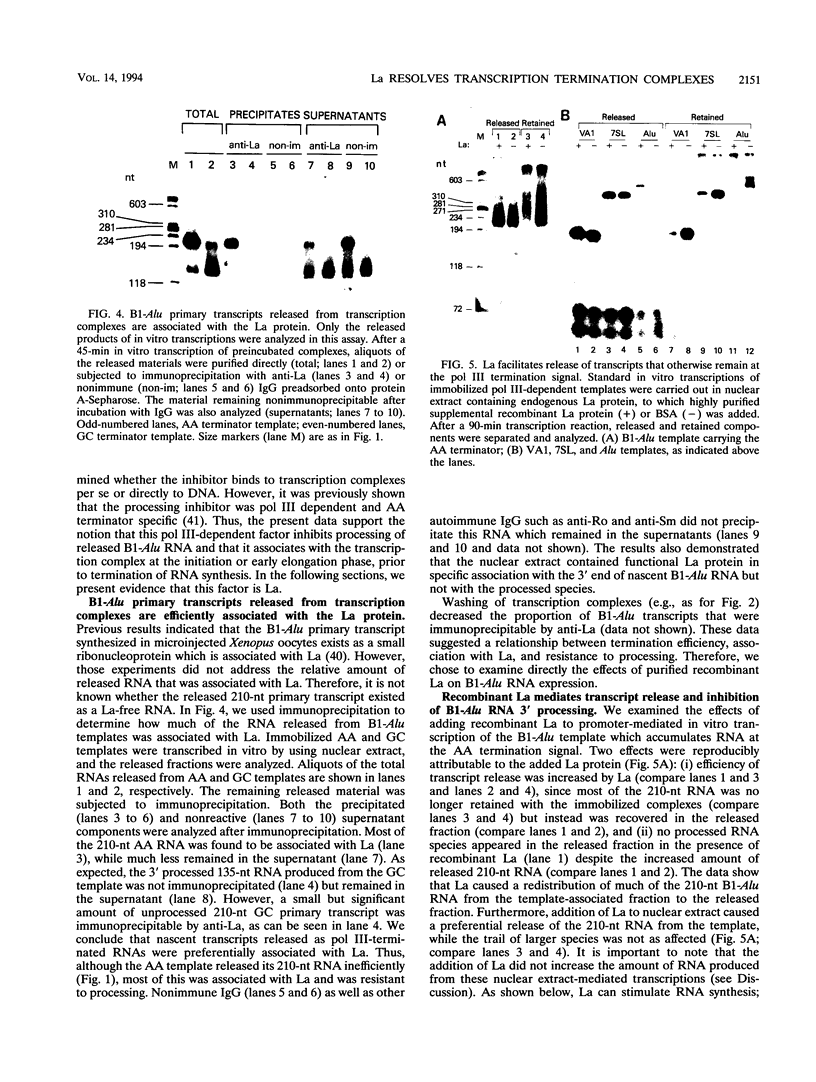
- Maraia R. J., Driscoll C. T., Bilyeu T., Hsu K., Darlington G. J. Multiple dispersed loci produce small cytoplasmic Alu RNA. Mol Cell Biol. 1993 Jul;13(7):4233–4241. doi: 10.1128/mcb.13.7.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraia R. J. The subset of mouse B1 (Alu-equivalent) sequences expressed as small processed cytoplasmic transcripts. Nucleic Acids Res. 1991 Oct 25;19(20):5695–5702. doi: 10.1093/nar/19.20.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
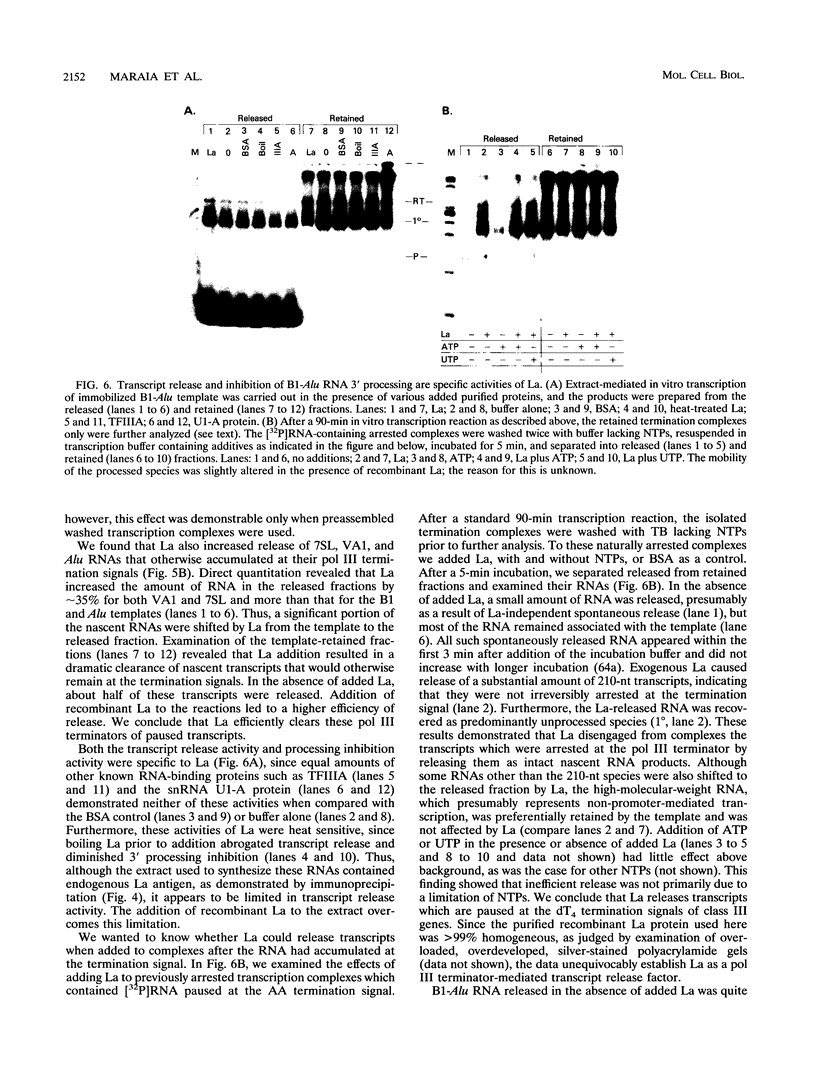
- Maraia R., Zasloff M., Plotz P., Adeniyi-Jones S. Pathway of B1-Alu expression in microinjected oocytes: Xenopus laevis proteins associated with nuclear precursor and processed cytoplasmic RNAs. Mol Cell Biol. 1988 Oct;8(10):4433–4440. doi: 10.1128/mcb.8.10.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera A. G., Hellmann U., Schmid C. W. A transpositionally and transcriptionally competent Alu subfamily. Mol Cell Biol. 1990 Oct;10(10):5424–5432. doi: 10.1128/mcb.10.10.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Francoeur A. M. La antigen recognizes and binds to the 3'-oligouridylate tail of a small RNA. Mol Cell Biol. 1984 Jun;4(6):1134–1140. doi: 10.1128/mcb.4.6.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazabraud A., Scherly D., Müller F., Rungger D., Clarkson S. G. Structure and transcription termination of a lysine tRNA gene from Xenopus laevis. J Mol Biol. 1987 Jun 20;195(4):835–845. doi: 10.1016/0022-2836(87)90488-8. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y. V., Lee H. S., Lejbkowicz F., Kenan D. J., Chan E. K., Agol V. I., Keene J. D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993 Jul;67(7):3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratani K., Hada T., Yamamoto Y., Kaneko T., Shigeto Y., Ohue T., Furuyama J., Higashino K. Inactivation of the cholinesterase gene by Alu insertion: possible mechanism for human gene transposition. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11315–11319. doi: 10.1073/pnas.88.24.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neznanov N. S., Oshima R. G. cis regulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Mar;13(3):1815–1823. doi: 10.1128/mcb.13.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neznanov N., Thorey I. S., Ceceña G., Oshima R. G. Transcriptional insulation of the human keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Apr;13(4):2214–2223. doi: 10.1128/mcb.13.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson K. E., Schmid C. W. Transcriptional inactivity of Alu repeats in HeLa cells. Nucleic Acids Res. 1986 Aug 11;14(15):6145–6158. doi: 10.1093/nar/14.15.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Henning D., Tan E., Busch H. Identification of a La protein binding site in a RNA polymerase III transcript (4.5 I RNA). J Biol Chem. 1983 Jul 10;258(13):8352–8356. [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Rosa M. D., Gottlieb E., Lerner M. R., Steitz J. A. Striking similarities are exhibited by two small Epstein-Barr virus-encoded ribonucleic acids and the adenovirus-associated ribonucleic acids VAI and VAII. Mol Cell Biol. 1981 Sep;1(9):785–796. doi: 10.1128/mcb.1.9.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Fordis C. M., Corsico C. D., Howard T. H., Howard B. H. Modulation of HeLa cell growth by transfected 7SL RNA and Alu gene sequences. J Biol Chem. 1991 Feb 15;266(5):3031–3038. [PubMed] [Google Scholar]
- Scherly D., Stutz F., Lin-Marq N., Clarkson S. G. La proteins from Xenopus laevis. cDNA cloning and developmental expression. J Mol Biol. 1993 May 20;231(2):196–204. doi: 10.1006/jmbi.1993.1275. [DOI] [PubMed] [Google Scholar]
- Schmid C., Maraia R. Transcriptional regulation and transpositional selection of active SINE sequences. Curr Opin Genet Dev. 1992 Dec;2(6):874–882. doi: 10.1016/s0959-437x(05)80110-8. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Tilghman S. M. Transient expression of a mouse alpha-fetoprotein minigene: deletion analyses of promoter function. Mol Cell Biol. 1983 Jul;3(7):1295–1309. doi: 10.1128/mcb.3.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., Brown D. D. Formation and stability of the 5 S RNA transcription complex. J Biol Chem. 1985 Feb 25;260(4):2483–2492. [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Mathews D. E., Durbin R. D., Burgess R. R. Tagetitoxin: a new inhibitor of eukaryotic transcription by RNA polymerase III. J Biol Chem. 1990 Jan 5;265(1):499–505. [PubMed] [Google Scholar]
- Steitz J. A. Immunoprecipitation of ribonucleoproteins using autoantibodies. Methods Enzymol. 1989;180:468–481. doi: 10.1016/0076-6879(89)80118-1. [DOI] [PubMed] [Google Scholar]
- Terns M. P., Lund E., Dahlberg J. E. 3'-end-dependent formation of U6 small nuclear ribonucleoprotein particles in Xenopus laevis oocyte nuclei. Mol Cell Biol. 1992 Jul;12(7):3032–3040. doi: 10.1128/mcb.12.7.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topfer F., Gordon T., McCluskey J. Characterization of the mouse autoantigen La (SS-B). Identification of conserved RNA-binding motifs, a putative ATP binding site and reactivity of recombinant protein with poly(U) and human autoantibodies. J Immunol. 1993 Apr 1;150(7):3091–3100. [PubMed] [Google Scholar]
- Ullu E., Weiner A. M. Human genes and pseudogenes for the 7SL RNA component of signal recognition particle. EMBO J. 1984 Dec 20;3(13):3303–3310. doi: 10.1002/j.1460-2075.1984.tb02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Arsdell S. W., Denison R. A., Bernstein L. B., Weiner A. M., Manser T., Gesteland R. F. Direct repeats flank three small nuclear RNA pseudogenes in the human genome. Cell. 1981 Oct;26(1 Pt 1):11–17. doi: 10.1016/0092-8674(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Andersen L. B., Saulino A. M., Gregory P. E., Glover T. W., Collins F. S. A de novo Alu insertion results in neurofibromatosis type 1. Nature. 1991 Oct 31;353(6347):864–866. doi: 10.1038/353864a0. [DOI] [PubMed] [Google Scholar]
- Watson J. B., Chandler D. W., Gralla J. D. Specific termination of in vitro transcription by calf thymus RNA polymerase III. Nucleic Acids Res. 1984 Jul 11;12(13):5369–5384. doi: 10.1093/nar/12.13.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis I. M. RNA polymerase III. Genes, factors and transcriptional specificity. Eur J Biochem. 1993 Feb 15;212(1):1–11. doi: 10.1111/j.1432-1033.1993.tb17626.x. [DOI] [PubMed] [Google Scholar]
- Wu J., Grindlay G. J., Bushel P., Mendelsohn L., Allan M. Negative regulation of the human epsilon-globin gene by transcriptional interference: role of an Alu repetitive element. Mol Cell Biol. 1990 Mar;10(3):1209–1216. doi: 10.1128/mcb.10.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. R., Scott R. W., Hamer D. H., Tilghman S. M. Construction and expression in vivo of an internally deleted mouse alpha-fetoprotein gene: presence of a transcribed Alu-like repeat within the first intervening sequence. Nucleic Acids Res. 1982 May 25;10(10):3099–3116. doi: 10.1093/nar/10.10.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vegvar H. E., Lund E., Dahlberg J. E. 3' end formation of U1 snRNA precursors is coupled to transcription from snRNA promoters. Cell. 1986 Oct 24;47(2):259–266. doi: 10.1016/0092-8674(86)90448-4. [DOI] [PubMed] [Google Scholar]