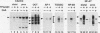Abstract
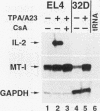
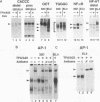
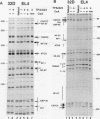
Interleukin-2 (IL-2) transcription is developmentally restricted to T cells and physiologically dependent on specific stimuli such as antigen recognition. Prior studies have shown that this stringent two-tiered regulation is mediated through a transcriptional promoter/enhancer DNA segment which is composed of diverse recognition elements. Factors binding to some of these elements are present constitutively in many cell types, while others are signal dependent, T cell specific, or both. This raises several questions about the molecular mechanism by which IL-2 expression is regulated. Is the developmental commitment of T cells reflected molecularly by stable interaction between available factors and the IL-2 enhancer prior to signal-dependent induction? At which level, factor binding to DNA or factor activity once bound, are individual regulatory elements within the native enhancer regulated? By what mechanism is developmental and physiological specificity enforced, given the participation of many relatively nonspecific elements? To answer these questions, we have used in vivo footprinting to determine and compare patterns of protein-DNA interactions at the native IL-2 locus in cell environments, including EL4 T-lymphoma cells and 32D clone 5 premast cells, which express differing subsets of IL-2 DNA-binding factors. We also used the immunosuppressant cyclosporin A as a pharmacological agent to further dissect the roles played by cyclosporin A-sensitive factors in the assembly and maintenance of protein-DNA complexes. Occupancy of all site types was observed exclusively in T cells and then only upon excitation of signal transduction pathways. This was true even though partially overlapping subsets of IL-2-binding activities were shown to be present in 32D clone 5 premast cells. This observation was especially striking in 32D cells because, upon signal stimulation, they mobilized a substantial set of IL-2 DNA-binding activities, as measured by in vitro assays using nuclear extracts. We conclude that binding activities of all classes fail to stably occupy their cognate sites in IL-2, except following activation of T cells, and that specificity of IL-2 transcription is enforced at the level of chromosomal occupancy, which appears to be an all-or-nothing phenomenon.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brabletz T., Pietrowski I., Serfling E. The immunosuppressives FK 506 and cyclosporin A inhibit the generation of protein factors binding to the two purine boxes of the interleukin 2 enhancer. Nucleic Acids Res. 1991 Jan 11;19(1):61–67. doi: 10.1093/nar/19.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel K., Hentsch B., Pfeuffer I., Serfling E. One base pair change abolishes the T cell-restricted activity of a kB-like proto-enhancer element from the interleukin 2 promoter. Nucleic Acids Res. 1991 Nov 11;19(21):5929–5936. doi: 10.1093/nar/19.21.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson K. A., Beverly B., Kang S. M., Lenardo M., Schwartz R. H. Transcriptional regulation of cytokine genes in nontransformed T cells. Apparent constitutive signals in run-on assays can be caused by repeat sequences. J Immunol. 1991 Nov 15;147(10):3601–3609. [PubMed] [Google Scholar]
- Brunvand M. W., Schmidt A., Siebenlist U. Nuclear factors interacting with the mitogen-responsive regulatory region of the interleukin-2 gene. J Biol Chem. 1988 Dec 15;263(35):18904–18910. [PubMed] [Google Scholar]
- Chen D., Rothenberg E. V. Molecular basis for developmental changes in interleukin-2 gene inducibility. Mol Cell Biol. 1993 Jan;13(1):228–237. doi: 10.1128/mcb.13.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Durand D. B., Shaw J. P., Bush M. R., Replogle R. E., Belagaje R., Crabtree G. R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988 Apr;8(4):1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Garrity P. A., Wold B. J. Effects of different DNA polymerases in ligation-mediated PCR: enhanced genomic sequencing and in vivo footprinting. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1021–1025. doi: 10.1073/pnas.89.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Nolan P. Nuclear transcription factors that bind to elements of the IL-2 promoter. Induction requirements in primary human T cells. J Immunol. 1991 Oct 15;147(8):2734–2739. [PubMed] [Google Scholar]
- Hentsch B., Mouzaki A., Pfeuffer I., Rungger D., Serfling E. The weak, fine-tuned binding of ubiquitous transcription factors to the Il-2 enhancer contributes to its T cell-restricted activity. Nucleic Acids Res. 1992 Jun 11;20(11):2657–2665. doi: 10.1093/nar/20.11.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992 Feb 15;148(4):1240–1250. [PubMed] [Google Scholar]
- Kamps M. P., Corcoran L., LeBowitz J. H., Baltimore D. The promoter of the human interleukin-2 gene contains two octamer-binding sites and is partially activated by the expression of Oct-2. Mol Cell Biol. 1990 Oct;10(10):5464–5472. doi: 10.1128/mcb.10.10.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. M., Tran A. C., Grilli M., Lenardo M. J. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992 Jun 5;256(5062):1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Kara C. J., Glimcher L. H. In vivo footprinting of MHC class II genes: bare promoters in the bare lymphocyte syndrome. Science. 1991 May 3;252(5006):709–712. doi: 10.1126/science.1902592. [DOI] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire K. L., Rothenberg E. V. Inducibility of interleukin-2 RNA expression in individual mature and immature T lymphocytes. EMBO J. 1987 Apr;6(4):939–946. doi: 10.1002/j.1460-2075.1987.tb04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire K. L., Yang J. A., Rothenberg E. V. Influence of activating stimulus on functional phenotype: interleukin 2 mRNA accumulation differentially induced by ionophore and receptor ligands in subsets of murine T cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6503–6507. doi: 10.1073/pnas.85.17.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. H., Wold B. J. c-myc inhibition of MyoD and myogenin-initiated myogenic differentiation. Mol Cell Biol. 1991 May;11(5):2842–2851. doi: 10.1128/mcb.11.5.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. R., Salser S. J., Wold B. Constitutive and metal-inducible protein:DNA interactions at the mouse metallothionein I promoter examined by in vivo and in vitro footprinting. Genes Dev. 1988 Apr;2(4):412–427. doi: 10.1101/gad.2.4.412. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Novak T. J., Chen D., Rothenberg E. V. Interleukin-1 synergy with phosphoinositide pathway agonists for induction of interleukin-2 gene expression: molecular basis of costimulation. Mol Cell Biol. 1990 Dec;10(12):6325–6334. doi: 10.1128/mcb.10.12.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., Rothenberg E. V. cAMP inhibits induction of interleukin 2 but not of interleukin 4 in T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9353–9357. doi: 10.1073/pnas.87.23.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak T. J., White P. M., Rothenberg E. V. Regulatory anatomy of the murine interleukin-2 gene. Nucleic Acids Res. 1990 Aug 11;18(15):4523–4533. doi: 10.1093/nar/18.15.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randak C., Brabletz T., Hergenröther M., Sobotta I., Serfling E. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 1990 Aug;9(8):2529–2536. doi: 10.1002/j.1460-2075.1990.tb07433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud G., Roux J., Pictet R., Grange T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell. 1991 Nov 29;67(5):977–986. doi: 10.1016/0092-8674(91)90370-e. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. V. The development of functionally responsive T cells. Adv Immunol. 1992;51:85–214. doi: 10.1016/s0065-2776(08)60487-3. [DOI] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Shaw J., Meerovitch K., Bleackley R. C., Paetkau V. Mechanisms regulating the level of IL-2 mRNA in T lymphocytes. J Immunol. 1988 Apr 1;140(7):2243–2248. [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Wang C. Y., Ho I. C., Bohjanen P. R., Petryniak B., June C. H., Miesfeldt S., Zhang L., Nabel G. J., Karpinski B. cis-acting sequences required for inducible interleukin-2 enhancer function bind a novel Ets-related protein, Elf-1. Mol Cell Biol. 1992 Mar;12(3):1043–1053. doi: 10.1128/mcb.12.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis A., Amsterdam A., Belanger C., Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev. 1991 May;5(5):880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Flanagan W. M., Edwards C. A., Crabtree G. R. Activation of early gene expression in T lymphocytes by Oct-1 and an inducible protein, OAP40. Science. 1991 Oct 25;254(5031):558–562. doi: 10.1126/science.1683003. [DOI] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Guidos C., Crabtree G. R. Cell type specificity and activation requirements for NFAT-1 (nuclear factor of activated T-cells) transcriptional activity determined by a new method using transgenic mice to assay transcriptional activity of an individual nuclear factor. J Biol Chem. 1990 Sep 15;265(26):15788–15795. [PubMed] [Google Scholar]
- Waterman M. L., Fischer W. H., Jones K. A. A thymus-specific member of the HMG protein family regulates the human T cell receptor C alpha enhancer. Genes Dev. 1991 Apr;5(4):656–669. doi: 10.1101/gad.5.4.656. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Williams T. M., Moolten D., Burlein J., Romano J., Bhaerman R., Godillot A., Mellon M., Rauscher F. J., 3rd, Kant J. A. Identification of a zinc finger protein that inhibits IL-2 gene expression. Science. 1991 Dec 20;254(5039):1791–1794. doi: 10.1126/science.1840704. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Oosterwegel M., Dooijes D., Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991 Jan;10(1):123–132. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]