Abstract
Objectives
Although dentin hypersensitivity is a common clinical condition and is generally reported by the patient after experiencing a sharp, short pain caused by one of several different external stimuli, it is often inadequately understood. The purpose of this paper is to discuss different available diagnostic approaches and assessment methods used in order to suggest a basis to diagnose, monitor, and measure these challenging painful conditions related to dentin hypersensitivity in daily practice and scientific projects properly.
Material and methods
A PubMed literature search strategy including the following MeSH terms were used as follows: “dentin sensitivity”[MeSH Terms] OR “dentin”[All Fields] AND “sensitivity”[All Fields] OR “dentin sensitivity”[All Fields] OR “dentin”[All Fields] AND “hypersensitivity”[All Fields] OR “dentin hypersensitivity”[All Fields] AND “diagnosis”[Subheading] OR “diagnosis”[All Fields] OR “diagnosis”[MeSH Terms] AND “assessment”[All Fields] AND (“methods”[Subheading] OR “methods”[All Fields] OR “methods”[MeSH Terms]. Furthermore, alternative terms such as “validity,” “reliability,” “root,” “cervical,” “diagnostic criteria,” and “hypersensitivities” were additionally evaluated.
Results
The literature search, also including the alternative terms and journals, revealed only a small number of specific papers related to valid diagnosis, diagnostic criteria, and assessment methods of dentin hypersensitivity. Outcomes from these publications showed that the response to different stimuli varies substantially from one person to another and is, due to individual factors, often difficult to assess correctly. Furthermore, the cause of the reported pain can vary, and the patient’s description of the history, symptoms, and discomfort might be different from one to another, not allowing a reliable and valid diagnosis.
Conclusions
The dental practitioner, using a variety of diagnostic and measurement techniques each day, will often have difficulties in differentiating dentin hypersensitivity from other painful conditions and in evaluating the success of a conducted therapy in a reliable way.
Clinical relevance
Correct diagnosis of dentin hypersensitivity including a patient’s history screening and a brief clinical examination in combination with the identification of etiologic and predisposing factors, particularly dietary and oral hygiene habits associated with erosion and abrasion, is essential. The relevant differential diagnosis should be considered to exclude all other dental conditions with similar pain symptoms.
Keywords: Dentin hypersensitivity, Diagnosis, Differential diagnosis, Diagnostic criteria, Dentin hypersensitivity assessment, Monitoring dentin hypersensitivity
Introduction
Previously, dentin hypersensitivity (DHS) was described as “an enigma being frequently encountered yet poorly understood” [1]. More recently, an internationally accepted and widely used definition in the international literature for dentin hypersensitivity is available [2]. Dentin hypersensitivity is characterized by distinctive short, sharp pain arising from exposed cervical dentin in response to various external stimuli that are typically thermal, evaporative, tactile, electrical, osmotic, or chemical, which cannot be ascribed to any other form of dental pathology, defect, or disease (Fig. 1) [3–5]. The definition provides a clinical description of the condition and identifies dentin hypersensitivity as a special clinical entity [6]. The most frequently experienced pain from dentin hypersensitivity is characterized by a rapid onset, sharp burst of pain of short duration strongly assigned to the application time and site of the used stimuli. Since several oral conditions may cause dental pain, such as untreated caries, a cracked tooth or marginal leakage around insufficient restorations, the diagnosis of dentin hypersensitivity can be very difficult [2, 7, 8]. Although there are numerous publications on all topics related to dentin hypersensitivity, a relatively high number of dental professionals are confused about the diagnosis, etiology, predisposing factors, and mechanism of this clinical condition [4, 9–12]. Time is needed to make a correct diagnosis, because dentin hypersensitivity is always a diagnosis of exclusion; it could only definitely be confirmed after all possible other conditions have been diagnostically eliminated. Therefore, the correct attribution of dental pain to dentin hypersensitivity is essential for dentists to develop and implement appropriate treatment options to help suffering patients effectively [4, 13]. However, despite an enormous number of products that are available for dental professionals and patients, a conclusive evidence of a successful treatment is still missing [14]. Although most of these agents have been proposed and developed to treat dentin hypersensitivity successfully, many clinical studies have shown contradictory results [15]. One explanation might be that in all pain studies, it is notoriously difficult to assess the subjective and individual different nature and complexity of pain [16]. Therefore, the correct and reliable diagnosis with valid measurement and assessment of dentin hypersensitivity is also a key factor in monitoring patients and judging therapeutic approaches in clinical trials.
Fig. 1.
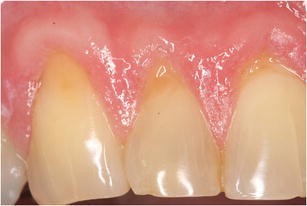
Patient with painful teeth after experiencing cold. Exposed dentin surfaces and signs of erosive lesions could explain the presence of dentin hypersensitivity
Material and methods
A PubMed literature search strategy including the following MeSH terms was used: “dentin sensitivity”[MeSH Terms] OR “dentin”[All Fields] AND “sensitivity”[All Fields] OR “dentin sensitivity”[All Fields] OR “dentin”[All Fields] AND “hypersensitivity”[All Fields] OR “dentin hypersensitivity”[All Fields] AND “diagnosis”[Subheading] OR “diagnosis”[All Fields] OR “diagnosis”[MeSH Terms] AND “assessment”[All Fields] AND (“methods”[Subheading] OR “methods”[All Fields] OR “methods”[MeSH Terms]. Furthermore, alternative and additional terms such as “validity,” “reliability,” “root,” “cervical,” “diagnostic criteria,” and “hypersensitivities,” and the possible combinations of these terms were evaluated. Additional handsearching was performed for the major oral medicine journals not included in PubMed. References of included studies and reviews were checked for further results. No language limitations were imposed. Date of last search was 20th of April 2012.
Criteria for considering studies for this overview
Publication related to the diagnosis of dentin hypersensitivity and different diagnostic procedures and criteria including tactile, thermal, and airblast stimuli, or assessment methodologies relevant for evaluation of patients’ subjective pain were selected.
Results
The actually performed PubMed search reveals that the number of international publications related to the diagnosis of dentin hypersensitivity is rarely available (the last search date was 20th of April 2012, Table 1). However, most of the selected 198 papers from the PubMed search found for diagnosis were related to clinical investigations evaluating the efficacy of different treatment products rather than for diagnosis per se which might be an indication that there are limited data available in the way of diagnosis and management of DHS. Indeed, only seven publications related to different aspects of the diagnosis of dentin hypersensitivity were identified that would be relevant for the purpose of the present overview [4, 6, 8, 17–19]. Expanding the PubMed search to other aspects of dentin hypersensitivity, further publications could be identified, including several papers relating specifically to diagnosis and diagnostic criteria [7, 12, 13, 20–23]. Regarding the assessment and measurement methods for dentin hypersensitivity, PubMed search identified only 13 relevant papers which could be included [5, 24–35]. Additional information could be found in four further papers dealing with therapeutic aspects and the clinical effect of different materials [13, 23, 36, 37].
Table 1.
The results of a PubMed search (last search date, 20th of April 2012) reveal that many more publications focus on the etiology and therapy of dentin hypersensitivity than on pathological, epidemiological, and especially on diagnostic aspects
| PubMed search strategy | Hits |
|---|---|
| “Dentin sensitivity/diagnosis”[MeSH] | 198 |
| “Dentin sensitivity/diagnosis”[MeSH] AND “Diagnostic Criteria”[All Fields] | 7 |
| “Dentin sensitivity/diagnosis”[MeSH] AND “Assessment Methods”[All Fields] | 13 |
| “Dentin sensitivity/etiology”[MeSH] | 686 |
| “Dentin sensitivity/pathology”[MeSH] | 76 |
| “Dentin sensitivity/epidemiology”[MeSH] | 51 |
| “Dentin sensitivity/therapy”[MeSH] | 1,155 |
Discussion
Typically, dentin hypersensitivity occurs when the external stimulus contacts exposed dentin surfaces with open and patent tubules [20]. The different stimuli trigger a rapid outflow of dentin fluid, and the following pressure change across the dentin activates baroreceptors near the pulp, leading to cause an immediate sharp pain [38]. Tactile, cold, evaporative, and osmotic stimuli trigger the nonphysiological fluid outflow. On the other hand, heat induces a slow retreat of dentin fluid, and the resultant pressure change activates the baroreceptors and nerve fibers in a less dramatic fashion, consistent with the observation that cold and evaporative stimuli are generally more painful to patients than heat [38]. The hydrodynamic theory of dentin hypersensitivity requires that dentin tubules are exposed at the dentin surface and patent to the pulp [39, 40]. Absi et al. demonstrated that the number of tubules in clinically hypersensitive teeth was eight times higher, and the tubules were two times larger in diameter and were mostly open compared with nonsensitive teeth [40, 41]. Furthermore, according to Poiseuille’s law, the fluid flow rate is proportional to the fourth power of the tubule radius, so that the fluid flow in hypersensitive teeth might probably be 16 times greater than that in nonsensitive teeth [2]. It is obvious that this difference in tubule diameter between hypersensitive and nonsensitive teeth might be of clinical relevance [38]. Therefore, a partial or complete tubule occlusion by therapeutic approaches or after physiological sclerosis may have significant effects on fluid flow and the corresponding symptoms and diagnosis [2]. As included in the definition of dentin hypersensitivity, the etiological factors must arise two specific changes in teeth. First, the dentin surface must be exposed and denuded, which requires the loss of enamel or gingival recession combined with loss of cementum. The second condition is the opening of the dentin tubules to allow the sensory mechanisms in the pulpal area following stimulation of the dentin surface [42].
Gingival recession and exposure of the underlying dentin could be caused by overzealous tooth brushing and improper tooth brushing technique, or by periodontal disease and its surgical and nonsurgical treatment [38]. Based on recently published studies, it appears that normal tooth brushing cannot cause significant enamel loss [42]. However, erosion from acidic foods and drinks in combination with tooth brushing can result in significant tooth wear on any aspect of the tooth surface, especially in the cervical areas [1, 43]. Investigators have concluded that gingival recession and erosion, rather than cervical enamel loss, may be the most important factor for dentin hypersensitivity [4].
Exposed dentin tubules are loosely occluded by a smear layer. On the basis of in vitro studies, it has been suggested that chemical reactions can remove the smear layer to open the exposed dentin tubules [38]. Further investigations reported that physical forces like tooth brushing alone are not able to remove the smear layer, opening exposed dentin tubules [16]. Dentin hypersensitivity is typically experienced by the middle-aged adult population, age range from 20 to 49 years, with a peak incidence between 30 and 39 years [22]. A slightly higher incidence of dentin hypersensitivity has been observed in females, which may reflect oral hygiene and dietary practices. Dentin hypersensitivity is most commonly observed in the buccal cervical area of permanent teeth, with canine, premolar, and incisor teeth being more frequently affected than molars [38]. Studies of the prevalence of dentin hypersensitivity have reported levels up to 57 % if patients in a general dental practice are evaluated [22, 44]. However, there are further studies suggesting that levels of 10–25 % are typical [45, 46]. The reported wide variations have been attributed to different methods of assessment, self-reported or professional clinical diagnosis, the population base and setting, and behavioral factors, such as oral hygiene habits and intake of acidic foods and drinks [38, 42]. Not surprisingly, levels of dentin hypersensitivity are higher, ranging from 65 to 98 %, in patients following periodontal treatment or surgery [47–49]. All these aspects, presence of denuded dentin surfaces, age, dental history, and the presence of predisposing factors should be considered when dealing with the aspect of dentin hypersensitivity diagnosis in order to allow a valid diagnosis.
General strategy for managing dentin hypersensitivity
Although our knowledge may have been previously incomplete, subsequent advances in our understanding have made a comprehensive approach to dentin hypersensitivity management possible. Specifically, the dental professional is advised to follow six steps with patients suffering from hypersensitivity teeth (Fig. 2) [4]:
Correct diagnosis of dentin hypersensitivity including a patient’s history screening and a brief clinical examination
Identification of etiologic and predisposing factors, particularly dietary and oral hygiene habits associated with erosion and abrasion
Differential diagnosis, to exclude all other dental conditions with similar pain symptoms
If present, treatment of all conditions with symptoms similar to dentin hypersensitivity
Removal or minimization of etiologic and predisposing factors through dietary advice and improved oral hygiene instruction
Recommendation or implementation of treatment based upon individual needs (in office and at home)
Fig. 2.
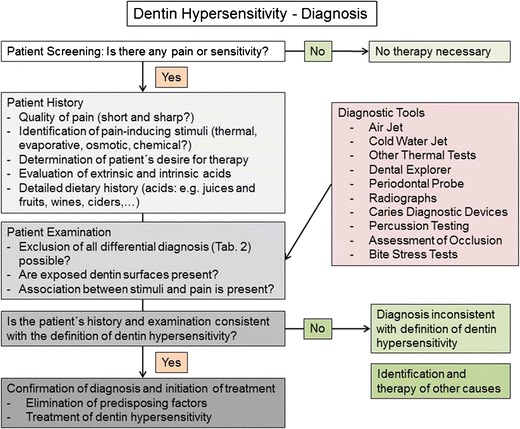
Clinical recommendation for valid diagnosis of dentin hypersensitivity modified on base of the algorithm published by the Canadian Advisory Board on Dentin Hypersensitivity in 2003 [4]
As mentioned and highlighted above, the exact diagnosis of dentin hypersensitivity is essential to consider successful treatment strategies and long-lasting pain relief for suffering patients. However, the results from the actually performed PubMed search undertaken for the present paper would indicate that there are limited data available in relation to the diagnosis of dentin hypersensitivity per se (Table 1). Unfortunately, even most of the 198 papers found for diagnosis are clinical investigations focusing on the success and comparison of different treatment options. Hence, this might be an indication that the issue of giving a correct and valid diagnosis of dentin hypersensitivity is either an easy or a difficult thing for dental professionals in daily practice. Regarding the definition of dentin hypersensitivity “Dentin hypersensitivity is characterized by a short sharp pain arising from exposed dentin in response to different stimuli and which cannot be ascribed to any other form of dental defect or pathology,” it is obvious that this definition contains two related parts. The first part is the clinical description and most relevant to establish a valid diagnosis. The second part strongly recommends considering numerous differential diagnosis to exclude other causes of dentinal pain to increase the probability for a correct diagnosis (Table 2).
Table 2.
A not necessarily exhaustive list of conditions which may results in similar symptoms to cervical dentin hypersensitivity [2, 7, 11]
| Differential diagnosis |
|---|
| Cracked tooth syndrome |
| Fractured restorations |
| Restorations left in traumatic occlusion |
| Chipped teeth |
| Dental caries, root caries |
| Postoperative sensitivity |
| Pulpal response to restorative treatment or certain materials |
| Marginal leakage of restorations |
| Pulpitis, pulpal status |
| Gingival inflammation |
| Palatogingival grooves |
| Vital bleaching procedures |
| Atypical odontalgia |
Although for the majority of all these differential conditions, the sensory mechanisms including the stimuli that may produce the patient’s pain may provide a similar outcome, the subsequent clinical management and treatment may be completely different for DHS. Nevertheless, a valid diagnosis of dentin hypersensitivity remains obvious after exclusion of all these dental and periodontal conditions that also might cause pain [4, 5, 12]. A recent Internet survey reported that most of the responding dentists used up to 12 different diagnostic tools in the case of patients suffering from dentin hypersensitivity, for example, air jet, dental explorer, or cold test (Table 3) [10].
Table 3.
An almost complete list of different tools used for the diagnosis of dentin hypersensitivity [10, 30]
| Diagnostic tools |
|---|
| Air jet |
| Cold water jet |
| Other thermal tests |
| Electrical devices |
| Dental explorer |
| Periodontal probe |
| Radiographs |
| Caries diagnostic devices |
| Percussion testing |
| Assessment of occlusion |
| Bite stress tests |
Time is needed to make a correct diagnosis; because a thorough patient history screening is required and dentin hypersensitivity is always a diagnosis of exclusion, it is confirmed only after all possible other conditions have been diagnostically eliminated. Unfortunately, a validated screening checklist that contains dentin hypersensitivity-related predisposing, initiating, and perpetuating risk factors identified in clinical or epidemiological studies is currently not available.
Clinical recommendations for valid diagnosis of dentin hypersensitivity
In order to avoid false diagnosis, underdiagnosis and further on an over- or undertreatment screening of patients for terms of dentin hypersensitivity should be routinely done. For every patient, especially patients reporting symptoms and pain, a verbal screening is recommended, during which she/he should be asked if any tooth hurts when eating or drinking hot, iced, cold, or acidic food or beverages. Moreover, the patients should be asked if the symptoms are present during oral hygiene procedures or following restorative procedures. If patients could confirm at least one of these questions, the individual history of the patient should be obtained. Dental professionals should ask for specific pain characteristics like site, character, severity, time, etc. Furthermore, the patient should be asked to identify the pain-related and pain-inducing stimuli. In the next step, dental professionals may ask and look for personal behavior patterns (e.g., extrinsic and intrinsic acids, consumption of high-acid drinks or food, and overzealous dental hygiene) and previous dental therapies (e.g., professional tooth cleaning, scaling, and other periodontal treatment; vital tooth bleaching; and restorative procedures). Afterwards, a clinical examination to confirm clinical signs associated with the definition of dentin hypersensitivity should be undertaken (e.g., dental erosion, gingival recession, and exposed cervical dentin).
In patients with suspected dentin hypersensitivity due to positive findings in the screening and history, the thorough differential diagnosis is very important to eliminate all other forms of orofacial pain, including pulpal inflammation, periodontal pain, cracked tooth syndrome, insufficient margins of restorations, atypical odontalgia, etc. All differential diagnosis (Table 2) must be excluded, before the diagnosis of dentin hypersensitivity is definitely formulated [50].
Furthermore, a specific dentin hypersensitivity-related clinical examination is obligatory in cases with positive results in the first examination steps. It could be suggested to carry out a tactile stimulation with a dental explorer scratching in the mesio-distal direction on the exposed dentin [51, 52]. In addition, it is strongly recommended to use a second stimulus to confirm the diagnosis in every patient [4]. An air syringe delivering a stream of air directly directed towards the affected and exposed dentin surface is one of the most often used stimuli [51, 52]. These tactile and evaporative stimuli should reliably provoke the dentin hypersensitivity-associated pain. If the diagnosis of dentin hypersensitivity could be confirmed in this way, the next step is to eliminate predisposing factors [53] (e.g., acid intake, improve, dietary habits, optimize oral hygiene procedures, etc.) and to start the treatment of the suffering patient either with home use or with in-office desensitizing products [4, 38].
Moreover, the suffering patients are known to have substantially decreased oral health-related quality of life in comparison with the general population [54]. Therefore, the integration of any kind of questionnaire focusing on the impact of DHS on oral health-related quality of life of these patients in the diagnostic process might be interesting for daily practice [54, 55]. Unfortunately, the two mostly accepted and validated questionnaires used in several clinical studies regarding the impact of DHS—the Oral Health Impact Profile [56] and the Dentin Hypersensitivity Experience Questionnaire [55]—contain at least 48 items. In the daily practice, we could not expect to complete a Quality of Life Questionnaire containing this huge number of items for each patient, but a simple and shortened questionnaire could help to measure the intensity and impact of pain-suffering patients who are experiencing DHS. An accepted and also validated alternative to record clinical oral health status relationships that affect quality of life for daily practice may be using the short form of Oral Health Impact Profile-14 or the 12-item General Oral Health Assessment Index [57–60]. Both questionnaires contain a manageable number of items requiring only a few minutes for suffering patients. Therefore, this might be a practicable alternative for the daily use in dental practice.
Clinical recommendations for valid assessment and monitoring of dentin hypersensitivity
The dental practitioner is confronted—within the daily practice and especially in clinical trials regarding the severity of symptoms or the efficiency of therapeutic interventions—with the need to assess and monitor discomfort and pain associated with dentin hypersensitivity. According to Gillam et al. the assessment of dentin hypersensitivity in clinical investigations is always claimed to be subjective and depends on the individual reaction of the examined patients to different stimuli [30, 50]. The perception of pain arising from exposed dentin surfaces is influenced by a number of different aspects, including the individual parameters of each patient, psychological factors, cultural aspects, and situational and emotional factors [50]. It is also described that patients accommodate to the applied stimuli used in diagnostic testing. Generally, patients report high values at the beginning when the pain stimuli is unknown, but once they are aware or used to the applied stimuli, the response may change significantly [50]. Furthermore, there are known interactions between the dentist and patient like Hawthorne, placebo, and nocebo effects [50, 61–63]. The placebo effect is a complex psychophysiological response caused by placebo administration. The two main theories that explain the placebo effect are “classic conditioning” and the “expectancy theory” [62, 64]. Certainly, they interact with each other, and patients learn to experience improvement after medical treatment, and the expectations through consulting a doctor bring symptomatic improvements. Especially in clinical studies, the Hawthorne effect is discussed. Because of the concentrated attention and observation, people temporarily change their behavior or performance [63, 65]. “Nocebo” is the opposite effect of placebo and is activated by negative expectations [61]. It may induce negative adverse effects in placebo treatments and reverse symptoms from positive to negative ones [66]. The most recent literature suggests that in the right circumstances, everybody may respond to placebo, so we are not able to exclude these effects or distinguish patients who react [67].
These aspects might explain the lack of clear and robust evidence in the dental literature and the sometimes contradictory results of clinical studies focusing on the success of different treatment options [14, 15]. The ability to quantify patient sensations to external stimuli on exposed dentin surfaces should allow record more accurately on the magnitude of the condition and could help to evaluate the different therapeutic strategies in a more objective and evident manner. In case of dentin hypersensitivity, two different assessment methodologies are described. Dentin hypersensitivity might either be evaluated in terms of a stimulus intensity required to provoke pain (stimulus-based assessment) or as a subjective evaluation of the pain produced by a defined stimulus (response-based assessment) [30, 33, 68]. Characteristic for all stimulus-based methods is the measurement of an individual pain threshold. On the other hand, the response-based methods assess pain severity after application of a standardized, reliable, and reproducible stimulus [5, 22]. Furthermore, these methods could be judged as acceptable if the used stimulation method is accepted as scientifically valid [30]. As mentioned above, several stimuli could induce dentinal pain, but not all are suited for quantifying dentin hypersensitivity in clinical practice [30, 68]. Tactile, cold, and evaporative air stimuli are physiological and easy controllable. Therefore, these stimuli are mostly used and widely recommended in various publications [69–71]. The value of other stimulation methods like the use of electrical stimuli is discussed controversially and often needs a special device with increasing costs [68]. It is important to address that recorded dentin hypersensitivity may be different for different stimuli [68, 72]. Therefore, it is recommended that at least two different hydrodynamic stimuli should be used [5]. Furthermore, it would appear from the results of a recently published study that the tactile stimulus using a dental explorer is less effective than thermal or evaporative stimulation [22]. If several stimuli are applied, the least severe stimulus should be always applied first to avoid a negative impact on the results of the stimulation [30]. Furthermore, the interval between stimulus applications should be sufficient to eliminate interactions between both stimuli. Unfortunately, the correct interval is still unknown and is likely to vary for different types of stimuli.
In response-based methods, the stimulus is held constant, and the subject’s response varies. An example of a response-based method is the use of a timed airblast. Therefore, each hypersensitive tooth will be isolated from the adjacent teeth (mesial and distal) by the placement of the examiner’s fingers over the adjacent teeth. Air will be delivered from a standard dental unit air syringe at 60 psi (±5 psi) and 70 °F (±3°F). The air will be directed at the exposed buccal surface of the hypersensitive tooth for 1 s from a distance of approximately 1 cm. Directly after stimulation, the subject response can be quantified by using a visual analog scale in which the patient places a mark on a 100-mm line labeled from no pain to worst pain or a validated graphic pain scale, such as the Faces Pain Scale [73]. This is considered preferable to a numerical rating scale where the subject rates pain intensity on a scale comprising several distinct categories. Another method of quantification is to use a verbal descriptor scale which uses word descriptors as a scaling technique to describe variations in pain according to the patient’s spontaneous report or by the use of a validated questionnaire [74, 75]. One disadvantage of verbal descriptor scales is that they could be restrictive because they may not offer enough descriptions that can be placed in a continuous and ascending or descending order of severity of pain. One commonly used scale is the Schiff cold air sensitivity scale [69, 70, 76–79]. This scale is mainly used to assess subject response to a stimulus like air or evaporative.
This scale is scored as follows:
Subject does not respond to air stimulus.
Subject responds to air stimulus but does not request discontinuation of stimulus.
Subject responds to air stimulus and requests discontinuation or moves from stimulus.
Subject responds to air stimulus, considers stimulus to be painful, and requests discontinuation of the stimulus.
In stimulus-based studies, the subject’s response is held constant at the pain threshold, and the stimulus is varied with increasing and decreasing intensities. An often used stimulus-based method might employ a calibrated probe (XiniX Research, Inc., Portsmouth, NH, USA) where the tactile pressure applied to the tooth with a dental explorer tip can be varied and increased in steps of 10 g using an electronic device [69, 77, 79]. Using this device, teeth can be evaluated for tactile dentin hypersensitivity as follows [69]. The patients were instructed to respond at the point where she/he first experienced pain after stroking the explorer tip with the preset force perpendicular to the tooth. The applied force could be increased by 10 g increments until the patient experienced discomfort. This force will be recorded for further analysis. An alternative could be thermo or electrical devices which have been developed in the past for applying graded thermal or electrical stimuli. However, these devices were discussed contradictory in the literature [34]. In selecting stimulus-based methods, it is important to realize that these have certain drawbacks. Repeated painful stimulation may cause a change in sensitivity. Anticipation of pain by the subject may influence outcome, especially with gradually increasing stimuli. In a recently published study, Ide et al. showed that the reproducibility of evaluation methods for dentin hypersensitivity is difficult to achieve, even when standardized techniques were used [35]. Furthermore, the fact that stimulus-based methods are often time consuming, which limit the number of teeth that can be tested with multiple stimuli in one appointment. Generally, outcome evaluation of dentin hypersensitivity treatment in clinical practice as well as in clinical trials should include at least two different stimuli, and if possible, it is recommended to use both approaches—stimulus- and response-based assessments [5].
Conclusion
Today, dentin hypersensitivity is essentially a diagnosis of exclusion. Therefore, the validity and quality of the diagnosis corresponds to the value of the numerous existing differential diagnoses. Finally, at least two different stimuli should be used to assess dentin hypersensitivity. Tactile, cold, and evaporative air stimuli are physiological and easy controllable. Therefore, these stimuli are mostly used and widely recommended for the clinical diagnosis of dentin hypersensitivity in dental practice in various publications [69–71]. Different pain scores could be used to assess the discomfort following any of the above-mentioned stimuli. For dental practice, the use of a continuous 100-mm visual analog scale could be recommended.
Furthermore, monitoring of dentin hypersensitivity is a challenging field. We have some standardized methods, but we should consider that pain negatively influencing the quality of life of suffering patients is still subjective and might be affected by a huge number of individual factors.
As performed in many studies, treatment effects of different therapies or materials could be expressed in terms of the degree of reduction, but in developing further therapeutic strategies, we should always keep in mind that for our patient, the presence or absence of pain is the most important result. The overall dental health impact of dentin hypersensitivity on a particular individual may ultimately correlate with the degree of discomfort and pain experienced [80]. Since it is known that dentin hypersensitivity has an influence on oral health-related quality of life [54], it could be recommended to include this pain-related dimension during the patient’s treatment in daily practice and in clinical trials. Beside several published and validated questionnaires [55, 57, 60], a commonly and widely used instrument for this purpose is the Oral Health Impact Profile-49 [81], which needs to be completed by the patient. In addition to the original version of this questionnaire, validated translations are available in many other languages [82–90]. The evaluation of the impact on the quality of life of patients suffering from dentin hypersensitivity might be a promising aspect in further clinical trials. Certainly, these questionnaires, including 48 and 49 items, were too large for the routine use in daily practice. An acceptable alternative to record clinical oral health status relationships that affect the quality of life for daily practice may be using the short form of Oral Health Impact Profile-14 or the 12-item General Oral Health Assessment Index. Finally, education of both the public and dental professionals should be encouraged to allow that patients affected by and suffering from dentin hypersensitivity receive an adequate treatment.
Acknowledgments
The author would like to thank the GABA International AG (Therwil, Switzerland) for the support and the possibility to be part of the GABA Forum held in Basel in 2011. Furthermore, the author sincerely thanks Dr. David Gillam for his support and all the helpful comments.
Conflict of interest
The author declares that he has no conflict of interest.
References
- 1.Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity: an enigma? A review of terminology, mechanisms, aetiology and management. Br Dent J. 1999;187:606–611. doi: 10.1038/sj.bdj.4800345. [DOI] [PubMed] [Google Scholar]
- 2.Addy M, Smith SR. Dentin hypersensitivity: an overview on which to base tubule occlusion as a management concept. J Clin Dent. 2010;21:25–30. [PubMed] [Google Scholar]
- 3.Dowell P, Addy M, Dummer P. Dentine hypersensitivity: aetiology, differential diagnosis and management. Br Dent J. 1985;158:97–98. doi: 10.1038/sj.bdj.4805542. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Advisory Board On Dentin Hypersensitivity Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–226. [PubMed] [Google Scholar]
- 5.Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–813. doi: 10.1111/j.1600-051X.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 6.Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51:323–332. doi: 10.2334/josnusd.51.323. [DOI] [PubMed] [Google Scholar]
- 7.Orchardson R, Gillam DG. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137:990–998. doi: 10.14219/jada.archive.2006.0321. [DOI] [PubMed] [Google Scholar]
- 8.Ide M. The differential diagnosis of sensitive teeth. Dent Update. 1998;25:462–466. [PubMed] [Google Scholar]
- 9.Amarasena N, Spencer J, Ou Y, Brennan D. Dentine hypersensitivity—Australian dentists’ perspective. Aust Dent J. 2010;55:181–187. doi: 10.1111/j.1834-7819.2010.01223.x. [DOI] [PubMed] [Google Scholar]
- 10.Cunha-Cruz J, Wataha JC, Zhou L, Manning W, Trantow M, Bettendorf MM, Heaton LJ, Berg J. Treating dentin hypersensitivity: therapeutic choices made by dentists of the northwest PRECEDENT network. J Am Dent Assoc. 2010;141:1097–1105. doi: 10.14219/jada.archive.2010.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillam DG, Bulman JS, Eijkman MA, Newman HN. Dentists’ perceptions of dentine hypersensitivity and knowledge of its treatment. J Oral Rehabil. 2002;29:219–225. doi: 10.1046/j.1365-2842.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 12.Chu CH, Lam A, Lo EC. Dentin hypersensitivity and its management. Gen Dent. 2011;59:115–122. [PubMed] [Google Scholar]
- 13.Trushkowsky RD, Oquendo A. Treatment of dentin hypersensitivity. Dent Clin North Am. 2011;55:599–608. doi: 10.1016/j.cden.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen S, Errboe M, Lescay Mevil Y, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev. 2006;3:CD001476. doi: 10.1002/14651858.CD001476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha-Cruz J, Stout JR, Heaton LJ, Wataha JC. Dentin hypersensitivity and oxalates: a systematic review. J Dent Res. 2011;90:304–310. doi: 10.1177/0022034510389179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West NX. Dentine hypersensitivity. Monogr Oral Sci. 2006;20:173–189. doi: 10.1159/000093362. [DOI] [PubMed] [Google Scholar]
- 17.Terry DA. Cervical dentin hypersensitivity: etiology, diagnosis, and management. Dent Today. 2011;30:61–62. [PubMed] [Google Scholar]
- 18.Cummins D. Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent. 2009;20:1–9. [PubMed] [Google Scholar]
- 19.Martinez-Ricarte J, Faus-Matoses V, Faus-Llacer VJ, Flichy-Fernandez AJ, Mateos-Moreno B. Dentinal sensitivity: concept and methodology for its objective evaluation. Med Oral Patol Oral Cir Bucal. 2008;13:E201–E206. [PubMed] [Google Scholar]
- 20.Thomas MS. Dentin hypersensitivity. J Am Dent Assoc. 2011;142:16–18. doi: 10.14219/jada.archive.2011.0001. [DOI] [PubMed] [Google Scholar]
- 21.Parolia A, Kundabala M, Mohan M. Management of dentinal hypersensitivity: a review. J Calif Dent Assoc. 2011;39:167–179. [PubMed] [Google Scholar]
- 22.Gillam DG, Aris A, Bulman JS, Newman HN, Ley F. Dentine hypersensitivity in subjects recruited for clinical trials: clinical evaluation, prevalence and intra-oral distribution. J Oral Rehabil. 2002;29:226–231. doi: 10.1046/j.1365-2842.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- 23.Gillam DG, Orchardson R. Advances in the treatment of root dentine sensitivity: mechanism and treatment principles. Endod Top. 2006;13:13–33. doi: 10.1111/j.1601-1546.2006.00209.x. [DOI] [Google Scholar]
- 24.Stark MM, Pelzner R (1982) Measurement of dentinal hypersensitivity. Compend Contin Educ Dent 3:S105–107 [PubMed]
- 25.Ash MM. Quantification of stimuli. Endod Dent Traumatol. 1986;2:153–156. doi: 10.1111/j.1600-9657.1986.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 26.Kanapka JA, Colucci SV. Clinical evaluation of dentinal hypersensitivity: a comparison of methods. Endod Dent Traumatol. 1986;2:157–164. doi: 10.1111/j.1600-9657.1986.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 27.Kleinberg I, Kaufman HW, Confessore F. Methods of measuring tooth hypersensitivity. Dent Clin North Am. 1990;34:515–529. [PubMed] [Google Scholar]
- 28.Kontturi-Nahri V, Narhi M. Testing sensitive dentine in man. Int Endod J. 1993;26:4. doi: 10.1111/j.1365-2591.1993.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 29.Orchardson R. Clinical measurement of hypersensitive dentine. Int Endod J. 1993;26:5–6. doi: 10.1111/j.1365-2591.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 30.Gillam DG, Newman HN. Assessment of pain in cervical dentinal sensitivity studies. A review. J Clin Periodontol. 1993;20:383–394. doi: 10.1111/j.1600-051X.1993.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 31.Kleinberg I, Kaufman HW, Wolff M. Measurement of tooth hypersensitivity and oral factors involved in its development. Arch Oral Biol. 1994;39(Suppl):63S–71S. doi: 10.1016/0003-9969(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 32.Orro M, Truong T, De Vizio W, Miller S, Chu TC, Boylan D. Thermodontic stimulator—a new technology for assessment of thermal dentinal hypersensitivity. J Clin Dent. 1994;5(Spec No):83–86. [PubMed] [Google Scholar]
- 33.Gillam DG, Bulman JS, Newman HN. A pilot assessment of alternative methods of quantifying dental pain with particular reference to dentine hypersensitivity. Community Dent Health. 1997;14:92–96. [PubMed] [Google Scholar]
- 34.Walline BW, Wagner JG, Marx DB, Reinhardt RA. Comparison of methods for measuring root and mucogingival sensitivity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:641–646. doi: 10.1067/moe.2000.109659. [DOI] [PubMed] [Google Scholar]
- 35.Ide M, Wilson RF, Ashley FP. The reproducibility of methods of assessment for cervical dentine hypersensitivity. J Clin Periodontol. 2001;28:16–22. doi: 10.1034/j.1600-051x.2001.280103.x. [DOI] [PubMed] [Google Scholar]
- 36.Zaidel L, Patel R, Mello S, Heu R, Stranick M, Chopra S, Prencipe M. Anti-hypersensitivity mechanism of action for a dentifrice containing 0.3 % triclosan, 2.0 % PVM/MA copolymer, 0.243 % NaF and specially-designed silica. Am J Dent. 2011;24(Spec No A):6A–13A. [PubMed] [Google Scholar]
- 37.Jones JA (2011) Dentin hypersensitivity: etiology, risk factors, and prevention strategies. Dent Today 30(108), 112–103 [PubMed]
- 38.Addy M, West N (1994) Etiology, mechanisms, and management of dentine hypersensitivity. Curr Opin Periodontol 1:71–77 [PubMed]
- 39.Ishikawa S. A clinico-histological study on the hypersensitivity of dentin. Kokubyo Gakkai Zasshi. 1969;36:278–298. doi: 10.5357/koubyou.36.278. [DOI] [PubMed] [Google Scholar]
- 40.Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280–284. doi: 10.1111/j.1600-051X.1987.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 41.Absi EG, Addy M, Adams D. Dentine hypersensitivity. The development and evaluation of a replica technique to study sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1989;16:190–195. doi: 10.1111/j.1600-051X.1989.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 42.West NX. Dentine hypersensitivity: preventive and therapeutic approaches to treatment. Periodontol. 2008;2000(48):31–41. doi: 10.1111/j.1600-0757.2008.00262.x. [DOI] [PubMed] [Google Scholar]
- 43.Zero DT, Lussi A. Erosion—chemical and biological factors of importance to the dental practitioner. Int Dent J. 2005;55:285–290. doi: 10.1111/j.1875-595x.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 44.Flynn J, Galloway R, Orchardson R. The incidence of ‘hypersensitive’ teeth in the West of Scotland. J Dent. 1985;13:230–236. doi: 10.1016/0300-5712(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 45.Bamise CT, Olusile AO, Oginni AO, Dosumu OO. The prevalence of dentine hypersensitivity among adult patients attending a Nigerian teaching hospital. Oral Health Prev Dent. 2007;5:49–53. [PubMed] [Google Scholar]
- 46.Fischer C, Fischer RG, Wennberg A. Prevalence and distribution of cervical dentine hypersensitivity in a population in Rio de Janeiro, Brazil. J Dent. 1992;20:272–276. doi: 10.1016/0300-5712(92)90043-C. [DOI] [PubMed] [Google Scholar]
- 47.Chabanski MB, Gillam DG, Bulman JS, Newman HN. Prevalence of cervical dentine sensitivity in a population of patients referred to a specialist Periodontology Department. J Clin Periodontol. 1996;23:993–997. doi: 10.1111/j.1600-051X.1996.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 48.Chabanski MB, Gillam DG, Bulman JS, Newman HN. Clinical evaluation of cervical dentine sensitivity in a population of patients referred to a specialist periodontology department: a pilot study. J Oral Rehabil. 1997;24:666–672. doi: 10.1046/j.1365-2842.1997.00552.x. [DOI] [PubMed] [Google Scholar]
- 49.Al-Sabbagh M, Beneduce C, Andreana S, Ciancio SG. Incidence and time course of dentinal hypersensitivity after periodontal surgery. Gen Dent. 2010;58:e14–e19. [PubMed] [Google Scholar]
- 50.Gillam DG, Orchardson R, Närhi MVO, Kontturi-Närhi V. Present and future methods for the evaluation of pain associated with dentine hypersensitivity. In: Addy M, Embery G, Edgar WM, Orchardson R, editors. Tooth wear and sensitivity. London: Martin Dunitz Ltd; 2000. pp. 283–297. [Google Scholar]
- 51.Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: Recent trends in management. J Conserv Dent. 2010;13:218–224. doi: 10.4103/0972-0707.73385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porto ICCM, Andrade AKM, Montes MAJR. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51:323–332. doi: 10.2334/josnusd.51.323. [DOI] [PubMed] [Google Scholar]
- 53.Bamise CT, Olusile AO, Oginni AO. An analysis of the etiological and predisposing factors related to dentin hypersensitivity. J Contemp Dent Pract. 2008;9:52–59. [PubMed] [Google Scholar]
- 54.Bekes K, John MT, Schaller HG, Hirsch C. Oral health-related quality of life in patients seeking care for dentin hypersensitivity. J Oral Rehabil. 2009;36:45–51. doi: 10.1111/j.1365-2842.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 55.Boiko OV, Baker SR, Gibson BJ, Locker D, Sufi F, Barlow AP, Robinson PG. Construction and validation of the quality of life measure for dentine hypersensitivity (DHEQ) J Clin Periodontol. 2010;37:973–980. doi: 10.1111/j.1600-051X.2010.01618.x. [DOI] [PubMed] [Google Scholar]
- 56.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11:3–11. [PubMed] [Google Scholar]
- 57.Locker D, Matear D, Stephens M, Lawrence H, Payne B. Comparison of the GOHAI and OHIP-14 as measures of the oral health-related quality of life of the elderly. Community Dent Oral Epidemiol. 2001;29:373–381. doi: 10.1034/j.1600-0528.2001.290507.x. [DOI] [PubMed] [Google Scholar]
- 58.John MT, Miglioretti DL, LeResche L, Koepsell TD, Hujoel P, Micheelis W. German short forms of the Oral Health Impact Profile. Community Dent Oral Epidemiol. 2006;34:277–288. doi: 10.1111/j.1600-0528.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 59.Hassel AJ, Rolko C, Koke U, Leisen J, Rammelsberg P. A German version of the GOHAI. Community Dent Oral Epidemiol. 2008;36:34–42. doi: 10.1111/j.1600-0528.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 60.Tubert-Jeannin S, Riordan PJ, Morel-Papernot A, Porcheray S, Saby-Collet S. Validation of an oral health quality of life index (GOHAI) in France. Community Dent Oral Epidemiol. 2003;31:275–284. doi: 10.1034/j.1600-0528.2003.t01-1-00006.x. [DOI] [PubMed] [Google Scholar]
- 61.Molin C. Placebo- and nocebo-factors in medicine and dentistry. Tandlakartidningen. 1991;83(820):822–823. [PubMed] [Google Scholar]
- 62.Ernst E. Placebo: new insights into an old enigma. Drug Discov Today. 2007;12:413–418. doi: 10.1016/j.drudis.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 63.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Addy M, West NX, Barlow A, Smith S. Dentine hypersensitivity: is there both stimulus and placebo responses in clinical trials? Int J Dent Hyg. 2007;5:53–59. doi: 10.1111/j.1601-5037.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- 65.De Amici D, Klersy C, Ramajoli F, Brustia L, Politi P. Impact of the Hawthorne Effect in a longitudinal clinical study: the case of anesthesia. Control Clin Trials. 2000;21:103–114. doi: 10.1016/S0197-2456(99)00054-9. [DOI] [PubMed] [Google Scholar]
- 66.Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. Jama. 2002;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- 67.Koshi EB, Short CA. Placebo theory and its implications for research and clinical practice: a review of the recent literature. Pain Pract. 2007;7:4–20. doi: 10.1111/j.1533-2500.2007.00104.x. [DOI] [PubMed] [Google Scholar]
- 68.Orchardson R, Collins WJ. Thresholds of hypersensitive teeth to 2 forms of controlled stimulation. J Clin Periodontol. 1987;14:68–73. doi: 10.1111/j.1600-051X.1987.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 69.Schiff T, Delgado E, Zhang YP, Cummins D, DeVizio W, Mateo LR. Clinical evaluation of the efficacy of an in-office desensitizing paste containing 8 % arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity. Am J Dent. 2009;22(Spec No A):8A–15A. [PubMed] [Google Scholar]
- 70.Nathoo S, Delgado E, Zhang YP, DeVizio W, Cummins D, Mateo LR. Comparing the efficacy in providing instant relief of dentin hypersensitivity of a new toothpaste containing 8.0 % arginine, calcium carbonate, and 1450 ppm fluoride relative to a benchmark desensitizing toothpaste containing 2 % potassium ion and 1450 ppm fluoride, and to a control toothpaste with 1450 ppm fluoride: a three-day clinical study in New Jersey, USA. J Clin Dent. 2009;20:123–130. [PubMed] [Google Scholar]
- 71.Kobler A, Kuss O, Schaller HG, Gernhardt CR (2008) Clinical effectiveness of a strontium chloride-containing desensitizing agent over 6 months: a randomized, double-blind, placebo-controlled study. Quintessence Int 39:321–325 [PubMed]
- 72.Narhi M, Hirvonen T, Huopaniemi T. The function of intrdental nerves in relation to the sensations induced by dental stimulation. Acupunct Electrther Res. 1984;9:107–113. doi: 10.3727/036012984816714785. [DOI] [PubMed] [Google Scholar]
- 73.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 74.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 75.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 76.Fu Y, Li X, Que K, Wang M, Hu D, Mateo LR, DeVizio W, Zhang YP. Instant dentin hypersensitivity relief of a new desensitizing dentifrice containing 8.0 % arginine, a high cleaning calcium carbonate system and 1450 ppm fluoride: a 3-day clinical study in Chengdu, China. Am J Dent. 2010;23(Spec No A):20A–27A. [PubMed] [Google Scholar]
- 77.Schiff T, Delgado E, Zhang YP, DeVizio W, Cummins D, Mateo LR. The clinical effect of a single direct topical application of a dentifrice containing 8.0 % arginine, calcium carbonate, and 1450 ppm fluoride on dentin hypersensitivity: the use of a cotton swab applicator versus the use of a fingertip. J Clin Dent. 2009;20:131–136. [PubMed] [Google Scholar]
- 78.Petrou I, Heu R, Stranick M, Lavender S, Zaidel L, Cummins D, Sullivan RJ, Hsueh C, Gimzewski JK. A breakthrough therapy for dentin hypersensitivity: how dental products containing 8 % arginine and calcium carbonate work to deliver effective relief of sensitive teeth. J Clin Dent. 2009;20:23–31. [PubMed] [Google Scholar]
- 79.Hamlin D, Williams KP, Delgado E, Zhang YP, DeVizio W, Mateo LR. Clinical evaluation of the efficacy of a desensitizing paste containing 8 % arginine and calcium carbonate for the in-office relief of dentin hypersensitivity associated with dental prophylaxis. Am J Dent. 2009;22(Spec No A):16A–20A. [PubMed] [Google Scholar]
- 80.Heaton LJ, Carlson CR, Smith TA, Baer RA, de Leeuw R. Predicting anxiety during dental treatment using patients’ self-reports: less is more. J Am Dent Assoc. 2007;138:188–195. doi: 10.14219/jada.archive.2007.0135. [DOI] [PubMed] [Google Scholar]
- 81.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11:3–11. [PubMed] [Google Scholar]
- 82.Al-Jundi MA, Szentpétery A, John MT. An Arabic version of the Oral Health Impact Profile: translation and psychometric properties. Int Dent J. 2007;57:84–92. doi: 10.1111/j.1875-595X.2007.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 83.Wong MC, Lo EC, McMillan AS. Validation of a Chinese version of the Oral Health Impact Profile (OHIP) Community Dent Oral Epidemiol. 2002;30:423–430. doi: 10.1034/j.1600-0528.2002.00013.x. [DOI] [PubMed] [Google Scholar]
- 84.Petricevic N, Celebic A, Papic M, Rener-Sitar K. The Croatian version of the Oral Health Impact Profile Questionnaire. Coll Antropol. 2009;33:841–847. [PubMed] [Google Scholar]
- 85.van der Meulen MJ, John MT, Naeije M, Lobbezoo F. The Dutch version of the Oral Health Impact Profile (OHIP-NL): translation, reliability and construct validity. BMC Oral Health. 2008;8:11. doi: 10.1186/1472-6831-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allison P, Locker D, Jokovic A, Slade G. A cross-cultural study of oral health values. J Dent Res. 1999;78:643–649. doi: 10.1177/00220345990780020301. [DOI] [PubMed] [Google Scholar]
- 87.John MT, Patrick DL, Slade GD. The German version of the Oral Health Impact Profile: translation and psychometric properties. Eur J Oral Sci. 2002;110:425–433. doi: 10.1034/j.1600-0722.2002.21363.x. [DOI] [PubMed] [Google Scholar]
- 88.Szentpetery A, Szabo G, Marada G, Szanto I, John MT. The Hungarian version of the Oral Health Impact Profile. Eur J Oral Sci. 2006;114:197–203. doi: 10.1111/j.1600-0722.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 89.Ide R, Yamamoto R, Mizoue T. The Japanese version of the Oral Health Impact Profile (OHIP): validation among young and middle-aged adults. Community Dent Health. 2006;23:158–163. [PubMed] [Google Scholar]
- 90.Yamazaki M, Inukai M, Baba K, John MT. Japanese version of the Oral Health Impact Profile (OHIP-J) J Oral Rehabil. 2007;34:159–168. doi: 10.1111/j.1365-2842.2006.01693.x. [DOI] [PubMed] [Google Scholar]


