Abstract
Background and Objectives: Stenosing tenosynovitis (trigger finger) is one of the most common causes of pain and disability in the hand, which may often require treatment with anti-inflammatory drugs, corticosteroid injection, or open surgery. However, there is still large room for improvement in the treatment of this condition by corticosteroid injection. The mechanical, viscoelastic, and antinociceptive properties of hyaluronic acid may potentially support the use of this molecule in association with corticosteroids for the treatment of trigger finger. This study examines the feasibility and safety of ultrasound-guided injection of a corticosteroid and hyaluronic acid compared, for the first time, with open surgery for the treatment of trigger finger.
Methods: This was a monocentric, open-label, randomized study. Consecutive patients aged between 35 and 70 years with ultrasound-confirmed diagnosis of trigger finger were included. Patients were randomly assigned to either ultrasound-guided injection of methylprednisolone acetate 40 mg/mL with 0.8mL lidocaine into the flexor sheath plus injection of 1mL hyaluronic acid 0.8% 10 days later (n = 15; group A), or to open surgical release of the first annular pulley (n = 15; group B). Clinical assessment of the digital articular chain was conducted prior to treatment and after 6 weeks, and 3, 6, and 12 months. The duration of abstention from work and/or sports activity, and any treatment complications or additional treatment requirements (e.g. physiotherapy, compression, medication) were also recorded.
Results: Fourteen patients (93.3%) in group A had complete symptom resolution at 6 months, which persisted for 12 months in 11 patients (73.3%), while three patients experienced recurrences and one experienced no symptom improvements. No patients in group A reported major or minor complications during or after corticosteroid injection, or required a compression bandage. All 15 patients in group B achieved complete resolution of articular impairment by 3 weeks after surgery, but ten patients were assigned to physiotherapy and local and/or oral analgesics for complete resolution of symptoms, which was approximately 30–40 days postsurgery. The mean duration of abstention from work and/or sport was 2–3 days in group A and 26 days in group B.
Conclusions: Although the limited sample size did not allow any statistical comparison between treatment groups, and therefore all the findings should be regarded as preliminary, the results of this explorative study suggest that ultrasound-guided injection of a corticosteroid and hyaluronic acid could be a safe and feasible approach for the treatment of trigger finger. It is also associated with a shorter recovery time than open surgery, which leads to a reduced abstention from sports and, in particular, work activities, and therefore may have some pharmacoeconomic implications, which may be further explored. In light of the promising results obtained in this investigation, further studies comparing ultrasound-guided injection of corticosteroid plus hyaluronic acid with corticosteroid alone are recommended in order to clarify the actual benefits attributable to hyaluronic acid.
Introduction
Stenosing tenosynovitis, otherwise known as trigger finger (TF), affects the flexor pollicis longus or flexor digitorum tendons, and causes a characteristic painful tendon snap or click on flexion and extension, and/or locking of the metacarpophalangeal or proximal interphalangeal joints of the involved digit. Most cases are idiopathic. Entrapment of the affected tendon at the first annular (A1) pulley occurs because of a difference in the diameter of the flexor tendon and its sheath as a result of thickening of the sheath and some localized tendon thickening. Chronic repetitive friction between the tendon and its sheath may then lead to an intratendinous nodule at the A1 pulley.[1]
TF is one of the most common causes of pain and disability in the hand with an incidence of around 28 cases per 100 000 population per year, or a lifetime risk of 2.6% in the general population.[1] In adults, it occurs more commonly in women than in men, usually in the dominant hand, and most often in the thumb or ring fingers.[1] The aim of treatment is abolition of pain and restoration of a full range of smooth motion in the involved digit(s).[2] Current treatment options include either conservative management by splinting,[1] treatment with anti-inflammatory drugs or corticosteroid injection (usually with local anesthetic) in the flexor tendon sheath,[2–10] or surgical release of the A1 pulley (via an open or percutaneous approach).[11–14]
Although there are no well controlled studies comparing treatment modalities, corticosteroid injection is widely accepted as first-line treatment of TF; surgery is generally reserved for cases of corticosteroid treatment failure or for patients with more severe articular locking and joint rigidity.[2,4,9,13] Certainly surgery is the definitive treatment, providing permanent resolution of symptoms,[4,13] but corticosteroid injection is often preferred, not only because a single injection is effective in a large proportion of patients, but also because it can be given in an office setting and is a relatively simple, low-cost procedure.[4] However, symptoms may not resolve with only one corticosteroid injection and the likelihood of success decreases with each subsequent injection (60% of 109 trigger digits after the first injection, 36% after the second, and 33% after the third; for those patients requiring more than one injection, average duration of relief from injection was 14 weeks, and ranged from 4 to 40 weeks).[4] There is some room for improvement in the treatment of TF by corticosteroid injection.
The glycosaminoglycan hyaluronic acid (HA) is an essential component of the connective tissue[15] and is naturally found in the synovial fluid. HA injection has been used in the effective and safe treatment of osteoarthritis of the hip, knee, and other joints with success rates varying from 68% to 95%.[16–21]
Several properties of HA support its use in combination with corticosteroid injection in patients with TF. We hypothesize that HA may act initially as a mechanical intermediary via its visco-supplementing properties (by immediate recovery of synovial fluid viscosity),[15,18,19,22,23] consequently enlarging the tendon passageway narrowed by the disease and thereby regaining enough space for the tendon to glide normally. Subsequently, for 3–7 days after injection, HA not only provides shock absorption via its rheologic (visco-elastic) properties, but it has an antinociceptive effect and also significantly decreases the expression of proinflammatory cytokines in the synovial space.[19] In addition, experimental studies show that HA has a direct effect on tendons by inhibiting and modulating fibroblast activity impaired by stenosing tenosynovitis.[19,23,24] To the best of our knowledge, there are no studies to date on the use of HA in the treatment of TF.
The aim of this explorative study was to establish the feasibility and safety of injection of a corticosteroid followed by HA in the treatment of TF, using surgery as the reference treatment, because in the study institution, surgery is the treatment of choice for TF, and a conservative infiltration treatment with corticosteroid is only seldom prescribed. The relatively new method for corticosteroid injection of ultrasound-guided injection was employed because of its very high long-term success rate (90% of digits at 1 year post injection),[25] and potential to be more accurate and safer than blind injection.[7] This procedure allows data to be obtained that is as free as possible from the potential errors associated with infiltrative procedures.
Methods
Subjects and Study Design
This was a prospective, open-label, randomized, single-center study. Consecutive patients aged between 35 and 70 years presenting with clinical signs and symptoms of stenosing tenosynovitis of the flexor tendons and in whom diagnosis was confirmed by ultrasound (in order to exclude other conditions that may be associated with the same symptoms, e.g. luxations, joint abnormalities, focal dystony) were eligible for enrollment. Ultrasound diagnosis was based on the criteria described previously by Ebrahim et al.,[26] which, briefly, were as follows: hypoechoic thickening of the A1 pulley; visualization of a nodule in the flexor superficialis tendon; bow-stringing of the pulley during flexion; and visualization of triggering (obstruction of the tendon passing through the osteofibrous canal in the pulley during flexion-extension) of the involved digit. Patients with grade IV TF or the following co-morbidities were excluded: diabetes mellitus; rheumatoid arthritis; hypercholesterolemia; hypotension; and hypertension. This choice was taken in order to avoid changes associated with alterations in the synovial and intratendinous microcirculation.
Disease severity was graded according to Froimson’s modification[27] of Quinnell’s classification,[28] as follows: grade I (pre-triggering) – pain and a history of catching, not demonstrable on clinical examination; grade II (active triggering) – demonstrable actively correctable catching; grade III (passive triggering) – demonstrable passively correctable catching (III A) or impossible active flexion (III B); and grade IV (contracture) – uncorrectable fixed flexion. This observational study was performed in accordance with the Declaration of Helsinki as part of routine clinical practice based on two treatment options; full ethical approval was therefore not required. The nature of the study was fully explained to participating patients who consented to use of their data for purposes of clinical study.
Interventions
Patients were randomly assigned to corticosteroid injection plus HA injection (group A) or open surgery (group B). In group A, the corticosteroid injection was administered according to methods detailed in Bodor and Flossman,[25] in a sterile environment under ultrasound guidance. Briefly, a Philips IU22 ultrasound system with high-frequency linear-array probe 17 MHz (figure 1) was positioned on the volar aspect of the hand and a 25-gauge needle was used to inject methylprednisolone acetate 40 mg/1 mL (Depo-medrol®, Pfizer, Italy) with 0.8 mL lidocaine chlorhydrate 2% (S.A.L.F., Bergamo, Italy) into the sheath of the flexor tendons, distally to the A1 pulley (figure 2). Ten days later, 1 mL 0.8% HA (Sinovial® Mini, Yaral® Mini, IBSA Institut Biochimique SA, Pambio-Noranco, Switzerland) was injected using the same technique (figure 3). The time period of 10 days was chosen because, in our experience, 10 days are required to observe the maximum effect of corticosteroid therapy.
Fig. 1.
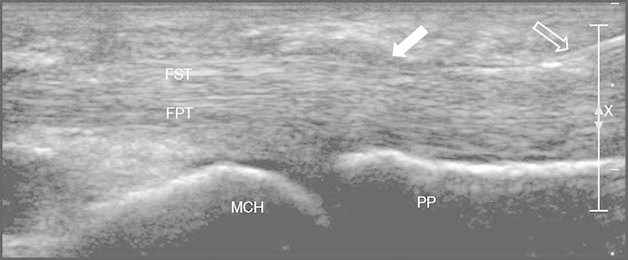
Puncture: longitudinal scan of the flexor tendons of the fourth finger using a high frequency (17 Mhz) linear probe over the metacarpophalangeal joint. The 25 G needle is clearly displayed (empty white arrow) with its tip at the tendon sheath distal to the A1 pulley (full white arrow). The X and bar on the right are markers of focus in the US display (X is the center, and the bar is the range of focus). FPT = flexor profundus tendon; FST = flexor superficialis tendon; MCH = metacarpal head; PP = proximal phalange.
Fig. 2.
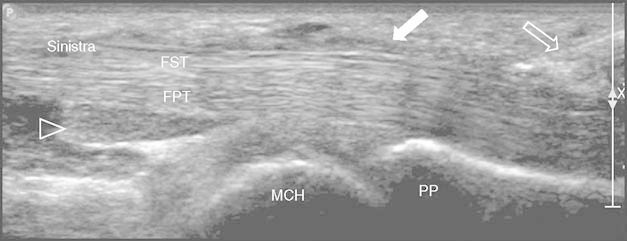
Corticosteroid injection: longitudinal scan of the flexor tendons of the fourth finger using a high frequency (17 Mhz) linear probe over the metacarpophalangeal joint. The 25 G needle is clearly displayed (empty white arrow) with its tip at the tendon sheath distal to the A1 pulley (full white arrow). The drug is injected into the proximal recess of the tendon sheath (empty arrowhead). The X and bar on the right are markers of focus in the US display (X is the center, and the bar is the range of focus). FPT = flexor profundus tendon; FST = flexor superficialis tendon; MCH = metacarpal head; PP = proximal phalange.
Fig. 3.
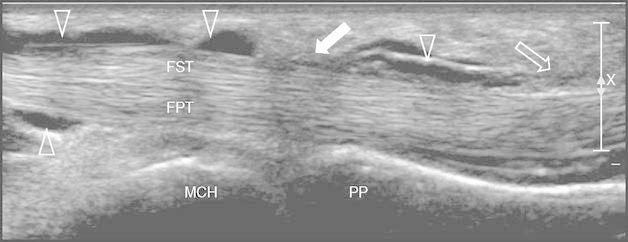
Hyaluronic acid injection: longitudinal scan over the flexor tendons of the fourth finger using a high frequency (17 Mhz) linear probe over the metacarpophalangeal joint. The 25 G needle is clearly displayed (empty white arrow) with its tip at the tendon sheath distal to the A1 pulley (full white arrow). Two weeks after corticosteroid injection, low-medium molecular weight hyaluronic acid is injected using the same technique into the synovial space (empty arrowheads) releasing the walls of the tendon sheath. The X and bar on the right are markers of focus in the US display (X is the center, and the bar is the range of focus). FPT = flexor profundus tendon; FST = flexor superficialis tendon; MCH = metacarpal head; PP = proximal phalange.
Group B patients underwent open surgery by conventional technique under locoregional anesthesia and a haemostatic pressure cuff inflated around the upper arm. The procedure was carried out on a day surgery basis and patients were discharged in the evening with a compression dressing to be kept in place for 4 days until it was changed in the outpatient clinic. Sutures were removed 2 weeks after surgery. At the first follow-up visit, patients were advised to mobilize the finger, depending on the level of pain experienced.
Endpoints and Assessments
Clinical assessment of the digital articular chain was conducted prior to treatment and after 6 weeks, and 3, 6, and 12 months. The duration of abstention from work and/or sports activity, and any treatment complications or additional treatment requirements (e.g. physiotherapy, compression, medication) were also recorded for each patient. The primary endpoint was the proportion of patients with a ‘satisfactory’ outcome; clinical outcome was deemed satisfactory if there was a resolution of symptoms in the first 6 weeks with no recurrence in the first 3 months, and if resolution persisted until the 6-month assessment. The change in symptom severity, graded according to the Quinnell-Green classification,[27,28] was also assessed in each patient before and after treatment.
All patients were assessed before and after treatment using the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire,[29] the Satisfaction Visual Analog Scale (SVAS),[29] and a pain Visual Analog Scale (VAS).[30] The DASH is a 30-item questionnaire that records patient-reported outcomes on a Likert scale from 1 to 5, giving a total score (ranging from 30 to 150), which is then converted to a percentage, where 0% indicates no disability and 100% is the most severe disability.[29] Due to the explorative nature of this study, we did not perform any calculation of statistical power or any kind of statistical comparison between groups.
Results
Demographics
A total of 10 men and 20 women, with an average age of 52.5 years, were enrolled between 1 January and 31 May 2007, and treated. Disease severity was grade II in 14 patients and grade III in 16 patients. All were single, idiopathic cases: the thumb was involved in 16 patients, the ring finger in seven, and the long finger in seven. No patient had more than one finger affected or had co-existing syndromes such as carpal tunnel or Dupuytren’s disease. Pain and associated dysfunction had been present for an average period of 3.5 months (range 1–6 months) prior to treatment; 40% of patients had difficulty undertaking daily life activities due to impaired grip strength, while the remaining 60% reported milder symptoms only partly interfering with their normal occupations and sports activity. Patients were equally distributed for age, sex, occupation, and severity and duration of symptoms between the two treatment groups.
Outcomes
At 6 months’ follow-up, complete symptom resolution was observed in 14 of 15 (93.3%) patients in group A, with a residual deficit of 7° in only one patient. At 12 months after treatment, 11 of 15 (73.3%) patients still had complete resolution of symptoms, three (20.0%) had experienced a recurrence, but the severity had passed from grade III to grade I, and one patient (6.7%) had no symptom improvement. All 15 patients in group B achieved complete resolution of articular impairment by 3 weeks after surgery, but ten patients needed physiotherapy, and local and/or oral analgesics for complete resolution of symptoms, which was approximately 30–40 days postsurgery.
Excluding very mild local pain for 12–36 hours, which was reported in three patients, no patients in group A reported major or minor complications during or after corticosteroid injection, or required a compression bandage. In group B, seven patients experienced scar pain, two patients had an abnormal flexion of the finger, and one patient presented a mild algodystrophic syndrome, which resolved after therapy. Recovery time was also faster with ultrasound-guided injection of a corticosteroid and HA than with surgery; the mean duration of abstention from work and/or sport was 2–3 days in group A and 26 days (range 20–30 days) in group B as shown in table I.
Table I.
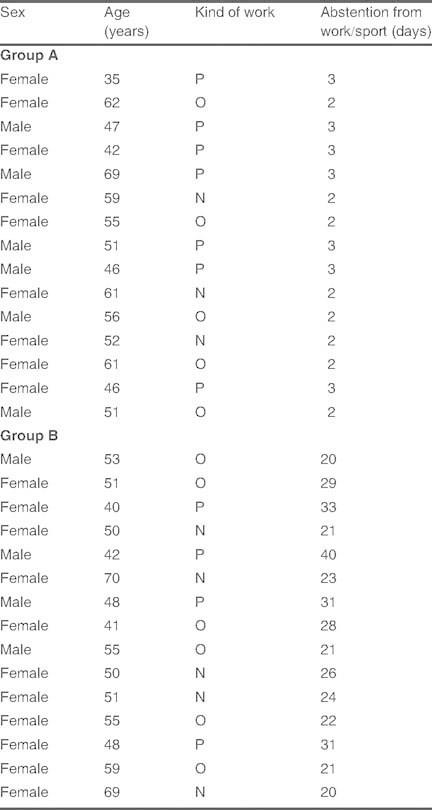
Duration of abstention from physical work/sport (P), office work (O), or normal household work (N) as a result of the treatment in group A (corticosteroid and hyaluronic acid injection) and group B (open surgery)
Patient-reported outcomes for pain, disability, and patient satisfaction assessed according to the VAS, DASH, and SVAS scores were satisfactory in group A patients and similar to those in group B patients (table II).
Table II.
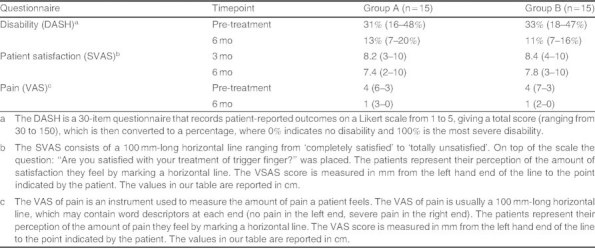
Mean scores (range) for disability (Disabilities of the Arm, Shoulder and Hand [DASH] questionnaire), patient satisfaction (Satisfaction Visual Analog Scale [SVAS]) and pain (visual analog scale [VAS]) in patients treated for trigger finger with ultrasound-guided injection of a corticosteroid and hyaluronic acid (group A) or open surgery (group B)
Discussion
Our findings show that ultrasound-guided injection of a corticosteroid and HA yielded satisfactory outcomes in 14 of 15 (93.3%) patients 6 months after treatment. The outcomes remained unchanged at 12 months’ clinical follow-up in 11 of 15 (73.3%) patients. This infiltrative therapy regimen also led to faster recovery with fewer complications and required less additional treatment than open surgery; however, this finding is somehow conflicting with other evidence, which has suggested that the higher costs associated with surgery may be ultimately offset by the benefit conferred through permanency of relief.[4] The faster recovery times associated with this infiltrative regimen, when compared with those following open surgery, led to an approximate 10-fold decrease in abstention from sporting and, in particular, work activities. This may have some pharmacoeconomic implications, which may be further explored. Patient-reported outcomes for disability, satisfaction, and pain were satisfactory in patients treated with ultrasound-guided corticosteroid and HA injection, and similar to those obtained in patients who received surgical treatment.
Open surgery is considered the gold standard for the treatment of TF,[4,13] and we therefore chose it as a control to assess the feasibility of ultrasound-guided injection of a corticosteroid and HA. As expected, in this study, open surgery was associated with a complete resolution of articular symptoms, while the conservative approach resulted, in a limited number of cases, in recurrences, possibly due to the fact that the anatomical structures remain unmodified. However, the ultrasound-guided injection of a corticosteroid and HA provided an overall favorable clinical outcome and some potential advantages (e.g. a short recovery time and few complications after the procedure), which deserve further investigation.
Corticosteroid injection into the sheath of the flexor tendons as a conservative treatment based on the anti-inflammatory effect of corticosteroids dates back to the 1950s. Since then, local corticosteroid injections have been assessed in many reports, with different outcomes, and long-term efficacy rates between 60% and 93% obtained with between one and four injections.[2,4–6,8] As emphasized in the review by Kelly et al.,[31] no literature reports are currently available on the use of HA to treat stenosing tenosynovitis of the flexor tendons, but the results of our study appear to compare favorably with those obtained in other studies utilizing injection of methylprednisolone acetate. A retrospective review of 76 digits in 50 patients treated with methylprednisolone acetate or triamcinolone diacetate showed complete resolution of symptoms in 82% of digits for a mean follow-up period of 35 months.[5] Of these, a single injection was needed for 42 digits, two injections for 11 digits, three injections for six digits, and four injections for three digits. A second study prospectively followed 77 digits in 58 patients for an average of 4.6 years following injection of methylprednisolone acetate.[10] A total of 61% of digits showed complete resolution of symptoms after a single injection, 27% reported recurrent episodes, and 12% failed or required surgery because of early recurrence. Finally, a double-blind, randomized study showed a success rate of 60% in patients treated with injection of methylprednisolone acetate plus anesthetic compared with 16% in those treated with anesthetic alone (p = 0.02).[8] However, in all of these studies, corticosteroid injection was not ultrasound-guided and no information regarding the severity of disease or duration of symptoms was reported, so direct comparison with our study cannot be made.
Such a wide variation in efficacy rates can be explained by many factors. Obviously, injection of the drug into the correct location will enhance the likelihood of a successful outcome and preclude many of the complications associated with the procedure. For this reason, ultrasound guidance is deemed the most accurate technique to ensure an optimal result, especially in an anatomically difficult location such as the hand, requiring injection into a virtual space such as the flexor tendon sheath, and avoiding iatrogenic injury.[7] Corticosteroid administration carries the risk of complications such as adipose necrosis, skin depigmentation, dermal or epidermal atrophy, infection, and tendon rupture.[1] These adverse effects have occurred following corticosteroid injection without ultrasound guidance; therefore, certain complications are presumably due to incorrect deployment of the drug,[3,8] as well as direct tendon puncture and the undesired effects of corticosteroids.
The use of ultrasound-guided injection of corticosteroids and HA may be associated with a relevant cost (in Italy, about €180 if the procedure is performed outside the National Healthcare System, on a private basis [2010 values]). However, these costs are likely to be lower than those determined by the gold standard for the treatment of TF (i.e. open surgery). Moreover, ultrasound-guided injection of corticosteroids and HA appears, in this preliminary study, to be associated with a high proportion of patients achieving disease resolution and with a short recovery time (in turn reducing abstention from working and sporting activities), without significant post-procedural complications. These potential advantages should be taken into consideration for a full evaluation of the cost/benefit ratio of ultrasound-guided injection of corticosteroids and HA.
It is important to point out that this study has a number of limitations that must be acknowledged and discussed to better clarify the potential implications on future research. A lack of a corticosteroid-only treatment arm means that any benefits of adding HA to the regimen of injection compared with corticosteroid alone cannot be shown. In light of the promising results obtained in this investigation, further study comparing ultrasound-guided injection of corticosteroid plus HA with corticosteroid alone, or exploring other treatment strategies (e.g. no ultrasound-guided injection, corticosteroid only vs surgery) is recommended. Also, due to small patient numbers in this study, it is not possible to analyze for any trends in the duration of symptoms or number of injections and success rates. Further studies with a larger sample size are likely to provide new insights on the effectiveness and safety of ultrasound-guided injection of corticosteroid plus HA. It also must be acknowledged that, due to the explorative nature of this study and the low number of patients enrolled, neither a calculation of power nor a statistical comparison between groups were performed.
Conclusion
Ultrasound-guided injection of corticosteroids and HA appears a safe and feasible option in the treatment of TF. While open surgery remains the reference treatment in terms of symptom resolution, the reduced need for physiotherapy and additional medications, suggesting lower costs, and in particular the short recovery time associated with ultrasound-guided injection of corticosteroids and HA may support a further evaluation of the use of this infiltrative therapy for the treatment of TF.
Acknowledgements
The authors declare no conflicts of interest that are directly relevant to the content of this study.
We thank Tracy Harrison and Luca Giacomelli of inScience Communications, a Wolters Kluwer business, who provided technical and native-English editing and journal styling prior to submission. This assistance was funded by IBSA Institut Biochimique SA, Pambio-Noranco, Switzerland.
References
- 1.Akhtar S., Bradley M.J., Quinton D.N., et al. Management and referral for trigger finger/thumb. BMJ. 2005;331(7507):30–3. doi: 10.1136/bmj.331.7507.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nimigan A.S., Ross D.C., Gan B.S. Steroid injections in the management of trigger fingers. Am J Phys Med Rehab. 2006;85(1):36–43. doi: 10.1097/01.phm.0000184236.81774.b5. [DOI] [PubMed] [Google Scholar]
- 3.Akhtar S., Burke F.D. Study to outline the efficacy and illustrate techniques for steroid injection for trigger finger and thumb. Postgrad Med J. 2006;82(973):763–6. doi: 10.1136/pmj.2006.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson L.S., Ptaszek A.J. Injection versus surgery in the treatment of trigger finger. J Hand Surg Am. 1997;22(1):138–44. doi: 10.1016/S0363-5023(05)80194-7. [DOI] [PubMed] [Google Scholar]
- 5.Clark D.D., Ricker J.H., MacCollum M.S. The efficacy of local steroid injection in the treatment of stenosing tenovaginitis. Plastic Reconstruct Surg. 1973;51:179–80. doi: 10.1097/00006534-197302000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Freiberg A., Mulholland R.S., Levine R. Nonoperative treatment of trigger fingers and thumbs. J Hand Surg Am. 1989;14(3):553–8. doi: 10.1016/S0363-5023(89)80024-3. [DOI] [PubMed] [Google Scholar]
- 7.Godey S.K., Bhatti W.A., Watson J.S., et al. A technique for accurate and safe injection of steroid in trigger digits using ultrasound guidance. Acta Orthop Belg. 2006;72(5):633–4. [PubMed] [Google Scholar]
- 8.Lambert M.A., Morton R.J., Sloan J.P. Controlled study of the use of local steroid injection in the treatment of trigger finger and thumb. J Hand Surg Br. 1992;17(1):69–70. doi: 10.1016/0266-7681(92)90014-s. [DOI] [PubMed] [Google Scholar]
- 9.Ring D., Lozano-Calderon S., Shin R., et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33(4):516–22. doi: 10.1016/j.jhsa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Anderson B., Kaye S. Treatment of flexor tenosynovitis of the hand (‘trigger finger’) with corticosteroids: a prospective study of the response to local injection. Arch Int Med. 1991;151(1):153–6. doi: 10.1001/archinte.1991.00400010155024. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y.C., Huang P.J., Tien Y.C., et al. Revision of incompletely released trigger fingers by percutaneous release: results and complications. J Hand Surg Am. 2006;31(8):1288–91. doi: 10.1016/j.jhsa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Gilberts E.C., Beekman W.H., Stevens H.J., et al. Prospective randomized trial of open versus percutaneous surgery for trigger digits. J Hand Surg Am. 2001;26(3):497–500. doi: 10.1053/jhsu.2001.24967. [DOI] [PubMed] [Google Scholar]
- 13.Lim M.H., Lim K.K., Rasheed M.Z., et al. Outcome of open trigger digit release. J Hand Surg Eur. 2007;32(4):457–9. doi: 10.1016/J.JHSB.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Park M.J., Oh I., Ha K.I. A1 pulley release of locked trigger digit by percutaneous technique. J Hand Surg Am. 2004;29(5):502–5. doi: 10.1016/j.jhsb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Stitik T.P., Levy J.A. Viscosupplementation (biosupplementation) for osteoarthritis. Am J Phys Med Rehab. 2006;85(11Suppl.):32–50. doi: 10.1097/01.phm.0000245677.20294.c2. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal A., Sempowski I.P. Hyaluronic acid injections for knee osteoarthritis: systematic review of the literature. Can Fam Physician. 2004;50:249–56. [PMC free article] [PubMed] [Google Scholar]
- 17.Juni P., Reichenbach S., Trelle S., et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthr Rheum. 2007;56(11):3610–9. doi: 10.1002/art.23026. [DOI] [PubMed] [Google Scholar]
- 18.Kotz R., Kolarz G. Intra-articular hyaluronic acid: duration of effect and results of repeated treatment cycles. Am J Orthop. 1999;28(11Suppl.):5–7. [PubMed] [Google Scholar]
- 19.Mitsui Y., Gotoh M., Nakama K., et al. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res. 2008;26:1032–7. doi: 10.1002/jor.20558. [DOI] [PubMed] [Google Scholar]
- 20.van den Bekerom M.P., Lamme B., Sermon A., et al. What is the evidence for viscosupplementation in the treatment of patients with hip osteoarthritis? Systematic review of the literature. Arch Orthop Trauma Surg. 2008;128(8):815–23. doi: 10.1007/s00402-007-0447-z. [DOI] [PubMed] [Google Scholar]
- 21.Migliore A., Tormenta S., Martin Martin L.S., et al. The symptomatic effects of intra-articular administration of hylan G-F 20 on osteoarthritis of the hip: clinical data of 6 months follow-up. Clin Rheum. 2006;25(3):389–93. doi: 10.1007/s10067-005-0052-x. [DOI] [PubMed] [Google Scholar]
- 22.Wiebkin O.W., Muir H. Influence of the cells on the pericellular environment: the effect of hyaluronic acid on proteoglycan synthesis and secretion by chondrocytes of adult cartilage. Philos Trans R Soc Lond B Biol Sci. 1975;271(912):283–91. doi: 10.1098/rstb.1975.0053. [DOI] [PubMed] [Google Scholar]
- 23.Wiig M., Abrahamsson S.O. Hyaluronic acid modulates cell proliferation unequally in intrasynovial and extrasynovial rabbit tendons in vitro. J Hand Surg Br. 2000;25(2):183–7. doi: 10.1054/jhsb.1999.0354. [DOI] [PubMed] [Google Scholar]
- 24.Campo G.M., Avenoso A., Campo S., et al. Reduction of DNA fragmentation and hydroxyl radical production by hyaluronic acid and chondroitin-4-sulphate in iron plus ascorbate-induced oxidative stress in fibroblast cultures. Free Radic Res. 2004;38(6):601–11. doi: 10.1080/10715760410001694017. [DOI] [PubMed] [Google Scholar]
- 25.Bodor M., Flossman T. Ultrasound-guided first annular pulley injection for trigger finger. J Ultrasound Med. 2009;28(6):737–43. doi: 10.7863/jum.2009.28.6.737. [DOI] [PubMed] [Google Scholar]
- 26.Ebrahim F.S., De Maeseneer M., Jager T., et al. US diagnosis of UCL tears of the thumb and Stener lesions: technique, pattern-based approach, and differential diagnosis. Radiographics. 2006;26(4):1007–20. doi: 10.1148/rg.264055117. [DOI] [PubMed] [Google Scholar]
- 27.Froimson A.I. Flexor tendon injury. In: Green D.P., Hotchkiss R.N., Pederson W.C., editors. Green’s operative hand surgery. 4th ed. New York (NY): Churchill Livingstone; 1999. p. 2029. [Google Scholar]
- 28.Quinnell R.C. Conservative management of trigger finger. Practitioner. 1980;224(1340):187–90. [PubMed] [Google Scholar]
- 29.Hudak P.L., Amadio P.C., Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29(6):602–8. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Ohnhaus E.E., Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1(4):379–84. doi: 10.1016/0304-3959(75)90075-5. [DOI] [PubMed] [Google Scholar]
- 31.Kelly M.A., Moskowitz R.W., Lieberman J.R. Hyaluronan therapy: looking toward the future. Am J Orthop. 2004;33(2Suppl.):23–8. [PubMed] [Google Scholar]


