Abstract
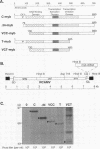
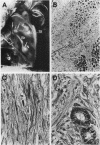
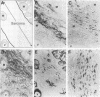
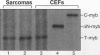
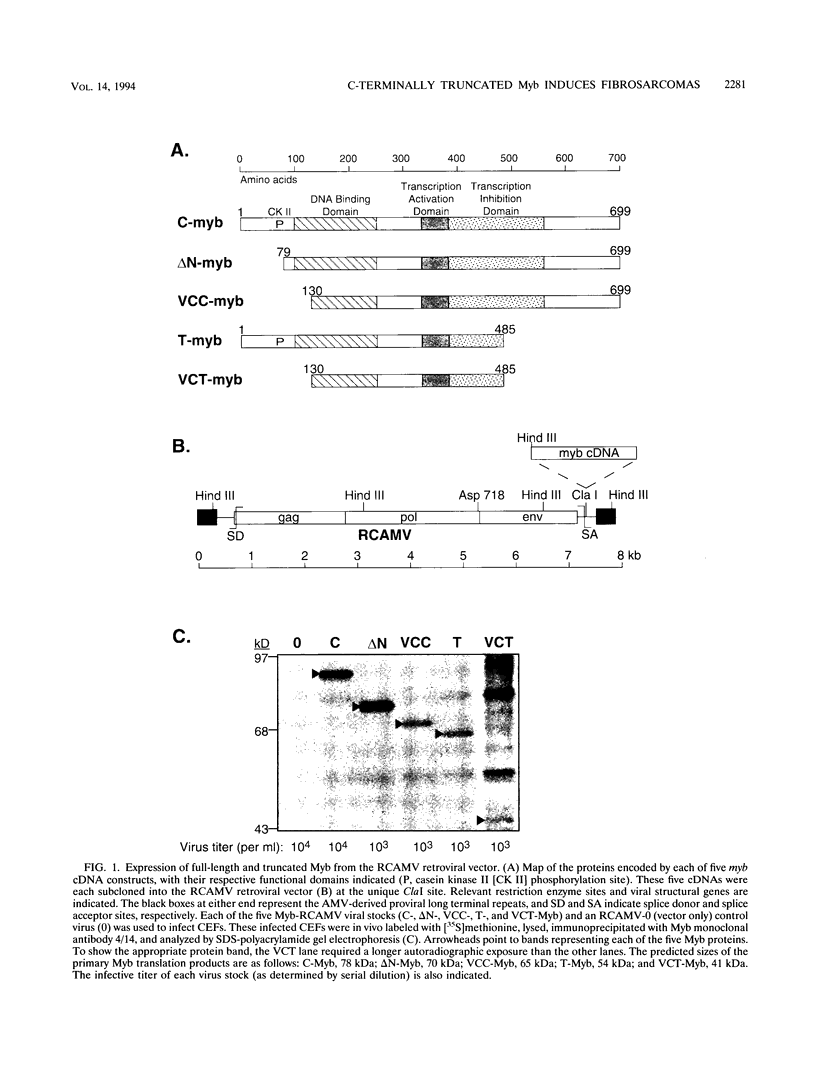
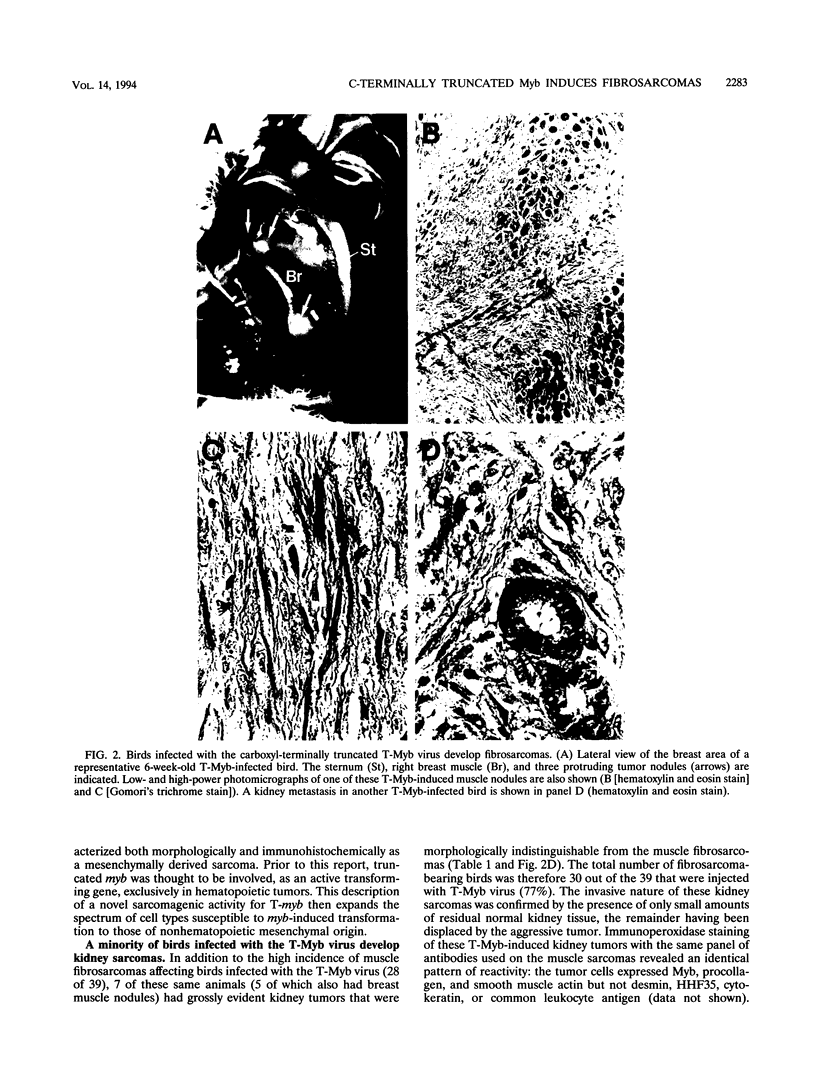
The myb oncogene encodes a DNA-binding transcriptional transactivator which can become a hematopoietic cell-transforming protein following the deletion of amino acid sequences from either its amino or carboxyl terminus. Although a number of hematopoietic tumors express terminally deleted variants of Myb, the involvement of truncated Myb in nonhematopoietic tumors has not been adequately investigated. To assess the full spectrum of Myb's oncogenic capability, a replication-competent retroviral vector (RCAMV) was used to express a full-length protein (C-Myb), an amino-terminally truncated protein (VCC- or delta N-Myb), a carboxyl-terminally truncated protein (T-Myb), or a doubly truncated protein (VCT-Myb) in vivo. These viruses were injected intravenously into 10-day chicken embryos, and the infected chicks were monitored for tumors. Approximately 4 to 8 weeks after hatching, the majority (30 of 39 [77%]) of animals infected with the T-Myb retrovirus (without 214 carboxyl-terminal residues) developed nodular muscle tumors which could be identified by both morphologic and immunohistochemical criteria as fibrosarcomas. Identically appearing tumors could also be found in the kidney of some T-Myb-infected animals. The T-Myb-induced fibrosarcomas expressed the appropriately sized T-Myb protein, contained an unaltered proviral T-myb gene, and showed clonal proviral integration sites. In comparison, no sarcomas were observed in any of the animals infected with the amino-terminally truncated (VCC- and delta N-Myb) or doubly truncated (VCT-Myb) viruses. A loss of carboxyl-terminal but not amino-terminal sequences can thus convert Myb into a potent in vivo transforming protein for nonhematopoietic mesenchymal cells. In comparison, a truncation of either or both ends of the protein can activate Myb into a hematopoietic cell-transforming protein.
Full text
PDF



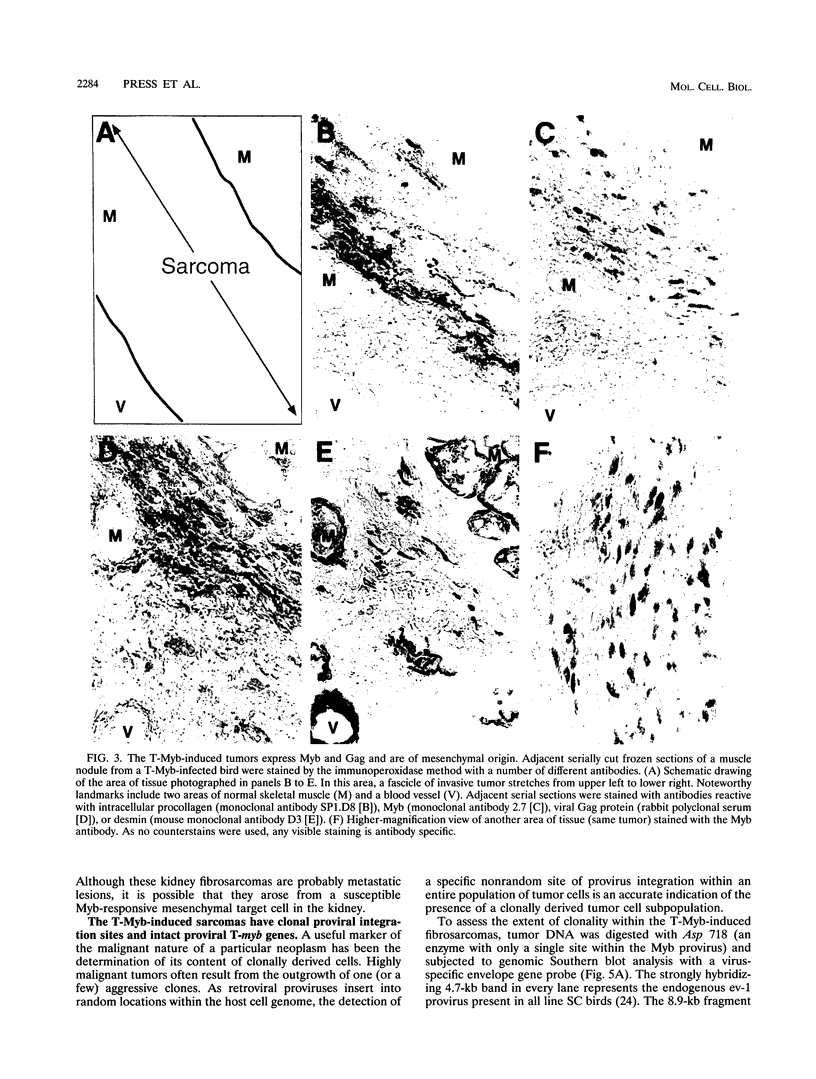
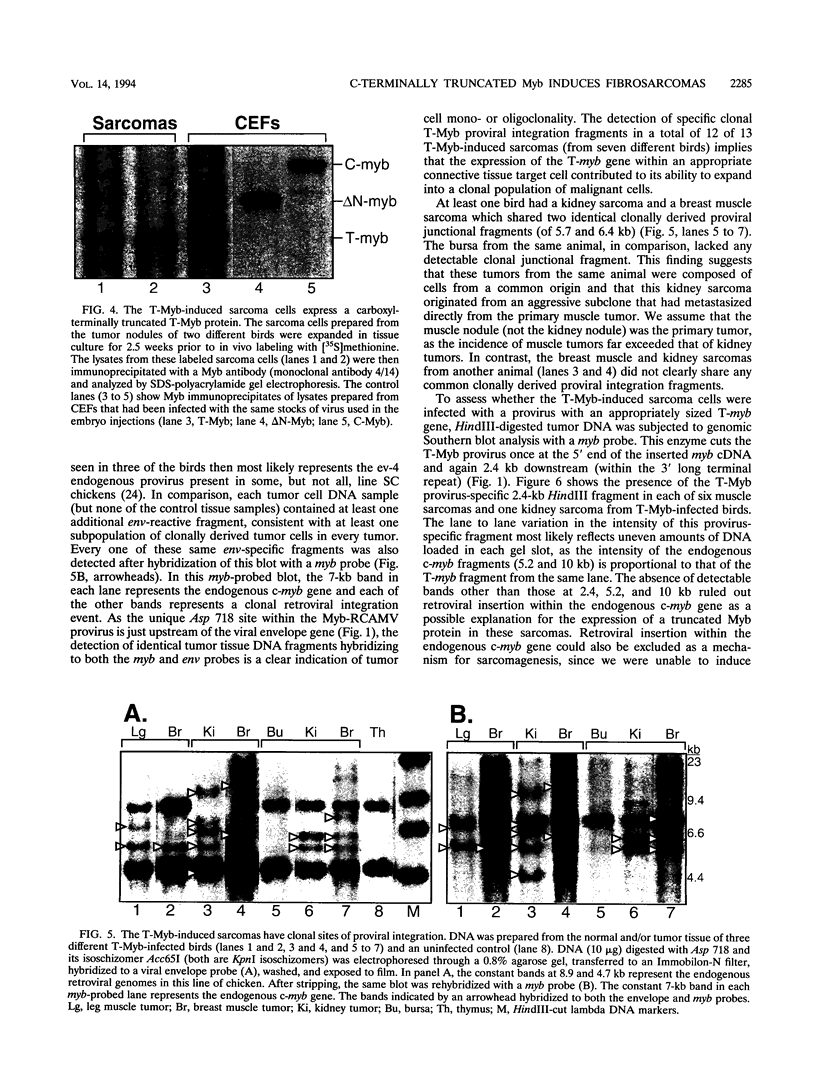
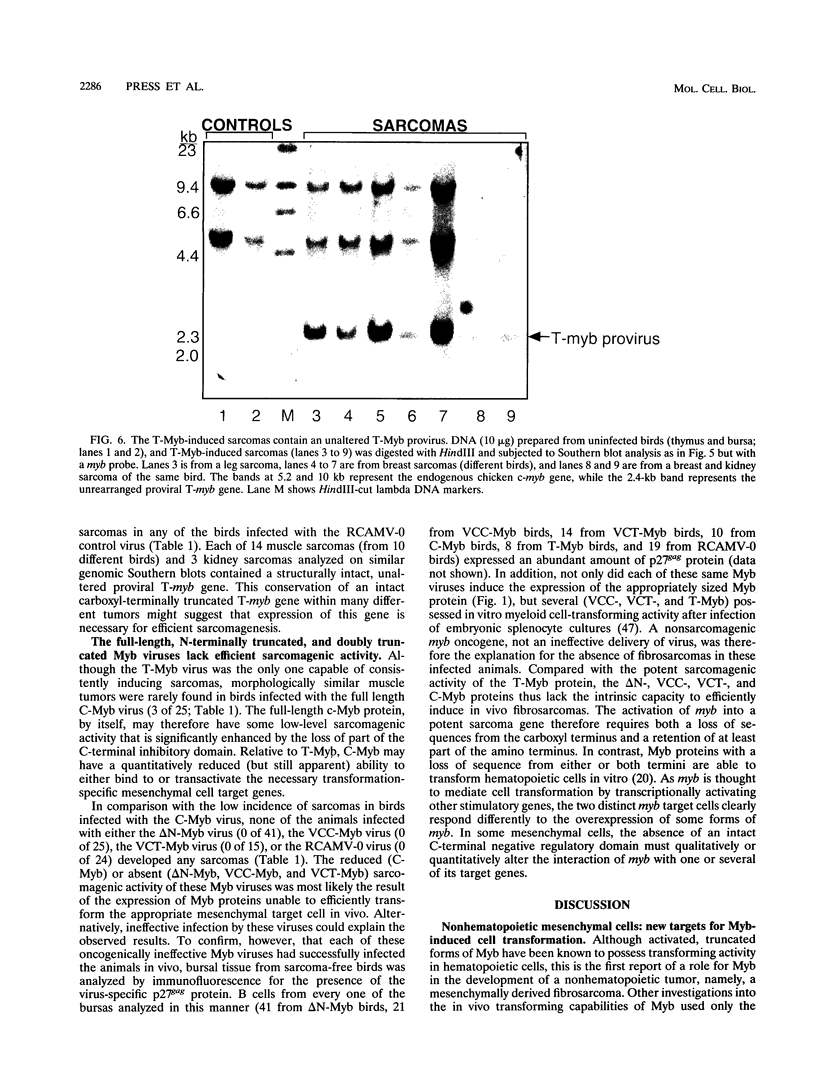




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfossi G., Gewirtz A. M., Calabretta B. An oligomer complementary to c-myb-encoded mRNA inhibits proliferation of human myeloid leukemia cell lines. Proc Natl Acad Sci U S A. 1989 May;86(9):3379–3383. doi: 10.1073/pnas.86.9.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H., Hansen J., Moelling K. Selective DNA binding of the human cellular myb protein isolated by immunoaffinity chromatography using a monoclonal antibody. Oncogene. 1987;1(4):395–401. [PubMed] [Google Scholar]
- Clarke M. F., Kukowska-Latallo J. F., Westin E., Smith M., Prochownik E. V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988 Feb;8(2):884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell J. P., Cogswell P. C., Kuehl W. M., Cuddihy A. M., Bender T. M., Engelke U., Marcu K. B., Ting J. P. Mechanism of c-myc regulation by c-Myb in different cell lineages. Mol Cell Biol. 1993 May;13(5):2858–2869. doi: 10.1128/mcb.13.5.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. W., Bloch A. Early decline in c-myb oncogene expression in the differentiation of human myeloblastic leukemia (ML-1) cells induced with 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1984 Feb;44(2):442–446. [PubMed] [Google Scholar]
- Danto S. I., Fischman D. A. Immunocytochemical analysis of intermediate filaments in embryonic heart cells with monoclonal antibodies to desmin. J Cell Biol. 1984 Jun;98(6):2179–2191. doi: 10.1083/jcb.98.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Bishop J. M. Isolation of monoclonal antibodies specific for products of avian oncogene myb. Mol Cell Biol. 1984 Dec;4(12):2843–2850. doi: 10.1128/mcb.4.12.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. L., Moore T. L., Kuehl W. M., Bender T., Ting J. P. Functional analysis of c-Myb protein in T-lymphocytic cell lines shows that it trans-activates the c-myc promoter. Mol Cell Biol. 1990 Nov;10(11):5747–5752. doi: 10.1128/mcb.10.11.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert D. L., Avdalovic N., Goldstein C. Follicular exclusion of retroviruses in the bursa of Fabricius. Virology. 1989 Jun;170(2):433–441. doi: 10.1016/0042-6822(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Foellmer H. G., Kawahara K., Madri J. A., Furthmayr H., Timpl R., Tuderman L. A monoclonal antibody specific for the amino terminal cleavage site of procollagen type I. Eur J Biochem. 1983 Jul 15;134(1):183–189. doi: 10.1111/j.1432-1033.1983.tb07549.x. [DOI] [PubMed] [Google Scholar]
- Frampton J., Leutz A., Gibson T., Graf T. DNA-binding domain ancestry. Nature. 1989 Nov 9;342(6246):134–134. doi: 10.1038/342134a0. [DOI] [PubMed] [Google Scholar]
- Garrido C., Grässer F., Lipsick J. S., Stehelin D., Saule S. Protein truncation is not required for c-myb proto-oncogene activity in neuroretina cells. J Virol. 1992 Nov;66(11):6773–6776. doi: 10.1128/jvi.66.11.6773-6776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S., Bishop J. M. Structure of the protein encoded by the chicken proto-oncogene c-myb. Mol Cell Biol. 1986 Nov;6(11):3677–3684. doi: 10.1128/mcb.6.11.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz A. M., Anfossi G., Venturelli D., Valpreda S., Sims R., Calabretta B. G1/S transition in normal human T-lymphocytes requires the nuclear protein encoded by c-myb. Science. 1989 Jul 14;245(4914):180–183. doi: 10.1126/science.2665077. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Buckmaster C., Ramsay R. G. Activation of c-myb by carboxy-terminal truncation: relationship to transformation of murine haemopoietic cells in vitro. EMBO J. 1989 Jun;8(6):1777–1783. doi: 10.1002/j.1460-2075.1989.tb03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Gough N. M., Dunn A. R., de Blaquiere J. Nucleotide sequence of cDNA clones of the murine myb proto-oncogene. EMBO J. 1985 Aug;4(8):2003–2008. doi: 10.1002/j.1460-2075.1985.tb03884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Grässer F. A., Graf T., Lipsick J. S. Protein truncation is required for the activation of the c-myb proto-oncogene. Mol Cell Biol. 1991 Aug;11(8):3987–3996. doi: 10.1128/mcb.11.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. L., Ramsay R. G., Kanei-Ishii C., Ishii S., Gonda T. J. Transformation by carboxyl-deleted Myb reflects increased transactivating capacity and disruption of a negative regulatory domain. Oncogene. 1991 Sep;6(9):1549–1553. [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H. Sequence of the long terminal repeat and adjacent segments of the endogenous avian virus Rous-associated virus 0. J Virol. 1982 Jul;43(1):191–200. doi: 10.1128/jvi.43.1.191-200.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries E. H., Danhof M. L., Hlozanek I. Characterization of endogenous viral loci in five lines of white Leghorn chickens. Virology. 1984 May;135(1):125–138. doi: 10.1016/0042-6822(84)90123-5. [DOI] [PubMed] [Google Scholar]
- Introna M., Golay J., Frampton J., Nakano T., Ness S. A., Graf T. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell. 1990 Dec 21;63(6):1289–1297. doi: 10.1016/0092-8674(90)90424-d. [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C., MacMillan E. M., Nomura T., Sarai A., Ramsay R. G., Aimoto S., Ishii S., Gonda T. J. Transactivation and transformation by Myb are negatively regulated by a leucine-zipper structure. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3088–3092. doi: 10.1073/pnas.89.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter M. R., Smith R. E., Hayward W. S. Rapid induction of B-cell lymphomas: insertional activation of c-myb by avian leukosis virus. J Virol. 1988 Apr;62(4):1423–1432. doi: 10.1128/jvi.62.4.1423-1432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Ramsay G., Bishop J. M., Moscovici M. G., Moscovici C., McGrath J. P., Levinson A. D. The product of the retroviral transforming gene v-myb is a truncated version of the protein encoded by the cellular oncogene c-myb. Cell. 1983 Jun;33(2):345–355. doi: 10.1016/0092-8674(83)90416-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Sippel A. E. The highly conserved amino-terminal region of the protein encoded by the v-myb oncogene functions as a DNA-binding domain. EMBO J. 1987 Sep;6(9):2719–2725. doi: 10.1002/j.1460-2075.1987.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryceve-Martinerie C., Soret J., Crochet J., Baluda M., Perbal B. Expression of a truncated v-myb product in transformed chicken embryo fibroblasts. FEBS Lett. 1987 Apr 6;214(1):81–86. doi: 10.1016/0014-5793(87)80017-0. [DOI] [PubMed] [Google Scholar]
- Ku D. H., Wen S. C., Engelhard A., Nicolaides N. C., Lipson K. E., Marino T. A., Calabretta B. c-myb transactivates cdc2 expression via Myb binding sites in the 5'-flanking region of the human cdc2 gene. J Biol Chem. 1993 Jan 25;268(3):2255–2259. [PubMed] [Google Scholar]
- Levy J. B., Iba H., Hanafusa H. Activation of the transforming potential of p60c-src by a single amino acid change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4228–4232. doi: 10.1073/pnas.83.12.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Christenson E., Litchfield D. W., Krebs E. G., Eisenman R. N. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature. 1990 Apr 5;344(6266):517–522. doi: 10.1038/344517a0. [DOI] [PubMed] [Google Scholar]
- McMahon J., Howe K. M., Watson R. J. The induction of Friend erythroleukaemia differentiation is markedly affected by expression of a transfected c-myb cDNA. Oncogene. 1988 Dec;3(6):717–720. [PubMed] [Google Scholar]
- Melani C., Rivoltini L., Parmiani G., Calabretta B., Colombo M. P. Inhibition of proliferation by c-myb antisense oligodeoxynucleotides in colon adenocarcinoma cell lines that express c-myb. Cancer Res. 1991 Jun 1;51(11):2897–2901. [PubMed] [Google Scholar]
- Merzak A., Dooghe Y., Pironin M., Perbal B., Vigier P. Cooperation between the H-ras oncogene and a truncated derivative of the v-myb oncogene in transformation of hamster embryo fibroblasts. Oncogene. 1992 Oct;7(10):2031–2039. [PubMed] [Google Scholar]
- Metz T., Graf T. Fusion of the nuclear oncoproteins v-Myb and v-Ets is required for the leukemogenicity of E26 virus. Cell. 1991 Jul 12;66(1):95–105. doi: 10.1016/0092-8674(91)90142-l. [DOI] [PubMed] [Google Scholar]
- Michelin S., Varlet I., Martinerie C., Perbal B., Sarasin A., Suárez H. G. v-myb transformation of Xeroderma pigmentosum human fibroblasts: overexpression of the c-Ha-ras oncogene in the transformed cells. Exp Cell Res. 1991 Oct;196(2):314–322. doi: 10.1016/0014-4827(91)90266-w. [DOI] [PubMed] [Google Scholar]
- Mucenski M. L., McLain K., Kier A. B., Swerdlow S. H., Schreiner C. M., Miller T. A., Pietryga D. W., Scott W. J., Jr, Potter S. S. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991 May 17;65(4):677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyaya R., Wolff L. New sites of proviral integration associated with murine promonocytic leukemias and evidence for alternate modes of c-myb activation. J Virol. 1992 Oct;66(10):6035–6044. doi: 10.1128/jvi.66.10.6035-6044.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W. C., Ewert D. L. Markers of B lymphocyte differentiation in the chicken. Hybridoma. 1990 Aug;9(4):331–350. doi: 10.1089/hyb.1990.9.331. [DOI] [PubMed] [Google Scholar]
- Pizer E. S., Baba T. W., Humphries E. H. Activation of the c-myb locus is insufficient for the rapid induction of disseminated avian B-cell lymphoma. J Virol. 1992 Jan;66(1):512–523. doi: 10.1128/jvi.66.1.512-523.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer E., Humphries E. H. RAV-1 insertional mutagenesis: disruption of the c-myb locus and development of avian B-cell lymphomas. J Virol. 1989 Apr;63(4):1630–1640. doi: 10.1128/jvi.63.4.1630-1640.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press R. D., Kim A., Ewert D. L., Reddy E. P. Transformation of chicken myelomonocytic cells by a retrovirus expressing the v-myb oncogene from the long terminal repeats of avian myeloblastosis virus but not Rous sarcoma virus. J Virol. 1992 Sep;66(9):5373–5383. doi: 10.1128/jvi.66.9.5373-5383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. G., Ishii S., Gonda T. J. Increase in specific DNA binding by carboxyl truncation suggests a mechanism for activation of Myb. Oncogene. 1991 Oct;6(10):1875–1879. [PubMed] [Google Scholar]
- Raschellà G., Negroni A., Skorski T., Pucci S., Nieborowska-Skorska M., Romeo A., Calabretta B. Inhibition of proliferation by c-myb antisense RNA and oligodeoxynucleotides in transformed neuroectodermal cell lines. Cancer Res. 1992 Aug 1;52(15):4221–4226. [PubMed] [Google Scholar]
- Reiss K., Ferber A., Travali S., Porcu P., Phillips P. D., Baserga R. The protooncogene c-myb increases the expression of insulin-like growth factor 1 and insulin-like growth factor 1 receptor messenger RNAs by a transcriptional mechanism. Cancer Res. 1991 Nov 1;51(21):5997–6000. [PubMed] [Google Scholar]
- Rosson D. Effects of 5' and 3' truncations of the myb gene on the transforming ability of avian myeloblastosis virus (AMV). Virology. 1990 Apr;175(2):562–567. doi: 10.1016/0042-6822(90)90441-s. [DOI] [PubMed] [Google Scholar]
- Rosson D., Reddy E. P. Nucleotide sequence of chicken c-myb complementary DNA and implications for myb oncogene activation. Nature. 1986 Feb 13;319(6054):604–606. doi: 10.1038/319604a0. [DOI] [PubMed] [Google Scholar]
- Saikumar P., Murali R., Reddy E. P. Role of tryptophan repeats and flanking amino acids in Myb-DNA interactions. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8452–8456. doi: 10.1073/pnas.87.21.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H., Kanei-Ishii C., Nagase T., Nakagoshi H., Gonda T. J., Ishii S. Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5758–5762. doi: 10.1073/pnas.86.15.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuur E. R., Baluda M. A. Expression of MYB proteins in avian hematopoietic tissues. Oncogene. 1991 Oct;6(10):1923–1929. [PubMed] [Google Scholar]
- Selvakumaran M., Liebermann D. A., Hoffman-Liebermann B. Deregulated c-myb disrupts interleukin-6- or leukemia inhibitory factor-induced myeloid differentiation prior to c-myc: role in leukemogenesis. Mol Cell Biol. 1992 Jun;12(6):2493–2500. doi: 10.1128/mcb.12.6.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Potter M., Mushinski J. F., Lavu S., Reddy E. P. Activation of the c-myb locus by viral insertional mutagenesis in plasmacytoid lymphosarcomas. Science. 1984 Nov 30;226(4678):1077–1080. doi: 10.1126/science.6093260. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Wolff L. Moloney murine leukemia virus-induced myeloid tumors in adult BALB/c mice: requirement of c-myb activation but lack of v-abl involvement. J Virol. 1987 Dec;61(12):3721–3725. doi: 10.1128/jvi.61.12.3721-3725.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Simons M., Rosenberg R. D. Antisense nonmuscle myosin heavy chain and c-myb oligonucleotides suppress smooth muscle cell proliferation in vitro. Circ Res. 1992 Apr;70(4):835–843. doi: 10.1161/01.res.70.4.835. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soret J., Kryceve-Martinerie C., Crochet J., Perbal B. Transformation of Brown Leghorn chicken embryo fibroblasts by avian myeloblastosis virus proviral DNA. J Virol. 1985 Jul;55(1):193–205. doi: 10.1128/jvi.55.1.193-205.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K., Bishop J. M. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989 Jul 14;58(1):85–93. doi: 10.1016/0092-8674(89)90405-4. [DOI] [PubMed] [Google Scholar]
- Zobel A., Kalkbrenner F., Guehmann S., Nawrath M., Vorbrueggen G., Moelling K. Interaction of the v-and c-Myb proteins with regulatory sequences of the human c-myc gene. Oncogene. 1991 Aug;6(8):1397–1407. [PubMed] [Google Scholar]
- al Moustafa A. E., Quatannens B., Dieterlen-Lièvre F., Saule S. Tumorigenic effects mediated in the avian embryo by one or more oncogenes associated with v-myc. Oncogene. 1992 Aug;7(8):1667–1670. [PubMed] [Google Scholar]