Abstract
Objective: The aim of this study was to confirm the efficacy, safety, and expected palatability of amlodipine orally disintegrating tablets (ODT) [RACTAB® formulation (Towa, Osaka, Japan)]. We report the re-analyzed results of 1687 cases in clinical settings obtained through postmarketing surveillance in Japan.
Method: Study subjects were patients receiving treatment for the first time with amlodipine ODT for hypertension under routine care. A multicenter central registration system was used for this prospective survey. The survey was conducted from October 2008 to October 2010. The observational period was 12 weeks, during which time surveys on outpatient blood pressure, adverse events, palatability, etc. were conducted.
Results: Blood pressure stabilized following treatment, and both systolic and diastolic blood pressures were favorably controlled. Adverse events observed were not significantly different from those observed during drug use trials of amlodipine formulations reported in 2003. Moreover, palatability of amlodipine ODT showed a 99.6% (227 of 228 cases) favorable patient acceptance, which is consistent with the initial design concept of RACTAB® formulation.
Conclusions: The results of this postmarketing surveillance study indicated that the efficacy, safety, and palatability of amlodipine ODT met our expectations (dissolves quickly in the mouth, tastes good, and is not rough on the tongue). Accordingly, amlodipine ODT are believed to be an easy-to-use formulation for prescribing doctors, dispensing pharmacists, and patients receiving treatment.
Introduction
Japan initiated its own universal medical care insurance system in 1961, whereby the whole population would receive equal medical care when needed. This system earned praise around the world.[1] However, Japan is no exception to the growing costs of medical care, with healthcare costs in 2009 reaching approximately ¥35 trillion, representing an increase of 3.5% over 2008.[2] One reason for this is the aging of society and declining birth rate, which have been markedly high in Japan. With the proportion of people aged ≥65 years being 22.7% in 2009, Japan has suddenly become an aged society in a manner unprecedented in the rest of the world.[3] This has increased the burden of insurance premiums on the youth and is a problem that could threaten the maintenance of the universal medical care insurance system.
The Japanese government aims to alleviate that burden and improve the financial state of the medical insurance system without compromising the quality of medical care and has started to reduce the cost of drugs included in medical care costs by increasing the availability of generic medicines (GEs). The cost of drugs accounts for approximately 20% of overall medical care costs in Japan, and according to the estimates of the Japan Generic Medicines Association (Tokyo, Japan), replacing long-term listed drugs with substitutable GEs may reduce the annual costs of drugs by more than ¥1 trillion.[4]
However, compared with Europe, the US, and other countries, the use of GEs has not spread in Japan as expected, and the GE market share (based on sales volume) only increased by 0.8% from 2003 to 2007 and remained at 17.2% in 2007.
Japan’s Ministry of Health, Labour and Welfare (MHLW) has therefore set forth the objective of raising the market share of GEs to ≥30% of the sales volume by 2012. In 2007, MHLW established the Action Programs for Promoting Safe Use of Generic Medicine to clarify the measures to be taken by state officials and generic pharmaceutical product manufacturers.[5] The goal was to improve (i) quality assurance; (ii) stable supply; (iii) supply of information; (iv) the environment for promoting GE use; and (v) the medical insurance system to help increase the GE market share.
RACTAB® is a formulation technology for an orally disintegrating tablet (ODT) that can be consumed without water, disintegrates quickly in a patient’s mouth,[6,7] and has characteristics such as being less likely to chip or crack when being supplied or dispensed,[8,9] and being versatile because of its simple manufacturing method.
Among all of the RACTAB® formulations released in to the market, rough feeling on the tongue is less with amlodipine ODTs because of the particle diameter being ≤100 μm. Moreover, the bitterness of the principal agent has been masked.[10]
Objective
The objective of this study was to confirm the efficacy, safety, and expected palatability of amlodipine ODT by re-analyzing the results of 1687 cases from a study of 2251 cases in clinical settings.[11] In these 1687 cases, amlodipine ODT (manufactured using RACTAB® technology) was administered.
Methods
Study Subjects
The study subjects were patients who (i) switched to amlodipine ODT from another amlodipine formulation (hereafter called substituted cases); (ii) were receiving antihypertensive treatment for the first time (hereafter called treatment-naïve cases); or (iii) switched to amlodipine ODT from non-amlodipine formulations and/or were administered with additional drugs etc. (hereafter called remaining cases). A multicenter central registration system was used for registration. The registry period for postmarketing surveillance was October 2008 to March 2010, and the survey period for the current study was October 2008 to October 2010. Because the study was an observational research of patients under routine care in accordance with Good Postmarketing Study Practice (GPSP) and not an interventional trial, no informed consent was required from study participants. Likewise, since the medication’s palatability was reported by the doctor as a part of the patient compliance instruction under routine care, no informed consent was required.
During the observational period of 12 weeks, surveys were conducted on patient background, pretreatment drugs, administration of the study drug with concomitant drugs, results of outpatient blood pressure and heart rate tests, as well as drug monitoring investigations and adverse events.
Of the 2251 cases investigated during postmarketing surveillance, amlodipine ODT was administered in 1687 cases (74.9%). Of these 1687 cases, 10 cases (0.6%) were excluded because the cases had not been consulted since the first prescription, and 11 cases (0.7%) were excluded because of deviation from GPSP (either the patient had started treatment prior to agreement or the registration period had deviated from the time between the start of treatment and treatment day 14). Therefore, 1666 cases were included for safety evaluation. Of the 1666 cases, an additional 120 cases (7.2%) were excluded from efficacy evaluation because the readings of outpatient blood pressure at treatment initiation or after the treatment had started were unavailable; therefore, 1546 cases were evaluated for efficacy (figure 1).
Fig. 1.

Case configuration.
Sex and age characteristics were studied in 812 men (48.7%) and 854 women (51.3%), with 695 patients (41.7%) aged <65 years and 971 patients (58.3%) aged ≥65 years. Children (aged <15 years) and pregnant women were not included. Of the 1666 patients, 423 patients (25.4%) were substituted cases (amlodipine besylate had previously been administered in these patients).
Of the 1546 cases evaluated for efficacy, 596 cases (38.6%) were treatment- naïve cases and 399 (25.8%) were substituted cases.
Adverse Reactions
Of all the adverse drug reactions (ADRs) noticed during the observation period, the present text refers to those ADRs that can be classified by the Medical Dictionary for Regulatory Activities System Organ Class (MedDRA SOC)[12] as nervous system disorders, heart and vascular disorders, gastrointestinal disorders, skin and subcutaneous tissue disorders, general disorders, and administration site conditions. Among these, dizziness, headache, arrhythmia, palpitations, feeling of hot flushes, nausea and vomiting symptoms, epidermal and dermal conditions, and asthenia have been reported by the Risk/Benefit Assessment of Drug - Analysis and Response (RAD-AR) Council (Japan) to frequently begin during the early period of antihypertensive drug administration.[13] When analyzing the onset time from the initiation of amlodipine ODT treatment, these ADRs were classified as ‘ADRs occurring early in treatment’.
Palatability
To determine palatability (whether the medicine was easy or difficult to ingest and the reasons for this), doctors involved in the survey interviewed patients by conducting a survey on the method of ingestion and palatability of the medicines at 12 weeks after treatment initiation or termination.
Results
Efficacy
After treatment initiation, systolic and diastolic blood pressures of the 596 treatment-naïve cases dropped significantly within 2 weeks (p < 0.001) and then declined moderately until a favorably stabilized transition was confirmed within 12 weeks. The changes in systolic and diastolic blood pressures between treatment initiation and after 12 weeks varied from 163.9 mmHg (95% CI 162.4, 165.4) to 136.3 mmHg (95% CI 135.1, 137.4) and from 94.5 mmHg (95% CI 93.5, 95.5) to 80.2 mmHg (95% CI 79.4, 81.1), respectively. In addition, heart rates were stabilized within 12 weeks after treatment initiation. In figure 2, the antihypertensive effect on different original systolic blood pressures in the 329 cases (21.3%) in which a single drug dose of 5 mg was administered is shown as an average value from treatment initiation to the time of final evaluation. When systolic blood pressure was ≥140 mmHg at treatment initiation, a significant antihypertensive effect was observed relative to the blood pressure value before treatment.
Fig. 2.

Cases where systolic blood pressure (SBP) has been lowered by the antihypertensive from treatment initiation (treatment-naïve cases: single drug [5 mg] dose [non-substituted antihyperintensive]). Paired t-test (time of final observation vs time of treatment initiation). NS = no significant difference; *** p < 0.001.
In the 399 substituted cases, blood pressure stabilized after substitution, and systolic and diastolic blood pressures were favorably controlled. In addition, heart rates were stabilized within 12 weeks of treatment initiation (figure 3).
Fig. 3.

Changes in blood pressure and heart rate (substituted cases). Average value and 95% confidence interval. Paired t-test (time of final observation vs time of treatment initiation). DBP = diastolic blood pressure; NS = no significant difference; SBP = systolic blood pressure; * p < 0.05, ** p < 0.01, *** p < 0.001.
Safety
Incidence of Adverse Drug Reactions (ADRs)
ADRs were observed in 43 cases (an incidence rate of 2.58%) of 1666 cases evaluated for safety. Primary ADRs were nervous system disorders (n = 12 cases; 0.72%), such as light-headed feeling or headaches, and abnormal test values (n = 8 cases; 0.48%), such as increased creatinine levels (table I). Serious ADRs were reported to include one case each of renal failure and arteriosclerosis obliterans, but the causal determination of each physician was ‘probably not related’.
Table I.
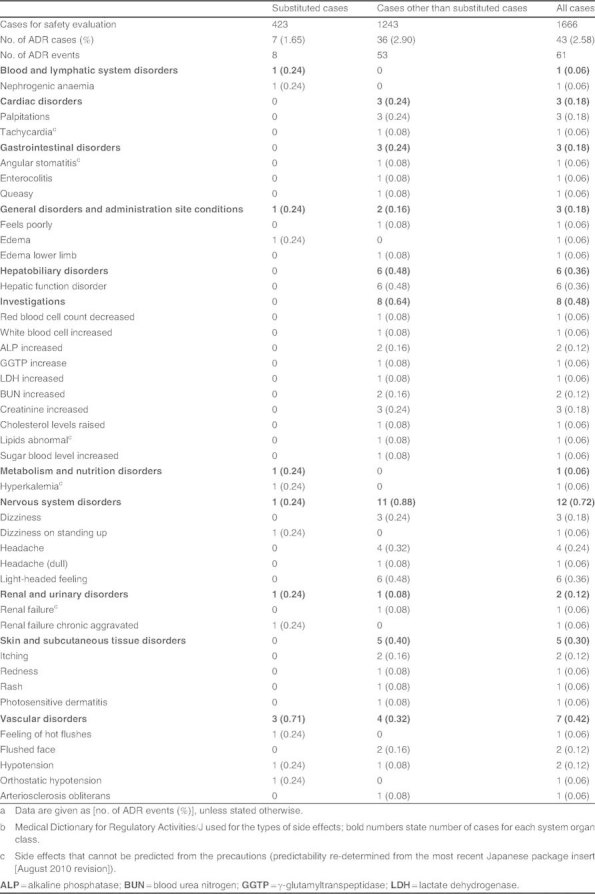
Types of adverse drug reactions (ADRs)a b
Among the substituted cases alone (n = 423 cases), ADRs were observed in seven cases (an incidence rate of 1.65%) but no serious ADRs were confirmed. The outcome of all seven cases was ‘recovered’ or ‘recovering’, excluding one case with an unknown outcome.
Onset Time of ADRs
In an analysis of the onset period of ‘ADRs occurring early in treatment’ in the 1243 cases other than the substituted cases in which amlodipine besylate was first administered as the active component, 32 ADR events were categorized as ‘ADRs occurring early in treatment’. Of them, 81% (n = 26) were ADRs occurring in <4 weeks of treatment initiation. Furthermore, of the 26 ADRs, 65% (n = 17) occurred in <2 weeks after treatment initiation (figure 4).
Fig. 4.

Onset of adverse drug reactions (ADRs) at different periods (excluding substituted cases).
On the other hand, in the 423 substituted cases, five ADR events were categorized as ‘ADRs occurring early in treatment’, with the onset in <4 weeks in two cases (40%; one each of hypotension and feeling of hot flushes) and <2 weeks in one case (20%; feeling of hot flushes).
Palatability
Regarding the palatability of amlodipine ODT, figure 5 shows the results of patient interviews conducted by the doctors involved in the survey 12 weeks after treatment initiation or termination. Of the 1355 cases in which a response was obtained on palatability, 228 patients (16.8%) recorded that the medicine was ‘mostly ingested without water’. Among them, 227 (99.6%) found the medicine ‘easy to ingest’ and one (0.4%) found it ‘difficult to ingest’. The reasons for the medicine being considered ‘easy to ingest’ included ‘dissolves quickly in the mouth’ (170 cases), ‘tastes good’ (129 cases), and ‘not rough on the tongue’ (56 cases), whereas the reason for the medicine being considered ‘difficult to ingest’ was ‘tastes bad’.
Fig. 5.

Amlodipine ODT palatability (228 patients took the tablets mostly without water). Reproduced from Towa Pharmaceutical Co., Ltd.,[11] with permission.
Discussion
Efficacy
Blood pressure after treatment initiation in the treatment-naïve cases was favorably stabilized within 12 weeks. Furthermore, an antihypertensive effect on systolic blood pressure was observed in those cases where a single drug dose of 5 mg was administered; the effect was relative to pretreatment blood pressure values. In the substituted cases, blood pressure stabilized after substitution of medication, and systolic and diastolic blood pressures were favorably controlled.
From these findings, amlodipine ODT was confirmed to have an efficacy at clinical sites identical to that of amlodipine formulations from other companies.[14,15] It was also confirmed to have lowered blood pressure relative to that at treatment initiation, without further increases in the heart rate. Therefore, amlodipine ODT was confirmed as an easy-to-use antihypertensive that moderately lowered blood pressure without burdening the heart.
Safety
Of the 1666 cases, 43 ADRs (2.58%), primarily nervous system disorders or abnormal test values, were observed. This, together with the onset frequency and trend, was not significantly different from results reported in drug use trials of other amlodipine formulations.
Patients who switched from amlodipine besylate to amlodipine ODT had fewer ADRs occurring more frequently in the early period of treatment compared with those in whom amlodipine besylate was administered for the first time (non-substituted cases [i.e. both treatment-naïve and remaining cases]) because amlodipine besylate had already been administered as the active component, although it was believed that the distribution of suspected ADRs might be different. Thus, ADRs reported[13] by the RAD-AR Council in the Antihypertensive drug use trial database construct research: final report as those that frequently occurred immediately after beginning treatment with antihypertensives and the same ADRs deemed by MedDRA SOC as ‘ADRs occurring early in treatment’ were analyzed for the onset time. In our study, 32 ‘ADRs occurring early in treatment’ (2.6%) occurred in the 1243 cases (excluding the substituted cases). Of those, 81% occurred in <4 weeks and 65% of those occurred in <2 weeks. On the other hand, five corresponding ADRs (1.2%) were observed in the 423 substituted cases. Onset was observed in <44 weeks for a single case each of hypotension and feeling of hot flushes, and <2 weeks for a single case of feeling of hot flushes. These results support the hypothesis of a reduction in ADRs in patients who switched from amlodipine besylate to amlodipine ODT.
These results suggest that no significant difference was observed in the onset of ADRs between amlodipine ODT and other amlodipine formulations.[15] Furthermore, for substituted cases whose data are unique to GEs, as expected, ‘ADRs occurring early in treatment’ had already been experienced before the substitution took place; therefore, fewer ADRs were observed than when amlodipine besylate was first used as the active component.
Palatability
Towa Pharmaceutical Co., Ltd.’s RACTAB® applies an intelligent formulation design to conventional ODT formulation technology. It was developed to increase usability and further enhance medication adherence and a patient’s quality of life with tablets that do not crack or chip in transit or in automatic tablet-packaging machines.[8,9] In particular, amlodipine ODT disintegrates quickly because of its rapidly disintegrating particles. The rough feeling on the tongue from some medication is comparatively less with amlodipine ODT because its particle diameter is ≤100 μm. It is less bitter because the principal agent is masked.[7]
Therefore, in this study, a survey item was established to confirm the medicine’s palatability during actual use. The results showed that 99.6% of patients (227 of 228 cases) rated amlodipine ODT as ‘easy to ingest’ with regard to ease of consumption when ingested mostly without water. The reasons provided were that it ‘dissolves quickly in the mouth’, ‘tastes good’, and ‘not rough on the tongue’. Therefore, the RACTAB® formulation had favorable patient acceptance, which is in line with its initial design concept. This study also suggests that it is a formulation that can be expected to enhance medication adherence and quality of life.
Conclusion
In Japan, which has not had the same success as other countries in promoting GEs, the MHLW and Towa Pharmaceutical Co., Ltd. have an objective of increasing the use and improving the reliability of GEs.
By re-analyzing the results obtained from Towa Pharmaceutical Co., Ltd. postmarketing surveillance of amlodipine ODT and amlodipine tablets, the safety and efficacy of amlodipine ODT were reaffirmed. Furthermore, it was possible to confirm the palatability of tablets formulated with RACTAB® technology, which has received high praise in reviews for its design concept.[9] Accordingly, amlodipine ODT is deemed to be a valuable formulation for prescribing doctors, dispensing pharmacists, and patients receiving treatment.
Acknowledgments
We extend our gratitude toward our collaborators in investigating the use of amlodipine ODTs and amlodipine tablets from Towa Pharmaceutical Co., Ltd. and toward doctors who supplied us with valuable data. With regard to manuscript preparation, we express our thanks to Dr Hiroshi Takeda, who works as an advisor in Towa Pharmaceutical Co., Ltd. and honored us with his constant helpful advice. All authors are employees of Towa Pharmaceutical Co., Ltd (Kiyoshi Kawashima was an employee until March 2011).
References
- 1.WHO. The world health report 2000 — health systems financing: the path to universal coverage. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministry of Health, LabourWelfare . Trends in medical care costs. 2011. [Google Scholar]
- 3.Cabinet Office. White paper on aged society: 2010 edition. 2011. [Google Scholar]
- 4.Japan Generic Medicines Association [online; in Japanese]. Available from URL: http://www.jga.gr.jp/medical/generic06.html [Accessed 2011 Jul 1]
- 5.Ministry of Health, LabourWelfare. Action programs for promoting safe use of generic medicine: 2007. 2011. [Google Scholar]
- 6.Okuda Y, Irisawa Y, Okimoto K, et al. A new formulation for orally disintegrating tablets using a suspension spraycoating method. Int J Pharm. 2009;382:80–7. doi: 10.1016/j.ijpharm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Okuda Y. Formulation design of OD tablets using RACTAB technology. 2011;71(1):21–5. [Google Scholar]
- 8.Okimoto K, Okuda Y, Taniguchi K, et al. Generic OD tablets (RACTAB) Gekkanyakuji (The Pharmaceuticals Monthly) 2008;50(11):1691–9. [Google Scholar]
- 9.Okuda Y. Amlodipine-OD tablets “TOWA” in consideration of usability. Tyouzai to Jouhou (Rx Info) 2009;15(8):936–40. [Google Scholar]
- 10.Furihata K, Hoshiyama T, Okuda Y, et al. Bioequivalence trial of amlodipine-OD tablets 2.5 mg “TOWA” and amlodipine-OD tablets 5mg “TOWA” in healthy human volunteers. Jpn JMed Pharm Sci. 2008;59(5):787–806. [Google Scholar]
- 11.Towa Pharmaceutical Co., Ltd. Pharmacovigilance & quality assurance division Pharmacovigilance & post marketing surveillance department. Drug use investigation of amlodipine-OD tablets “TOWA”/tablets “TOWA” — safety, efficacy and sensory perceptions of OD tablets ”TOWA”. Jpn JMed Pharm Sci. 2011;65(2):243–59. [Google Scholar]
- 12.Medical Dictionary for Regulatory Activities System Organ Class (MedDRA SOC) [online]. Available from URL: http://www.meddramsso.com/public_about_meddra.asp [Accessed 2011 Nov 17]
- 13.RAD-AR Risk/Benefit Assessment of Drugs-AnalysisResponse Council, Japan. Antihypertensive drug use trial database construct research — final report: 2003. 2011. [Google Scholar]
- 14.Cross BW, Kirby MG, Miller S, et al. A multicentre study of the safety and efficacy of amlodipine in mild to moderate hypertension. Br J Clin Pract. 1993;47(5):237–40. [PubMed] [Google Scholar]
- 15.Komoto A, Kumamaru H, Houzawa H, et al. Test on safety and efficacy in usage of Norvasc tablets for hypertension-drug use trial. J New Rem & Clin. 2003;52(12):1553–72. [Google Scholar]


