Abstract
Objective
To perform an initial qualitative comparison of the different procurement models in India to frame questions for future research in this area; to capture the finer differences between the state models through 53 process and price parameters to determine their functional efficiencies.
Design
Qualitative analysis is performed for the study. Five states: Tamil Nadu, Kerala, Odisha, Punjab and Maharashtra were chosen to ensure heterogeneity in a number of factors such as procurement type (centralised, decentralised or mixed); autonomy of the procurement organisation; state of public health infrastructure; geography and availability of data through Right to Information Act (RTI). Data on procurement processes were collected through key informant analysis by way of semistructured interviews with leadership teams of procuring organisations. These process data were validated through interviews with field staff (stakeholders of district hospitals, taluk hospitals, community health centres and primary health centres) in each state. A total of 30 actors were interviewed in all five states. The data collected are analysed against 52 process and price parameters to determine the functional efficiency of the model.
Results
The analysis indicated that autonomous procurement organisations were more efficient in relation to payments to suppliers, had relatively lower drug procurement prices and managed their inventory more scientifically.
Conclusions
The authors highlight critical success factors that significantly influence the outcome of any procurement model. In a way, this study raises more questions and seeks the need for further research in this arena to aid policy makers.
Keywords: Public Health
Article summary.
Article focus
Qualitative analysis of the different procurement models in India.
Analysis of the models based on 53 process and price parameters.
Highlighting some critical success factors determining the efficiency of the procurement model.
Key messages
Detailed understanding of the advantages and disadvantages of pooled procurement methods, mixed procurement methods and decentralised procurement methods.
Highlighting the importance of the state contexts in which the models operate.
Importance of autonomy in procurement organisations for better efficiency as explained with the examples of models from Tamil Nadu and Kerala.
Strengths and limitations of this study
Macroview of the different kinds of procurement models and in-depth process analysis of each model.
Possible guiding tool for policy-makers and future researchers.
Availability of essential medicines at the public health facilities was not assessed as part of this study. It is the primary indicator of efficacy of a procurement system, so all the qualitative findings mentioned in the paper will have to recognise the lack of these data and interpret the findings appropriately.
Time and resource constraints have limited our primary data to two districts in each state. However, efforts were made to include both urban and rural ones in the study.
Quantifying the ‘impact’ of each of the procurement systems is rather ambiguous due to the lack of concrete indicators to record aspects like corruption, governance and so on. Thus, this section is qualitatively recorded with the help of a few indicators composed based on the existing literature and some aspects specific to public procurement systems.
Introduction
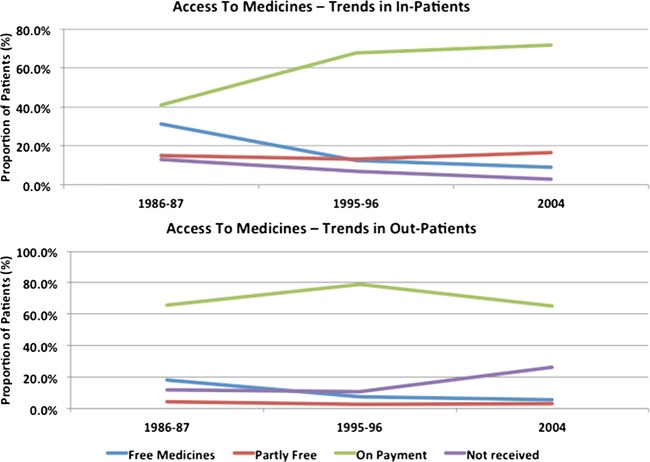
Over the years, India has seen a tremendous growth in the pharmaceutical sector. Yet it is grappling with a large population that is denied basic access to healthcare and essential medicines. According to a WHO report on the world's medicines situation, almost 68% of the people in India have limited or no access to essential medicines.1 Inadequate medicine access poses a major barrier to the objective of delivering essential healthcare and the more recently talked about universal healthcare. According to the United Nations Development Group,2 medicine access is defined as “having medicines continuously available and affordable at public or private health facilities or medicine outlets that are within one hour walk from the homes of the people.” Fulfilment of all these factors is arguably low in developing countries like India. Figure 1 shows a decreasing trend in the supply of free medicines since 1986 and also a corresponding increase in the number of people not receiving any medicines at all for outpatient care.3–5
Figure 1.
Access to medicines in India.
Private health expenditure constitutes almost 70% of the total health expenditure of which drugs form a massive component with anywhere between 20% and 65% in India and other transitional economies compared with 18% in Organization for Economic Cooperation and Development (OECD) countries.6 The burden of purchasing medicines is very high in India, accounting for the second largest bulk of expenditure after food. The high cost of medicine purchase in India and relatively low public health investment is exacerbating the lack of access to essential medicines. It is now well known, accepted and documented that out-of-pocket (OOP) payment for healthcare has pushed many people into poverty. Bearing the costs of a single hospitalisation, 35% of people fall below the poverty line and OOP medical costs alone may push 2.2% of the population below the poverty line in 1 year.7 Figure 2 below gives a glimpse of the healthcare spending in India for 2004–2005 across various states.8
Figure 2 .
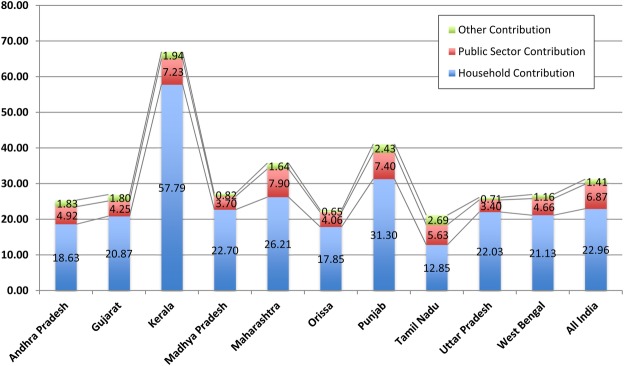
Healthcare spending in India 2004–2005 (figures in USD).
Strengthening the public sector availability of quality drugs is one of the long-term, sustainable ways to relieve a large number of people for whom medical expenditure may be catastrophic. This paper, focusing on the public drug procurement models in India, will detail five main factors of the systems—low financial burden, good quality, timely availability, minimal wastage and transparency—that are important to improve access to medicines. Although the rational usage of drugs and medical awareness among the people is equally important to determine the success of the public procurement systems, this paper only deals with the supply side of the medicines access issue. Accordingly, the objective of the paper is to understand and compare the public drug procurement systems in five Indian states—Kerala, Maharashtra, Odisha, Punjab and Tamil Nadu—on the basis of a set of predetermined comparison factors and also explore whether the success of the procurement models depends on some crucial intangible elements beyond the procurement process or price.
Methodology
The study was designed to compare the public drug procurement models of a sample of states on a set of 53 predetermined parameters. These parameters reflect each of the five main objectives of comparison, viz. low financial burden, good quality, timely availability, transparency and wastage elimination through an efficient supply chain.
The sample states were chosen to ensure heterogeneity in a number of factors such as procurement type (centralised, decentralised or mixed); autonomy of the procurement organisation; state of public health infrastructure and geography. Based on these parameters, the sample of states initially chosen were Kerala, Tamil Nadu, Maharashtra, Punjab, Uttar Pradesh and West Bengal. Consequently, Right to Information (RTI)i applications were sent to the concerned Public Information Officers to seek drug procurement and process data. However, owing to a lack of data responses despite multiple appeals from Uttar Pradesh and West Bengal, these states were replaced with Odisha. Table 1 provides an overview of the sampling methodology. It is also noteworthy that some of the sample states are primarily agrarian systems while the others are at different points of industrialisation.
Table 1.
Sample states for the study
| Sampling attribute | Kerala | Tamil Nadu | Maharashtra | Odisha | Punjab |
|---|---|---|---|---|---|
| Procurement type | Centralised | Mixed | Primarily decentralised | Mixed | Primarily decentralised |
| Autonomy | Fully autonomous | Fully autonomous | Government owned | Government owned | Government owned |
| Health infrastructure | Good | Good | Poor | Poor | Good |
| Geography | South | South | Mid-West | Mid-East | North |
The procurement type mentioned in table 1 is used to refer to the model wherein the state drug procurement budget is divided between the centralised, decentralised and mixed methods of acquiring medicines. Autonomy refers to the extent of government involvement in the decisions of the procurement organisation; ‘fully autonomous’ implies minimal involvement while ‘government owned’ indicates a high degree of involvement. The rating of health infrastructure as ‘good’ and ‘poor’ has been based on the perceived condition of the infrastructure such as the drug warehouses, transportation facilities, community health centre, primary health centre and district hospital conditions.
For an overview of the context, brief information about the sample states is presented in table 2.9–12
Table 2.
Overview of sample states
| Parameter | Kerala | Maharashtra | Odisha | Punjab | Tamil Nadu |
|---|---|---|---|---|---|
| Total population | 33387677 | 112372972 | 41947358 | 27704236 | 72138958 |
| Urban/rural population ratio (%) | 91.3 | 82.6 | 20 | 60 | 94 |
| Annual per capita income | 59179 | 83471 | 36923 | 67473 | 72993 |
| Annual per capita expenditure—rural | 22020 | 13836 | 9816 | 19788 | 13920 |
| Annual per capita expenditure—urban | 28956 | 29244 | 18576 | 25308 | 23376 |
| Total per capita health expenditure | 2952 | 1576 | 995 | 1813 | 933 |
| Public component (%) | 10.8 | 22.1 | 18 | 18 | 26.6 |
| Private component (%) | 86.3 | 73.3 | 79.1 | 76.1 | 60.7 |
| Number of subcentres | 4575 | 10579 | 6688 | 2950 | 8706 |
| Number of primary health centres | 697 | 1816 | 1279 | 394 | 1277 |
| Number of community health centres | 226 | 376 | 231 | 129 | 256 |
| Number of district hospitals | 14 | 35 | 32 | 20 | 29 |
| Birth rate (/1000 population) | 14.7 | 17.9 | 21.5 | 17.6 | 15.8 |
| Death rate (/1000 population) | 6.8 | 6.6 | 9.2 | 7 | 7.2 |
| Infant death rate (/1000 live-births) | 13 | 33 | 71 | 43 | 35 |
| Maternal death rate (per 100000 live-births) | 110 | 130 | 303 | 192 | 111 |
| Total fertility rate (children per woman) | 1.7 | 2 | 2.4 | 2 | 1.6 |
Primary data for the study were gathered through key informant analysis, in which semistructured interviews were conducted with executive leadership teams of the drug procurement cells and public health officials in the sample states in March–April 2012, and RTI responses from sample states. The information gathered from the key informant analysis was corroborated with the field staff by way of semistructured interviews with stakeholders of primary health centres, community health centres and district hospitals and qualitative observation during the authors’ warehouse visits.
Secondary data on expenditures, budgets and indicators were compiled from datasets published by the National Sample Survey Office, Ministry of Health and Family Welfare (Bulletin on Rural Health Statistics in India) and Office of the Registrar General & Census Commissioner of India (Sample Registration Survey). This study is intended to be a qualitative assessment with the objective of framing questions for future research, and therefore no statistical techniques were used.
Findings
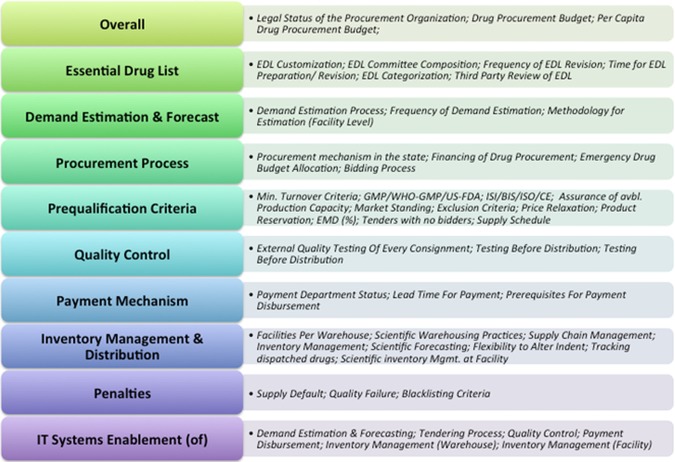
The procurement processes followed in the sample states were evaluated against a predetermined set of 53 parameters (including price). See figure 3 for the list of predetermined parameters used for comparison.
Figure 3.
Overview of comparison parameters.
The detailed comparison tables on the procurement process and prices are presented in tables 3 and 4. In many instances, the process followed was very different from the one given in the manuals. The information captured below relates to the processes that were actually followed.
Table 3.
Procurement process comparison across the sample states
| Parameter | Kerala | Odisha | Tamil Nadu | Punjab | Maharashtra |
|---|---|---|---|---|---|
| Legal status of procurement organisation | Autonomous (KMSCL) | Government owned (part of DHS) | Autonomous (TNMSC) | Government owned (PHSC) | Government owned |
| Drug procurement budget (USD) | 36.3 million (2011–2012) | 8.1 million (2010–2011) | 39.8 million (2010–2011) | 3.4 million (0.4 million state budget+3 million user fees) | 87.5 million (2010–2011) |
| Per capita drug procurement budget (USD) | 51 | 8.8 | 22.5 | 5.8 | 35.6 |
| Essential drug list | |||||
| Customised state EDL | Yes | Yes | Yes | Yes | No, but it has a drug list comprising 1850 drugs |
| Composition of EDL committee | Multistakeholder committee | Multistakeholder committee | Multistakeholder committee | Multistakeholder committee | Multistakeholder committee |
| Frequency of EDL revision (years) | 1 | 2 | 1 | 1 | N/A |
| Time for EDL preparation/revision (months) | 2–3 | 7–8 | 2–3 | 4 | N/A |
| EDL categorisation | Yes (8 product-based categories) | Yes (2 demography-based lists) | Yes (product-based categories) | Yes | N/A |
| Third party review of EDL | No | Yes (by WHO experts) | No | No | N/A |
| Demand estimation and forecast | |||||
| Demand estimation process | Aggregation of facility indents | Aggregation of facility indents | Aggregation of facility indents | Aggregation of facility indents | Facility-level indenting |
| Frequency of demand estimation (years) | 1 | 1 | 1 | 1 | 1 |
| Methodology for estimation (facility level) | 10–15% over previous year's indent; performed by pharmacist | No scientific method; usually performed by computer operator/clerk | 10% of the previous year consumption | N/A | 10% of previous year consumption |
| Procurement process | |||||
| Procurement mechanism in the state | Centralised | 80% centralised; 20% decentralised | 90% centralised; 10% decentralised | 12.5% centralised; 87.5% decentralised | Centralised rate contracting; decentralised purchasing |
| Financing of drug procurement | State budget allocation | State budget allocation | State budget allocation | State budget allocation and user charges | State budget allocation |
| Emergency drug budget allocation | Yes (additional funds released) | No (purchased from existing budget) | Yes (additional funds released) | No | Yes (additional funds released) |
| Tendering process | |||||
| Bidding process | Two-bid system | Two-bid system | Two-bid system | Two-bid system | Two-bid system |
| Prequalification criteria | |||||
| Minimum turnover criteria (INR/USD) | 10 crore/2.1 million | 10 crore/2.1 million | 3 crore/0.7 million | 50 crore/10.7 million | 10 crores/2.1 million |
| GMP/WHO-GMP/US-FDA | Required | Required | Required | Required | WHO-GMP required |
| ISI/BIS/ISO/CE | Required | Required | N/A | N/A | N/A |
| Assurance of available production capacity | Required (MPMASS) | None | Production capacity certificate | N/A | Production Capacity Certificate |
| Market standing (years) | 2 | 3 | 3 | 3 | 3 |
| Exclusion criteria for factory inspections | Supply to premier institutions | None | None | None | N/A |
| Price relaxation for SSIs/PSUs | Yes (SSI—10%; PSU—15%) | Yes (SSI—10%; additional 3% for ISO certification) | Yes (SSI—15%) | PSU produced antibiotics | None (20% quantity reserved if SSI matches L1 rate) |
| Product reservation for SSIs/PSUs | None | 31 items (from SSIs) | None | None | None |
| EMD | 1% of tender value | 1–5% of tender value | 1% of tender value (maximum upto 50000 INR), exempted for SSI | Differs for each drug | INR 25000 |
| Process for tenders with no bidders (in order of priority) | Retender (revised prequalifications); limited tender; short tender; direct purchase | Retender (same prequalifications)—open until bids are received | Retender (limited and short-tender process is used) | Pharmacy-based purchasing | Retendering, limited tendering or direct purchase |
| Supply schedule | 60 days—40% of PO quantity; 90 days—70%; 120 days—100% | 60 days—50% of PO quantity; rest before specified date | Starting from 30 days and has to end by 60 days, otherwise specified | 30 days to 3 months from the time of issue of PO | Within 3 months from the issue of PO |
| Quality control | |||||
| External quality testing of every consignment | Empanelled private labs | No external quality testing (supplier's internal quality certificate) | Empanelled private and government labs | Empanelled government labs | No external quality testing (supplier's internal quality certificate) |
| Testing before distribution | Mandatory | Not mandatory | Mandatory | Mandatory | Not mandatory |
| Lead time for quality testing | ∼15 days | Within 8 weeks | 15 days for tablets and capsules; 1 month for suspensions | 1 month | N/A |
| Payment mechanism | |||||
| Payment department status | Autonomous (managed by contractual staff) | Government (Account General's Office) | Autonomous (managed by contractual staff)-IT enabled | Government | Government (Directorate of Accounts and Treasuries) |
| Lead time for payment (days) | ∼30 | ∼90 | 30 | Minimum 30 | ∼90 |
| Pre-requisites for payment disbursement | Warehouse material receipt, external quality certificate | Warehouse material receipt, supplier's internal quality certificate | Warehouse material receipt, external quality certificate | Warehouse material receipt, quality certificates from labs | Facility material receipt, internal quality certificate |
| Inventory management and distribution | |||||
| Facilities (All) catered to per warehouse (average) | ∼290 | ∼235 | ∼411 | N/A | N/A |
| Scientific warehousing practices | Yes | No | Yes | No | No |
| In-house/outsourced supply chain management | Outsourced | In-house | In-house | In-house | In-house (facility level) |
| Inventory management | Dynamic (flexibility of second PO) | Static (only single PO is issued) | Dynamic (flexibility of second PO) | Static | 25% flexibility for quantity maintained |
| Scientific consumption/inventory forecasting | Yes (inventory management software) | No | Yes (inventory management software) | No | No |
| Flexibility for facilities to alter indent | Yes (just before dispatch) | No | Yes | Yes | No |
| Tracking dispatched/delivered drugs | Currently passbook (volume based; online in future) | No tracking | Passbook (value based) | N/A | No |
| (Scientific) Inventory management at facility | No (online in future) | No | Use first in first out (FIFO) principle | No | No |
| Penalty | |||||
| Penalty for supply schedule default | 10% of the unexecuted supply; unexecuted supply purchased at the cost of supplier in case of inability to supply | N/A | 0.5% per day to maximum of 15% of the tender amount | N/A | 0.5% of the value of unsupplied goods per week up to 5 weeks, after which unexecuted supply purchased at the cost of supplier |
| Penalty for quality failure | Supplier blacklisted with forfeiture of security deposit | Suppliers have to replace the entire PO quantity or risk blacklisting | Supplier blacklisted with forfeiture of security deposit | Forfeiture of EMD | Supplier blacklisted with forfeiture of security deposit |
| Blacklisting criteria | Defaulting on 3 POs or more with less than 50% supply; blacklisted by any other procurement agency on quality grounds | Quality failure after material supply | Defaulting continuously on 3 POs with less than 50% supply, quality failure, blacklisted by national or other state level agencies | Defaulting continuously on 3 POs with less than 50% supply, quality failure, blacklisted by national or other state level agencies | Supply default after extension period; quality failure |
| IT enablement processes | |||||
| Demand estimation & forecasting | Yes | No | Yes | No | No |
| Tendering process | Yes | No | Yes | No | Yes |
| Quality control | No | Yes | No | No | |
| Payment disbursement | Yes | No | Yes | No | No |
| Inventory management (warehouse) | Yes | Yes | Yes | No | No |
| Inventory management (facility) | No | No | Yes | No | No |
PO,purchase order; PSU,Public Sector Undertaking; SSI,Small Scale Industries.
Table 4.
Price comparison of 32 randomly selected drugs across the sample states
| Name of drug | Dosage | Unit | Price (INR) |
||||
|---|---|---|---|---|---|---|---|
| Kerala 2012 | Tamil Nadu 2012 | Odisha 2009 | Maharashtra 2011 | Punjab 2010 | |||
| Adrenaline | 1 mg/1 ml | Ampoule | 2.89 | 1.21 | 1.46 | 1.80 | N/A |
| Albendazole | 400 mg | Tablet | 0.81 | 0.57 | 0.49 | 0.61 | 0.64 |
| Aminophylline | 25 mg/ml | Ampoule | n/a | 2.60 | 2.91 | 4.90 | N/A |
| Amitriptyline | 25 mg | Tablet | 0.22 | 0.15 | 0.15 | 0.19 | N/A |
| Amlodipine | 5 mg | Tablet | 0.16 | 0.06 | 0.09 | 0.10 | 0.13 |
| Atenolol | 50 mg | Tablet | 0.125 | 0.11 | 0.13 | 0.14 | 0.14 |
| Benzyl penicillin | 10 lakh IU | Vial | 3.68 | 3.08 | 4.20 | 4.88 | N/A |
| Carbamazepine | 200 mg | Tablet | 0.59 | 0.54 | 0.42 | 0.53 | N/A |
| Cefotaxime | 250 mg | Vial | 4.73 | 3.94 | 5.40 | 5.14 | N/A |
| Ciprofloxacin | 500 mg | Tablet | 1.09 | 1.04 | 0.87 | 1.07 | 1.86 |
| Co-trimoxazole | 40 mg+200 mg per 5 ml | Bottle | n/a | 5.91 | 5.90 | 6.74 | N/a |
| Diclofenac | 25 mg/ml | Ampoule | 1.33 | 1.08 | 1.04 | 1.40 | 2.70 |
| Dicyclomine | 10 mg/ml | Ampoule | 1.34 | 0.88 | 1.17 | 1.37 | N/A |
| Dopamine | 40 mg/ml | Vial | 6.4 | 5.40 | 5.53 | 7.87 | N/A |
| Erythromycin | 250 mg | Tablet | 1.27 | 1.23 | 0.81 | 1.03 | N/A |
| Folic acid | 5 mg | Tablet | 0.06 | 0.06 | 0.06 | 0.08 | 0.05 |
| γ-Benzene hexachloride | 1% w/v | Bottle | 12.5 | 9.63 | 12.77 | 10.18 | N/A |
| Glibenclamide | 5 mg | Tablet | 0.12 | 0.07 | 0.08 | 0.08 | N/A |
| Glycopyrrolate | 0.2 mg/ml | Ampoule | 5.22 | 1.65 | 3.25 | 3.51 | N/A |
| Hydrocortisone | 100 mg | Vial | 11 | 10.50 | 7.45 | 11.38 | 7.39 |
| Ketamine | 50 mg/ml | Vial | n/a | 16.27 | 14.60 | 17.10 | N/A |
| Lignocaine | 2% w/v | Vial | 7.75 | 4.54 | 3.80 | 6.30 | 4.40 |
| Metformin | 500 mg | Tablet | 0.24 | 0.19 | 0.18 | 0.19 | N/A |
| Methyl ergometrine | 0.2 mg/ml | Ampoule | 1.85 | 1.33 | 1.71 | 2.75 | N/A |
| Norfloxacin | 400 mg | Tablet | 0.78 | 0.79 | 0.57 | 0.76 | N/A |
| Oxytocin | 5 IU/ml | Ampoule | n/a | 1.16 | 1.65 | 1.51 | N/A |
| Pentazocine | 30 mg/ml | Ampoule | 3.05 | 2.41 | 2.58 | 3.51 | 3.60 |
| Phenobarbitone | 30 mg | Tablet | 0.28 | 0.09 | 0.12 | 1.43 | 0.11 |
| Phenytoin | 100 mg | Tablet | 0.36 | 0.16 | 0.11 | 1.60 | N/A |
| Promethazine | 25 mg | Ampoule | 1.68 | 1.19 | 1.10 | 1.60 | N/A |
| Ranitidine | 50 mg | Ampoule | 1.31 | 0.81 | 0.98 | 1.40 | 2.20 |
| Thiopentone | 500 mg | Ampoule | 21.5 | 16.60 | 17.20 | 11.85 | N/A |
Discussion
An efficient drug distribution system ensures availability of the right medicines in sufficient quantities procured at the lowest prices to secure the maximum therapeutic value to the largest number of beneficiaries with the available & additional resources.
Broadly speaking, the two main beneficiaries in this context are the government and the patient. On the one hand, rationality dictates that any government in a resource-constrained setting would expect that an effective procurement system would ensure availability of quality medicines while optimising the finances to ensure the best outcomes. It is also in the interest of the government to run this system transparently to promote competition and thus efficiency. On the other hand, a patient expects that good quality medicines are available at all times, free of cost (see figure 4 for an expectation map of both beneficiaries). Leadership, technical capability and information technology overarching the expectations in the exhibit below are the pre-requisites for running a system efficiently. The capability of each state's procurement system to enhance IT usage and administrative capabilities driven by a strong leader is prerequisite.
Figure 4.
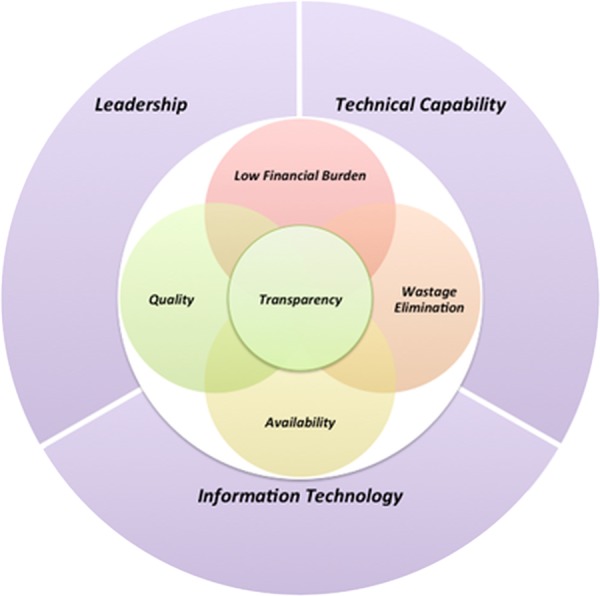
Combined expectation mapping of beneficiaries of a public procurement system.
Low financial burden
Low financial burden to the government exchequer is an important aspect of the public drug procurement systems because of limited resources. Some of the parameters among the 53 comparatives that reflect a procurement system's capacity to reduce the financial burden are the extent of capital expenditure for establishing the systems, costs for procurement, storage and transportation, the preciseness of the Essential Drug List (EDL) to suit the state health burden and finally the prices at which drugs are procured.
The procurement process adopted bears some strong repercussions on the budgets, which include both the capital expenditure and operating expenditure to run the system. For completely/predominantly centralised pooled procurement models like Tamil Nadu, Kerala and Odisha, it is imperative to have an optimum number of warehouses to cater to all the public health facilities. Additionally, the system requires adequate transportation facilities to transfer supplies from warehouses to user institutions and IT enablement to manage the entire system, necessitating a considerable initial capital expenditure. With a budget of Indian Rupee (INR) US$39.8 million and US$36.3 million in FY2010 for Tamil Nadu and Kerala, respectively, the states have been able to make capital investments—this also includes the cash surplus generated through management fees that the autonomous procurement agencies charge. Kerala has about 19 warehouses and Tamil Nadu about 25, most of which comply with scientific standards of inventory management. Odisha, with a budget of INR US$8.1 million for FY2011, is unable to make the necessary investments to fully realise the benefits of a centralised pooled procurement model.
Maharashtra follows the system of centralised rate contracting and decentralised purchasing where the suppliers directly deliver the medicines to the facilities. While transportation costs are not borne by the state, its cost is built into the drug price. This system also requires significantly large storage facilities at each user institution, thereby increasing the overall cost. Punjab was not considered in this analysis because it follows a mixed system with drugs worth about US$0.4 million being purchased in a centralised manner, whereas user charges collected by district hospitals, accounting for US$3 million, are utilised to directly purchase drugs from the open market.
A well-formulated and localised EDL is imperative to make optimal use of the limited financial resources. Tamil Nadu, Kerala and Odisha purchase about 260 drugs each year as a part of EDL, whereas in Maharashtra, centralised rate contracting (decentralised purchasing) is carried out for about 1850 drugs. Though the decentralised purchasing model offers more flexibility for facilities, the administrative costs of finalising rate contracts for 1850 drugs and empanelling the suppliers is by no measure insignificant.
Finally, drug price is the largest expenditure component. Theoretically, centralised procurement offers volume discounts, thereby reducing the financial burden; however, Annexure 2, which compares the prices of 32 drugs across the five states, reveals that Tamil Nadu may not necessarily have the lowest price despite the greater quantities. Despite the bulk discounts, some drugs are cheaper in states with arguably inefficient centralised/predominantly centralised models like Odisha and Punjab and states with decentralised models like Maharashtra. Owing to the larger population and public preference for the government's health system and good health infrastructure, it is safe to assume that the quantities for procurement in Tamil Nadu would be significantly higher than in Odisha, Kerala or Punjab. Then the question that remains unanswered is how the other states are able to procure at prices lower than Tamil Nadu. The reasons could be many. For instance, supplier location—more than half of the suppliers to Tamil Nadu are from within the state. The same statistic for Kerala is 14%, for Maharashtra 34% and for Odisha, a surprising 0%! With insufficient data, we are unable to confidently conclude the financial burden of all the variants of the procurement models. But perhaps a good lead to follow is to think about what is causing unexpected discrepancies in prices.
Wastage elimination
Eliminating wastage of drugs (through mishandling or expiry) is necessary (but not sufficient) to optimise expenditure and ensure availability. Eliminating wastage is predicated upon effective inventory management, which deals with requirement gathering, analysing consumption patterns and forecasting demand. Trained pharmacists using weekly, quarterly and annual consumption data are supposed to estimate demand each year. However, in reality, the previous year's data are inflated by 10–15% in most states. In Orissa, however, owing to the lack of trained personnel, clerks/computer operators perform these tasks.
Kerala was able to mitigate this inaccuracy in estimation by introducing the option of issuing a second purchase order (PO). The initial PO given to the supplier is only for 75% of the tender quantity. The procurement authorities have the option to either not issue the second PO or issue it for 25% or 50% of the tender quantity, thereby building in a flexibility of 25%. All the other states have a rather static inventory management.
Furthermore, Kerala and Tamil Nadu use software tools to monitor stock levels and manage inventory and distribution. The warehouses in Punjab, Odisha and Maharashtra manage the inventory manually by recording data into ledgers. These systems are not designed to store all types of drugs in a scientific manner. These practices not only lead to wastage of material but also precious warehouse space (in case of oversupply).
Availability
In the centralised model of pooled procurement, the distribution is managed centrally and the onus of the procurement agency is to ensure availability at the user institutions. The public health centres in Punjab and Maharashtra are at the mercy of the suppliers, owing to their decentralised purchasing model, whose supply is often sporadic due to various reasons like delayed payments, lack of proper planning, etc. This impacts availability at the time of need and could potentially lead to wastage.
Quality
A procurement organisation has two levers to ensure that only quality drugs enter the system: (1) prequalification criteria to filter out unqualified suppliers and (2) external quality testing protocols. When these levers are used together, quality is ensured while still keeping the prices low. States that have stringent external quality testing protocols can afford to keep the minimum turnover criterion low. For instance, Tamil Nadu has empanelled laboratories to which every sample from each batch is sent for quality testing before distributing to user institutions and the minimum turnover criterion is set at US$0.7 million (INR 3 crores). Kerala too has similar quality testing protocols but has a higher minimum turnover criterion (set at US$2.1 million/INR 10 crores) to enforce faith in the public system. Odisha and Maharashtra do not have any quality testing protocols in place, apart from the supplier's internal quality certificate, and have therefore set the minimum turnover criterion at INR 10 crores, assuming that higher volumes are more likely to be generated by suppliers with high quality products.
Additionally, states that have external quality testing protocols also have policies that provide price relaxation to Small Scale Industries (SSIs) and Public Sector Undertakings to encourage local industry. Such preference treatment does not exist in Odisha or Punjab. Maharashtra reserves 20% of quantities for SSIs only if they match the L1 rates; thus, they do not get any price preferences.
An important aspect of the prequalification criteria is also the good manufacturing practices (GMP) certificate. This certificate ensures that the facility follows the stipulated guidelines according to the industrial benchmarks and thus can guarantee a certain level of quality. Maharashtra demands a WHO-GMP certificate, which is deemed to be more strict and is reviewed every 2 years.
Transparency
A public procurement system is accountable to various stakeholders, so it is important that transparency is maintained in all its activities. Certain conditions need to be established for a more open and efficient functioning. TNMSC and KMSCL are autonomous organisations that are headed by an appointed Director who may be a civil services officer with a very good technical and administrative background. The idea of having an autonomous organisation in the public sector is to enable it to function more transparently by avoiding the plausible procedural delays and also to enable it to make decisions of contracting and outsourcing that are best suited for the prosperity of the organisation. On the other hand, Odisha, Punjab and Maharashtra have procurement cells that are a part of the Directorate of Health Services in the state. A clear difference in the efficiency of the processes can be seen between the autonomous organisations and the state-run organisations—in terms of lead times for payments, quality control and in the usage of IT systems and so on. In an autonomous system, most of the staff are contractual based on their technical capabilities, which may not be the case in state-run procurement organisations.
A multistakeholdership in the organisation may be a useful tool for bringing in more transparency and representation, providing it is well coordinated. Right from the formation of the EDL to the award of the tenders, open and multistakeholder decision-making may help to keep the system more transparent. All the states under the purview of the study have a multistakeholder decision-making body.
It is deemed to be good practice to have a separate payment processing team from the tender award team in order to keep transactions more transparent. All the states make the payments based on the receipt of stock in the warehouse and a quality certificate (either internal or external). The processing of payments through the public channels like the Auditor General's Office or the Directorate of Accounts & Treasury usually takes much longer, as was noted in Maharashtra, Odisha and Punjab, compared with the autonomous payment departments of TNMSC and KMSCL.
Conclusion
In conclusion, we opine that the critical success factors of each model need to be carefully analysed to see if they are valid in the state contexts. It is important for policy makers to understand in detail the tangible and intangible aspects that go into running a successful model before trying to replicate it. Also, in some states, the existing structures may serve the purpose, but there may be a need to review several aspects of the current method of procurement, to make it more efficient. Sometimes, scrapping existing structures for new procedures may be a herculean task, which needs to be well thought out before undertaking. Based on the qualitative observations made, the authors assert that some of the critical success factors that define the success of any procurement system are: effective leadership and political support; multistakeholder participation for political buy-in; sufficient budget allocation to meet drug demand and administrative costs; outsourcing of non-core services like IT, quality testing, supply chain management, etc; autonomous procurement agency, well-defined and localised EDL; scientific demand estimation and forecasting; effective prequalification criteria to promote competition and enforce quality; protocols for regular inspection of supplier premises; mandatory external quality testing; prompt payment to suppliers; autonomous payment body; scientific warehousing and inventory management; real-time stock monitoring (both at the warehouse and facility levels) and robust IT systems.
Supplementary Material
Contributors: PVS was involved in the conceptualisation and study design and analysis of the findings. AT and RK was involved in the conceptualisation and study design, field data collection and analysis of the findings. MC was involved in the conceptualisation and study design and analysis of the findings. All authors read and approved the final manuscript.
Funding: Center for Health Market Innovations.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Right to information act: Right to Information Act 2005 mandates timely response to citizens’ requests for government information.
References
- 1.WHO The world medicines situation. WHO, 2004
- 2.UNDG Indicators for monitoring the millennium development goals New York: United Nations, 2003 [Google Scholar]
- 3.National Sample Survey Office National Sample Survey 60th Round New Delhi: Ministry of Statistics and Program Implementation, 2004–2005 [Google Scholar]
- 4.National Sample Survey Office National Sample Survey Round 52 New Delhi: Ministry of Statistics and Program Implementation, 1995–1996 [Google Scholar]
- 5.National Sample Survey Office National Sample Survey Round 42 New Delhi: Ministry of Statistics and Program Implementation, 1986–1987 [Google Scholar]
- 6.Cameron A, Ewen M, Ross-Degnan D, et al. Medicines prices, availability and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet 2009;373:240–9 [DOI] [PubMed] [Google Scholar]
- 7.Health, Nutrition, Population Sector Unit, India, World Bank India: raising the sights—better health systems for India's poor New Delhi, India: The World Bank, 2001
- 8.Ministry of Health and Family Welfare Report of the National Commission on macroeconomics and health New Delhi: Government of India, 2005 [Google Scholar]
- 9.Census Report New Delhi: Government of India, 2011 [Google Scholar]
- 10.National Sample Survey Office Level and pattern of consumer expensiture-66th Round New Delhi: Ministry of Statistics and Program Implementation, 2009–2010 [Google Scholar]
- 11.Ministry of Health and Family Welfare New Delhi, India: Bulletin on Rural Health Statistics in India, 2008
- 12.SRS Bulletin. 2010–2011. Sample Registration Survey, New Delhi, India.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.