Abstract
Background: Neoadjuvant anti-tumor activity of an alternating taxane- and anthracycline-based dose-dense regimen in patients with operable, noninflammatory large breast cancer was investigated.
Objective: The objective is to study the rate of pathological complete response in patients with breast cancer receiving dose-dense chemotherapy sequentially with gemcitabine plus docetaxel and vinorelbine plus epirubicin.
Methods: Women (n = 74) with clinical stage II or III breast cancer were enrolled in this open-label, multicenter study to receive six 2-weekly courses of gemcitabine 1000 mg/m2 plus docetaxel 75 mg/m2 on days 1 and 15, and vinorelbine 25 mg/m2 plus epirubicin 100mg/m2 on days 29 and 43. Patients with an objective response on day 56 then received another cycle of gemcitabine/ docetaxel on day 57 and of vinorelbine/epirubicin on day 71. Conservative surgery was scheduled for all patients.
Results: Of the patients enrolled, 30% had triple-negative breast cancer (TNBC). The pathologic complete response (pCR) rate was 22% overall, but was higher in TNBC than patients without TNBC (40.9% vs 14.0%; p=0.028). Among patients with a pCR, patients with TNBC had similar recurrence-free survival (RFS) and overall survival (OS) to patients without TNBC. Among those without a pCR, RFS rates for patients with TNBC were significantly lower than for patients without TNBC (p=0.04). The most common severe hematologic toxicity was neutropenia.
Conclusions: Administering four drugs in a dose-dense alternating sequence gave a high pCR in patients with operable, invasive breast cancer. Patients with TNBC with a pCR had similar OS to patients without TNBC, whereas patients with TNBC without a pCR had poorer survival rate than their non- TNBC counterparts.
Introduction
Neoadjuvant chemotherapy reduces tumor burden, thus allowing the possibility of breast-conserving surgery.[1,2] It is believed to treat clinically undetectable micrometastases[3] and allows for monitoring of tumor response to therapy.[4] Neoadjuvant therapy has recently become more commonly used in patients with larger tumors and/or lymph node involvement at diagnosis. Although no single chemotherapy regimen is specifically recommended over other regimens, regimens tend to be anthracycline-based with the addition of a taxane for high-risk, node-positive disease.
A pathologic complete response (pCR) rate of 26% was achieved in a small phase I/II study of docetaxel in combination with gemcitabine and epirubicin given as intensive (six cycles) neoadjuvant therapy in patients with early breast cancer, suggesting that this combination is highly active.[18] ]fore, the anti-tumor activity of gemcitabine plus docetaxel and of vinorelbine plus epirubicin given as neoadjuvant therapy to patients with large, operable, invasive breast tumors was investigated. To achieve appropriate dose intensity of each agent whilst limiting cumulative toxicity, an alternating sequence of these two combination regimens was designed, which were given every 2 weeks.
First, a pilot safety study of the regimen in 13 patients with breast cancer was conducted, which demonstrated promising efficacy (own unpublished data). Given these encouraging results, the current study was conducted. We propose this regimen to be referred to as GTEN (Gemzar® Taxotere® Epirubicin Navelbine®).
Materials and Methods
This was an open-label, prospective, phase II trial conducted in two centers in Paris, France. All patients provided written informed consent and the study was conducted with local Ethics Committee approval and in accordance with Good Clinical Practice and the Declaration of Helsinki.
Patients
Women aged 18–75 years with chemotherapy-naïve, histologically-proven breast adenocarcinoma with a clinically-determined tumor size of ≥3 cm or local lymph node involvement, assessed clinically, and adequate blood cell counts, liver function, renal function and cardiac function were enrolled. Patients were excluded if they had inflammatory breast cancer, known overexpression (an immunohistochemical [IHC] score of ≥2+) of HER2 or metastatic disease.
Study Protocol
Patients received between four and six 2-week cycles of chemotherapy, starting with two cycles of docetaxel 75 mg/m2 plus gemcitabine 1000 mg/m2 (cycle 1 on day 1 and cycle 2 on day 15) followed by two cycles of vinorelbine 25 mg/m2 plus epirubicin 100 mg/m2 (cycle 3 on day 29 and cycle 4 on day 43). ]after, patients without an objective response (determined clinically or radiologically), underwent surgery with axillary lymph node dissection; those with an objective response received one cycle of docetaxel plus gemcitabine at the same dose (cycle 5 on day 57) followed by one cycle of vinorelbine plus epirubicin at the same dose (cycle 6 on day 71). For patients who had received six cycles, at the final assessment on day 85, patients without an objective response were treated off-protocol whilst those with an objective response underwent surgery with axillary lymph node dissection. Thus, all patients in the study were scheduled to undergo conservative surgery. Figure 1 summarizes the protocol description.
Fig. 1.
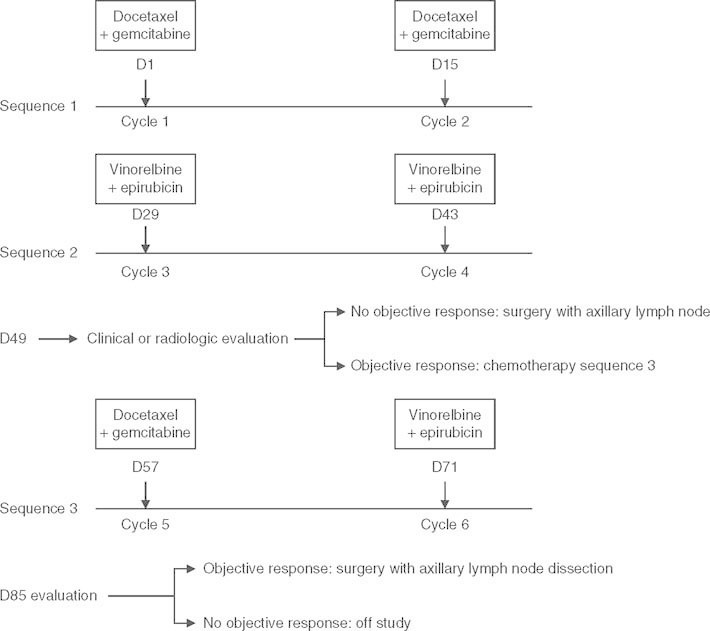
The timing of chemotherapy administration and evaluation of tumor response. D = day.
Treatment was delayed by 1 week for hematologic toxicity, defined as an absolute neutrophil count of <1.0 × 109/L and/or platelet count of <100 × 109/L in the 24 hours prior to treatment.
All patients received filgrastim 5 μg/kg subcutaneously from the 5th to 8th day after each chemotherapy infusion.
Planned Interim Analyses
Treatment response was assessed at two interim analyses (based on the Simon two-stage minimax design[19] ) as follows: if more than three complete pathologic responses were observed in the first 21 evaluable patients at the first analysis, then a further 28 patients were enrolled. If the cumulative total number of pathologic responses was ≥11 at the second analysis of evaluable patients (of the 49 enrolled), the treatment was considered effective and enrollment could continue. If at either analysis these criteria were not met, the study would be discontinued.
Clinical Response Criteria
The pCR was determined by microscopic examination of the excised tissues from the breast and regional lymph nodes, and was defined as no residual disease in either tissue. A lymph node response was defined as an absence of axillary lymph node involvement as determined by microscopic examination of excised tissue after treatment (pN-) in patients who had lymph node involvement at baseline (N+). The objective response by clinical or radiological criteria was determined according to Response Evaluation Criteria in Solid Tumors (RECIST 1.0).[20]
Efficacy Endpoints and Assessments
The primary efficacy endpoint was the proportion of patients with a pCR. Secondary efficacy endpoints included overall survival (OS).[defined as the time from tumor diagnosis to death], recurrence-free survival (RFS).[defined as patients alive and without recurrent disease as from the first day of tumor diagnosis to the time of first local or metastatic recurrence or tumor-related death], and the rate of metastatic and local recurrence. Exploratory analyses included the proportion of patients with residual disease, an analysis of lymph node response by baseline lymph node involvement, and IHC analyses of tissue from the initial biopsy for ER, PR, and HER2 status.[21] ER (clone 6F11, diluted 1 : 30) and PR (clone PGR-312, diluted 1 : 100) monoclonal antibodies were purchased from Novocastra (Newcastle upon Tyne, UK), and HER2 (clone A0485, diluted 1 : 600) from Dako (Glostrup, Denmark). An exploratory analysis was also conducted for treatment response and survival parameters in TNBC and non-TNBC.
Clinical tumor evaluation, breast ultrasound, and mammography were performed after the fourth and sixth cycles of chemotherapy.
Safety and Laboratory Assessments
Patients had a complete laboratory and vital sign (temperature, pulse, blood pressure) assessment 1 week prior to study inclusion and also during the study. Tests performed included a weekly complete blood count, vital signs measurement on treatment administration days, and blood biochemistry before each cycle, and measurement of cardiac parameters by ultrasound or scintigraphy after two cycles and at the end of every cycle ]after. Adverse events and toxicities were assessed at every cycle according to National Cancer Institute Common Toxicity Criteria version 2.[22]
Statistical Methods
To achieve a statistical power of 80%, assuming a minimum acceptable pCR rate of 15% and that 85% of patients would be evaluable, enrollment of 57 patients was required (in two stages, see the ‘Planned Interim Analyses’ section). The intent-to-treat (ITT) and safety populations included patients who received at least one cycle of study drug, and the per-protocol population included those evaluable for response and who received the scheduled study medication (at least three cycles of study drug).
Categorical variables were compared using the Chi-squared test or the Fisher exact test and continuous variables were compared using ANOVA between TNBC and non-TNBC.
We compared Kaplan-Meier curves for OS and RFS with the log-rank test for several variables (e.g. age, tumor size, positivity of lymph node, clinical stage, histologic grade, positivity of hormone receptors, TNBC subtypes, pCR). Unadjusted hazard ratios (HR) were calculated for each studied variable. All p-values were two-sided. Analyses were undertaken with Stata 9.2 (Stata corporation).
Results
Seventy-four patients were enrolled between December 2002 and December 2006, and all were included in the ITT and safety populations. One patient received chemotherapy but refused surgery, thus ] were 73 patients in the per-protocol population. Patient baseline demographic and disease characteristics are given in table I. Most patients had invasive ductal tumors (89%), tumor-node-metastasis (TNM) clinical stage II (81%), and Elston and Ellis histologic grade II (44%) or III disease (23%). Contrary to enrollment criteria, a small proportion of patients with HER2-positive (HER2+) disease were enrolled (two patients expressed ++ HER2). Twenty-two patients had TNBC and 51 had non-TNBC (data missing for one patient). Histologic grade was significantly higher in TNBC than patients without TNBC (p = 0.001).
Table I.
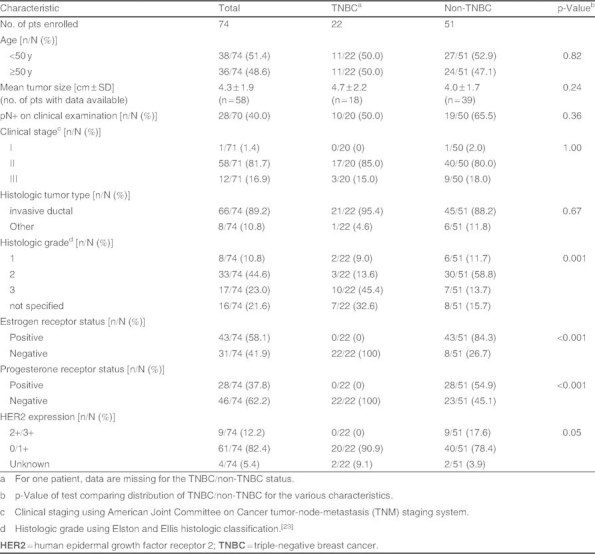
Patient (pt) clinical characteristics
Treatment
Three patients had at least three cycles of chemotherapy. Five patients received the first four cycles of chemotherapy but proceeded to surgery ]after because they did not have an objective response at the first assessment. Sixty-five patients received more than four cycles, of whom 35 underwent mastectomy and 30 breast-conserving surgery; all patients had lymph node dissection. Eight patients discontinued chemotherapy because of adverse events and ]fore did not receive all six chemotherapy cycles. At the final assessment (day 85), only one patient was without an objective response and was treated off-protocol by surgery.
Mean cumulative doses were docetaxel 215 mg/m2 (95% of planned dose), gemcitabine 2878 mg/m2 (95% of planned dose), vinorelbine 68 mg/m2 (90% of planned dose), and epirubicin 275 mg/m2 (91% of planned dose).
All patients (n = 32) who underwent tumorectomy received radiotherapy, as well as those who underwent mastectomy with T3 or greater tumor size or with three or more lymph node involvement. No further chemotherapy was prescribed. All patients with positive ER and/or PR received hormonal therapy. Two patients with HER over-expression received adjuvant trastuzumab.
Treatment Response
A pCR was observed in 21.9% of patients (table II). Of the 28 of 70 patients who had positive lymph nodes on clinical examination, over one-third of patients had a lymph node response. Of note, pCR occurred in significantly more TNBC than patients without TNBC (40.9% vs 14.0%; p = 0.028). The corresponding proportion of patients with residual disease was ]fore significantly lower in the TNBC than non-TNBC group (p = 0.028). More patients with a lymph node response tended to have non-TNBC than TNBC disease (44.4% vs 20.0%), although this difference was not significant.
Table II.
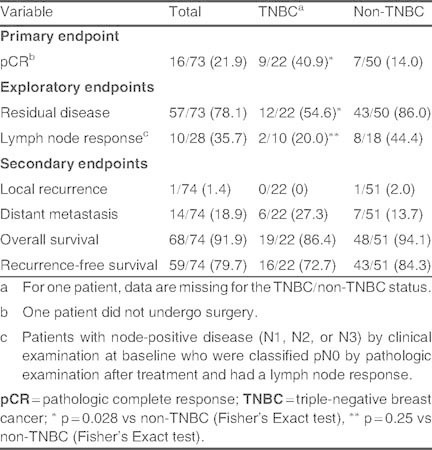
Treatment response and patient outcomes [n/N (%)]
Most of the patients with no lymph node involvement prior to neoadjuvant therapy remained node-negative at surgery (35 of 42 patients). Seven patients with clinically lymph node negative at baseline, had lymph node positive, diagnosed pathologically, after surgery. Of the 28 patients with node-positive disease prior to treatment, ten had no evidence of disease at surgery (table III). Moreover, the proportion of patients who experienced a local recurrence was very low (1.4%), and distant metastases were reported in 19% of patients after neoadjuvant therapy (table II). The difference between patients with TNBC and non-TNBC for these two endpoints was not significant.
Table III.
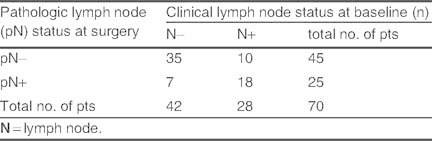
Lymph node response to treatment by baseline lymph node status in evaluable patients (pts).[n = 70]
Survival Parameters
After a median follow-up of 3.18 years, OS and RFS rates were 91.9% and 79.7%, respectively (table II). The 3- and 5-year estimates of OS were 95% and 81%, respectively, and of RFS were 84% and 65%, respectively. Notably, ] were no statistically significant differences between patients with TNBC or with non-TNBC in terms of survival parameters (table II).
We conducted a univariate analysis to identify prognostic factors for OS and RFS (table IV). Baseline lymph node involvement or advanced disease were prognostic of poorer RFS, and baseline high clinical stage was prognostic of poorer OS. However, pCR did not appear to be a prognostic factor for either RFS or OS.
Table IV.
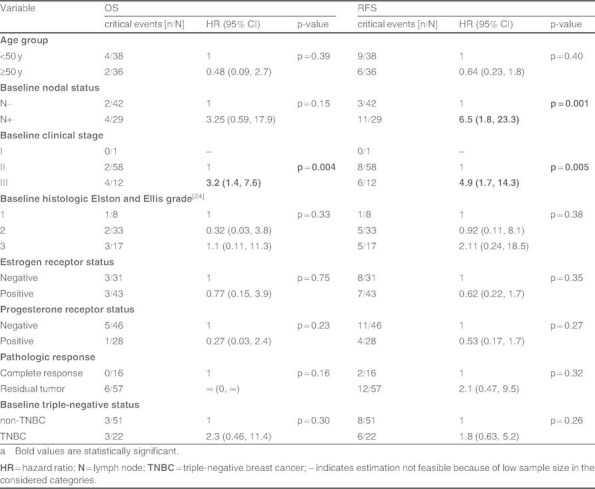
Unadjusted analysis using the Cox’s model for overall survival (OS) and recurrence-free survival (RFS) of prognostic factors in the intent-to-treat population (n = 74)a
In patients with TNBC achieving a pCR, OS was similar to that of patients without TNBC with a pCR (5-year estimates of OS = 100% for both). Among patients without a pCR, ] was a trend towards lower OS in patients with TNBC than non-TNBC (3- and 5-year OS estimates were 84% and 42% for TNBC, and 97% and 82% for non-TNBC; p = 0.07) but the difference was not significant. Similarly, among patients with a pCR, the RFS of patients with TNBC was no different from that in patients with non-TNBC (3- and 5-year RFS estimates were 100% and 75% for TNBC and, 100% and 67% for non-TNBC; HR 0.6; 95% CI 0.03, 8.8; Log rank test: p = 0.66). Notably, however, of those patients without a pCR, RFS rates were significantly lower in patients with TNBC than those with non-TNBC (3- and 5-year RFS estimates were 62% and 62% for TNBC, and 89% and 79% for non-TNBC; HR 3.3; 95% CI 0.99, 10.8; Log rank test: p = 0.04).[figure 2].
Fig. 2.
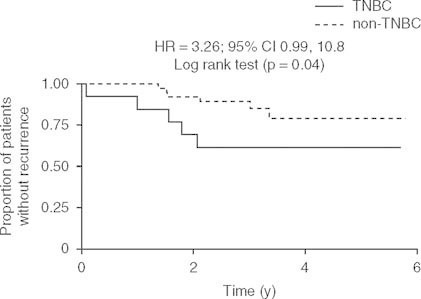
Recurrence-free survival in patients with breast cancer without a pathologic complete response after neoadjuvant treatment with gemcitabine plus docetaxel alternating with vinorelbine plus epirubicin according to triple-negative status. HR = hazard ratio; TNBC = triple-negative breast cancer.
Safety
Chemotherapy was discontinued before all six cycles were administered because of toxicity of at least grade 3 severity in four patients and of grade 1 or 2 severity in a further four patients, but all eight received surgery as per the study protocol (after a median of four [range of two to five] cycles of treatment).
Treatment-emergent adverse events are summarized in table V, of which neutropenia was the most frequently reported. Grade 4 febrile neutropenia occurred in two patients. Two patients experienced grade 2 peripheral neuropathy related to docetaxel treatment. Interstitial pneumopathy of grade 3 severity occurred in one patient and was considered to be related to docetaxel (table V), but did not lead to respiratory failure. Only one patient developed a grade 3 cardiotoxicity (acute heart failure), which was considered to be related to epirubicin.
Table V.
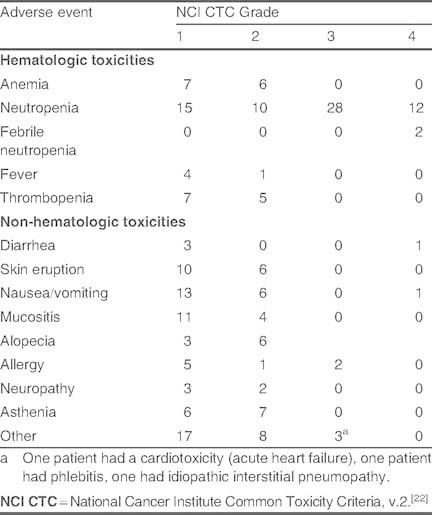
No. of treatment-emergent adverse events in the safety population (n = 74)
Treatment was delayed by 1 week for 46 patients (62%).
In addition, about one-fifth of patients had more than one adverse event of at least grade 3 severity (n = 14) and one-half of patients experienced at least one such adverse event (n = 39). Four patients experienced serious treatment-related adverse events; two cases of febrile neutropenia, one case of allergic reaction, and one case of pneumonia (the latter related to docetaxel treatment). ] were no treatment-related deaths.
Discussion
Our patients had operable, non-inflammatory, clinical stage II–III breast cancer, of whom most had HER2-negative (HER2-) disease. After treatment with two to three cycles each of alternating gemcitabine plus docetaxel followed by vinorelbine plus epirubicin, about one-fifth of patients had a complete response and survival rates were high (median follow-up 3.18 years).
The pCR rate falls within the range reported in other neoadjuvant chemotherapy trials in patients with operable breast cancer (6–29%).[4] is similar to that reported after the commonly used neoadjuvant combination of docetaxel plus doxorubicin plus cyclophosphamide in a similar patient population (21%).[17] and is higher than that reported for concomitantly administered neoadjuvant combination regimens using a 3- or 4-weekly dosing schedule (12–15%).[3,25,26] However, caution should be applied when making such comparisons because of differences in patient populations and in definitions of pCR across studies.
Recent studies assessing sequential and/or alternating chemotherapy have generally been in the adjuvant setting.[16,27] In one study, investigators concluded that sequential chemotherapy allows dose intensity to be maintained,[16] and our study results support this. Our use of a dose-dense regimen is, in turn, supported by their results with a combination of doxorubicin, cyclophosphamide, and paclitaxel given in a 2-weekly regimen that did not adversely affect the risk of local recurrence of disease and significantly (p < 0.05) improved OS and disease-free survival compared with a 3-weekly regimen.[16] No such therapeutic advantage was shown in a neoadjuvant setting comparing dose-dense epirubicin plus cyclophosphamide with standard fluorouracil plus epirubicin plus cyclophosphamide in patients with locally advanced or inflammatory breast cancer.[28] However, our dose-dense regimen was associated with good OS and RFS, and local disease recurrence occurred only in 1.4% of patients.
In our exploratory analysis, baseline clinical stage was prognostic of OS and RFS, baseline tumor grade of OS, and baseline lymph node status of RFS. We determined nodal response, which was observed in 36% of our patients. This good lymph node response may have contributed, in part, to the good survival outcomes in our study, because negative axillary nodes after neoadjuvant therapy predict favorable long-term outcomes.[23]
Approximately one-third of our patients had TNBC, which is higher than expected since TNBCs typically comprise 15% of breast cancer cases.[15] Our enrollment was slightly skewed in favor of TNBC because we aimed to exclude patients with HER2+ disease, yet we did not select based on hormone receptor status in addition to this (i.e. we did not preselect for TNBC). We postulate that due to the aggressive nature of TNBC, and that these tumors are often larger,[6–8] patients with TNBC are more likely to be candidates for neoadjuvant therapy. To our knowledge, ] is no direct evidence in the literature to support this, but two studies in patients with stage II–III breast cancer receiving neoadjuvant therapy reinforce our hypothesis because they too reported about one-third of patients with TNBC.[10,13]
It has been suggested that patients with TNBC are candidates for more aggressive chemotherapy.[29] Our dose-dense alternating sequence of anthracycline- and taxane-based combinations showed significantly better activity in patients with TNBC than in those with non-TNBC (pCR 40.9% vs 14.0%; p = 0.028). Our results are concordant with several studies demonstrating a better response to neoadjuvant therapy in TNBC than non-TNBC.[10,11,13,14] The anti-tumor activity of our regimen in patients with TNBC was also associated with improved long-term prognosis approaching that of patients without TNBC; between-group differences in OS or RFS were not significant. In contrast to our results, other studies have found that despite a higher pCR in patients with TNBC, the risk of death and relapse was significantly higher in TNBC than patients without TNBC,[10,11] or in TNBC (basal-like tumor subtype) than in luminal tumor subtypes.[13] Progression-free survival was 63% in TNBC versus 76% in non-TNBC (HR 1.86; 95% CI 1.39, 2.50) after neoadjuvant therapy in one such study,[11] but importantly, this difference was no longer apparent after more than 3 years of follow-up (corresponding to our median follow-up time). Our exploratory analyses revealed that improved survival in patients with TNBC was exclusively in those who had a pCR. Amongst patients without a pCR, the prognosis of patients with TNBC was significantly (p = 0.04) poorer than patients without TNBC (figure 2). These observations are concordant with previous reports;[11,13] for example, Carey et al.[13] found that patients with basal-like tumors with residual disease have higher relapse rates and poorer prognosis than those without basal-like tumors who did not achieved a pCR after anthracycline-based neoadjuvant therapy.
Numerous study limitations must be considered when interpreting our results, including the small patient population, relatively short follow-up, lack of molecular/hormone receptor data for all patients, and that the study was powered to examine pCR, making all other analyses exploratory in nature. Our inclusion criteria were not strictly adhered to, as nine patients were HER2+. Despite these limitations, we were able to demonstrate that a dose-dense taxane-based regimen, with therapy delivered over a shorter total period of time, allows for sequential administration of a dose-dense anthracycline-based regimen, which may help to limit resistance.[15]
Only one patient experienced a severe treatment-related cardiotoxic adverse event. During follow-up, ] was no late cardiotoxicity, secondary acute myelogenous leukemia, or myelodysplastic syndrome; however, longer follow-up may be required before such adverse events become apparent. No patients experienced severe neuropathy and ] were only two serious or severe treatment-related pulmonary adverse events (grade 3 interstitial pneumopathy and serious pneumonia). As expected, neutropenia was the most frequently reported toxicity overall, and the most common severe adverse event despite filgrastim support. However, no patients were hospitalized for neutropenia or febrile neutropenia. We conclude that overall, the majority of adverse events were manageable and, given that ] were no treatment-related deaths, this four-drug regimen was associated with an acceptable tolerability profile.
Conclusion
A regimen of dose-dense combinations of gemcitabine plus docetaxel and vinorelbine plus epirubicin, given in an alternating sequence was associated with favorable survival rates in patients with TNBC, but only in those patients with a pCR. Dose intensity was conserved with just three cycles of each combination to produce a good pCR rate and an acceptable tolerability profile. We recommend that this regimen be further explored in a prospective study in patients with TNBC.
Acknowledgements
We thank Tracy Harrison and Jenna Mitchell of inScience Communications, a Wolters Kluwer business, who provided medical writing and journal styling assistance, respectively, which was funded by Association pour la Recherche sur les Therapeutiques Innovantes en Cancerologie, Paris, France.
The study was sponsored by Association pour la Recherche sur les Therapeutiques Innovantes en Cancerologie, Paris, France. The study was independently designed, conducted, and managed. Data were also independently collected and analyzed. Preparation, review, and approval of the manuscript were done by all authors, independently of the sponsor.
All authors certify that no actual or potential conflict of interest in relation to this article exists, notably any financial or personal relationships with other people or organizations that could inappropriately influence (bias) their work.
References
- 1.Bafaloukos D. Neo-adjuvant therapy in breast cancer. Ann Oncol. 2005;16(Suppl.2):ii174–81. doi: 10.1093/annonc/mdi704. [DOI] [PubMed] [Google Scholar]
- 2.Hennessy B., Hanrahan E., Valero V. Neoadjuvant therapy of breast cancer. Am J Cancer. 2006;5:411–25. doi: 10.2165/00024669-200605060-00007. [DOI] [Google Scholar]
- 3.Van Praagh I., Cure H., Leduc B., et al. Efficacy of a primary chemotherapy regimen combining vinorelbine, epirubicin, and methotrexate (VEM) as neoadjuvant treatment in 89 patients with operable breast cancer. Oncologist. 2002;7:418–23. doi: 10.1634/theoncologist.7-5-418. [DOI] [PubMed] [Google Scholar]
- 4.Mieog J., van der Hage J., van de Velde C. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;2:CD005002. doi: 10.1002/14651858.CD005002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dent R., Trudeau M., Pritchard K.I., et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z., Liu G., Yang W., et al. Triple-negative breast cancer types exhibit a distinct poor clinical characteristic in lymph node-negative Chinese patients. Oncol Rep. 2008;20:987–94. [PubMed] [Google Scholar]
- 7.Rakha E.A., El-Sayed M. G., et al. Prognosticmarkers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 8.Tian X.S., Cong M.H., Zhou W.H., et al. Clinicopathologic and prognostic characteristics of triple-negative breast cancer. Onkologie. 2008;31:610–4. doi: 10.1159/000162288. [DOI] [PubMed] [Google Scholar]
- 9.Carey L.A., Perou C.M., Livasy C.A., et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 10.Keam B., Im S.A., Kim H.J., et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007;7:203. doi: 10.1186/1471-2407-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liedtke C., Mazouni C., Hess K., et al. Response to neoadjuvant therapy and long-term survival in patients with triplenegative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Pinilla S.M., Sarrio D., Honrado E., et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12:1533–9. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 13.Carey L.A., Dees E.C., Sawyer L., et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 14.Rouzier R., Perou C.M., Symmans W.F., et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–85. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 15.Cleator S., Heller W., Coombes R.C. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–44. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 16.Citron M.L., Berry D.A., Cirrincione C., et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–9. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G., Kummel S., Vogel P., et al. Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst. 2008;100:552–62. doi: 10.1093/jnci/djn089. [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss A., Huober J., Sinn H.P., et al. Gemcitabine, epirubicin and docetaxel as primary systemic therapy in patients with early breast cancer: results of a multicentre phase I/II study. Eur J Cancer. 2004;40:2432–8. doi: 10.1016/j.ejca.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.European Organisation for Research and Treatment of Cancer. RECIST version 1.0. 2000 [online]. Available from URL: http://www.eortc.be/Recist/Default.htm [Accessed 2009 Dec 17]
- 21.Hammond M.E.H., Hayes D.F., Dowsett M., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2009;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute. Common Toxicity Criteria Manual 1 June 1999 version 2.0 [online]. Available from URL: http://www.ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf [Accessed 2008 Dec 17]
- 23.Rastogi P., Anderson S.J., Bear H.D., et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 24.Robbins P., Pinder S., de Klerk N., et al. Histological grading of breast carcinomas: a study of interobserver agreement. Hum Pathol. 1995;26:873–9. doi: 10.1016/0046-8177(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 25.Chua S., Smith I.E., A’Hern R.P., et al. Neoadjuvant vinorelbine/epirubicin (VE) versus standard adriamycin/cyclophosphamide (AC) in operable breast cancer: analysis of response and tolerability in a randomised phase III trial (TOPIC 2) Ann Oncol. 2005;16:1435–41. doi: 10.1093/annonc/mdi276. [DOI] [PubMed] [Google Scholar]
- 26.Conte P.F., Donati S., Gennari A., et al. Primary chemotherapy with gemcitabine, epirubicin and taxol (GET) in operable breast cancer: a phase II study. Br J Cancer. 2005;93:406–11. doi: 10.1038/sj.bjc.6602723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fountzilas G., Skarlos D., Dafni U., et al. Postoperative dosedense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with highrisk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16:1762–71. doi: 10.1093/annonc/mdi366. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P., Mauriac L., Welnicka-Jaskiewicz M., et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a doseintensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 2003;21:843–50. doi: 10.1200/JCO.2003.05.135. [DOI] [PubMed] [Google Scholar]
- 29.Mehta R.S. Dose-dense and/or metronomic schedules of specific chemotherapies consolidate the chemosensitivity of triple-negative breast cancer: a step toward reversing triple-negative paradox [letter] J Clin Oncol. 2008;26:3286–8. doi: 10.1200/JCO.2008.17.1116. [DOI] [PubMed] [Google Scholar]


