Abstract
Background
Osteochondritis dissecans (OCD) has been defined as a localized process in which a focus of subchondral bone and adjacent articular cartilage separates from the surrounding bone. With the knee being the most common location for OCD development and the propensity for this lesion to be found in those who participate in sports, a repetitive microtrauma hypothesis for its cause has gained favor. However, the cause of OCD remains controversial, as does the most appropriate treatment for the varying degrees of OCD lesions.
Case Description
We present a unique case of three OCD lesions in one knee. The patient was a young, athletic boy who developed three separate OCD lesions in his right knee over the course of 4 years. Temporally, the OCD lesions developed first in the lateral femoral condyle, then in the medial femoral condyle, and finally in the trochlea.
Literature Review
Our literature review yielded a few reports of bicondylar OCD lesions. We identified no previous reports of three separate OCD lesions found in a single joint.
Purposes and Clinical Relevance
This report illustrates how a uniquely affected knee with three OCD lesions was treated in three different ways with resolution of symptoms. Each of the OCD lesions was evaluated individually and treatment for each based on the severity of the lesion from the physical examination, imaging studies, and arthroscopic findings.
Introduction
Osteochondritis dissecans (OCD) is an uncommon joint condition most commonly affecting the knee, which is the joint implicated in almost 75% of cases [7]. Reported incidence rates based on knee radiographs vary widely, from only 0.0003% [9] to as high as 4% [2]. Yearly incidence of OCD is reportedly between 0.02% and 0.03% [4] with a ratio of 2:1 when comparing rates of males to females [9]. The recently noted increase in incidence rates and the decrease in the male-to-female ratio have been attributed to the growing popularity of youth sports for both sexes [2].
Bilateralism is reported in 15% [4] to 40% [16] of patients with OCD lesions of the femoral condyle. Multiple lesions within one knee, on the other hand, are extraordinarily rare. There have only been two reported cases of such bicondylar defects [7]. We describe a unique case of a patient who developed three separate OCD lesions within the same knee: one lesion of the lateral femoral condyle (LFC), one lesion of the medial femoral condyle (MFC), and one lesion of the trochlea.
Case Report
A 15-year-old boy had been experiencing intermittent right knee pain for several years. At age 11 years, the patient presented to another orthopaedic surgeon with lateral knee pain. He was first diagnosed with an OCD lesion of the LFC. He was treated with nonweightbearing for 10 weeks. Subsequently, he wore a hinged knee brace (varus unloader) for 2 years. Radiographs from this time period suggested healing of this lesion as the radiodensity of the progeny fragment gradually increased to the radiodensity of the parent bone (Fig. 1). His pain resolved. He played basketball and baseball (except catcher) without difficulty. At age 15 years 6 months, about 1 month before presentation to the senior author (JLC), the patient began to experience popping, swelling, and a sense of instability. At this point, the knee pain localized primarily medially. The symptoms were aggravated by cutting and pivoting maneuvers. On physical examination, right knee ROM was 0° to 130°. There was a small effusion. Radiographs of the right knee demonstrated an OCD lesion of the MFC (Fig. 2A). The boundary between the progeny fragment and parent bone was radiolucent and distinct. The distal femoral and proximal tibial physes remained open. MRI of the right knee again demonstrated an OCD lesion of the MFC (Fig. 2B–C). There was a change in articular cartilage contour at the margin of the lesion. There was curvilinear high signal at the boundary between the progeny fragment and parent bone, which possibly extended to the articular surface. The normalized length of this lesion was 0.35 (26 mm/75 mm), and the normalized width was 0.27 (26 mm/97 mm). Of note, this lesion of the MFC could be seen on the previous radiographs (Fig. 1).
Fig. 1A–C.
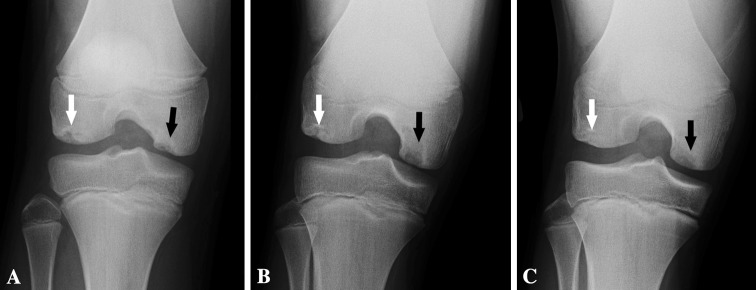
AP radiographs demonstrate healing of LFC OCD lesion (white arrows) at (A) age 12 years 9 months, (B) 13 years 1 month, and (C) 13 years 5 months. Of note, an OCD lesion of the MFC can also be seen in these images (black arrows), which appears to become less radiodense and to enlarge somewhat over this time period.
Fig. 2A–C.
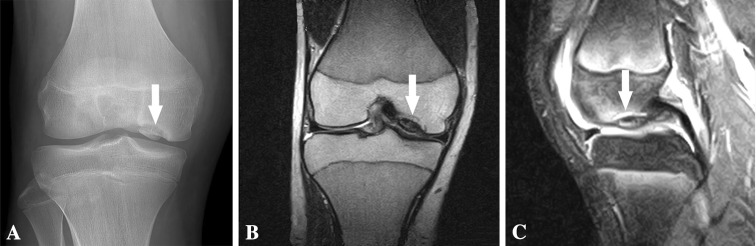
(A) A preoperative AP radiograph and (B) coronal and (C) sagittal MR images at age 15 years 7 months show the MFC lesion (arrows).
In addition, MRI revealed an approximately 32-mm proximal-distal and 22-mm medial-lateral OCD lesion of the lateral aspect of the trochlea (Fig. 3A–B). There was a 3- to 4-mm bony deficit between the progeny fragment and parent bone. Interestingly, this defect was likely to have developed between ages 14 and 15 years, since it was not visualized on the prior lateral radiographs (Fig. 3C) but was easily visualized on the radiographs at presentation (Fig. 3D).
Fig. 3A–D.
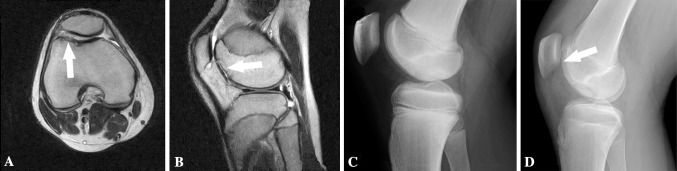
(A) Axial and (B) sagittal MR images show an OCD lesion of the trochlea (arrows) at 15 years 7 months. (C) A lateral radiograph demonstrates no appreciable OCD lesion of the trochlea at 13 years 9 months. (D) A lateral radiograph shows an OCD lesion of the trochlea (arrow) at age 15 years 7 months.
The OCD lesion on the MFC did not appear stable, due primarily to the curvilinear high signal at the boundary between the progeny fragment and parent bone, which possibly extended to the articular surface. Unstable OCD lesions are generally regarded to have a lower healing potential than stable OCD lesions. Therefore, the family was told the probability of healing the MFC lesion and trochlea lesion with nonoperative management was likely substantially less than 10%. Consequently, the surgeon discussed with the family the risks, benefits, and alternatives to surgical arthroscopy of the right knee with evaluation of the stability of both OCD lesions, possible biopsy of cartilage (for possible subsequent autologous chondrocyte implantation), and possible drilling, as well as possible right distal femur open repair of OCD nonunion with ipsilateral proximal tibia autogenous bone graft and internal fixation. The family decided to proceed with this treatment course.
With respect to the OCD lesion of the LFC, arthroscopic evaluation revealed no notable chondral defects (Fig. 4). Specifically, there was no appreciable evidence of the healed OCD lesion of the LFC.
Fig. 4.
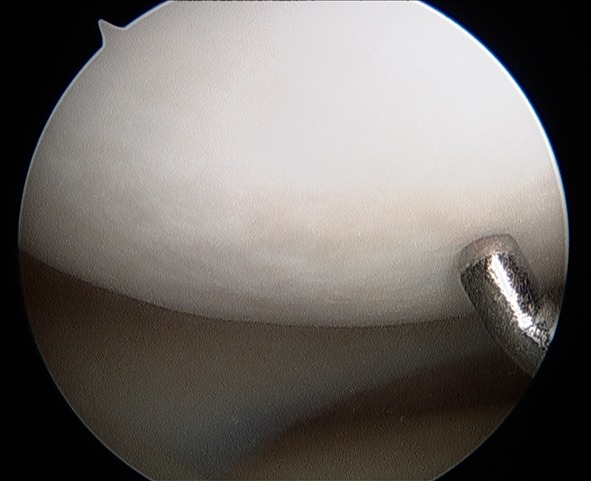
An arthroscopic image demonstrates no appreciable defect and no unstable region of the LFC at age 15 years 9 months.
With respect to the OCD lesion of the MFC, arthroscopic evaluation revealed an approximately 25-mm-diameter OCD lesion on the lateral aspect of the extension surface of the MFC (Fig. 5). There was a definite change of the articular cartilage contour at the margin of this lesion. The lesion was ballotable anteriorly and medially (Fig. 5A). An arthrotomy was sharply established from the proximal aspect of the patella to the superior aspect of the medial meniscus. A freer was placed along the medial margin of the OCD lesion. The lesion easily opened like a trap door, remaining hinged laterally (Fig. 5B). The fibrous tissue at the interface of the progeny and parent bone was removed. The sclerotic rims of the progeny fragment and of the parent base were removed with a bur. The margins of the base of the lesion were drilled with a 2-mm pin to stimulate healing. Autogenous bone graft was then placed under the OCD trap door (Fig. 5B). The trap door was closed. The OCD fragment was secured with two variable-pitch screws (Herbert/Whipple® bone screws; Zimmer, In, Warsaw, IN, USA) (Fig. 5C). Fibrin sealant (Tisseel®; Baxter International Inc, Deerfield, IL, USA) was applied to the articular cartilage interface of the parent and progeny to seal the healing bone from the synovial fluid (Fig. 5D).
Fig. 5A–D.
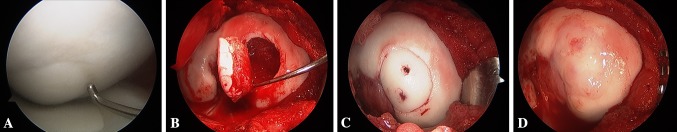
Arthroscopic images show (A) an unstable, ballotable 20-mm medial-lateral by 30-mm proximal-distal MFC lesion at age 15 years 9 months, (B) placement of bone graft behind the trap door of the progeny fragment, (C) fixation of the fragment with variable-pitch screws, and (D) placement of fibrin sealant at the lesion periphery and screw heads.
With respect to the OCD lesion of the trochlea, arthroscopic evaluation revealed a 20-mm medial-lateral by 30-mm proximal-distal OCD lesion of the lateral aspect of the trochlea. The articular cartilage was grossly unstable yet hinged medially (Fig. 6A–B). This cartilage was determined to be nonfunctional and nonsalvageable. The chondral fragments were resected and the fibrous tissue was curetted until healthy bone was visualized circumferentially. Subsequently, the drill was utilized to create multiple holes in the parent bone of sufficient depth to allow marrow cells to enter into the defect. The bony defect was most pronounced inferiorly (approximately 5 mm deep). This region was packed with autogenous bone graft (Fig. 6C). Fibrin sealant (Tisseel®) was carefully applied to the bone graft to seal the healing bone from the synovial fluid and prevent migration of the autograft material.
Fig. 6A–C.
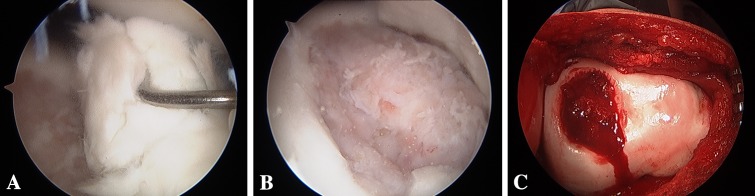
Arthroscopic images depict (A, B) an unstable OCD lesion of the trochlea with complex, multidirectional cartilage fissuring at age 15 years 9 months (C) followed by excision of unsalvageable progeny fragment, placement of autogenous bone graft, and placement of fibrin sealant.
In light of the size and location of the OCD lesion of the trochlea, autologous chondrocyte implantation was considered to be a possible salvage procedure in case of clinical failure of the drilling and bone grafting. Two full-thickness articular cartilage biopsies were harvested and sent to the proper destination.
The patient strictly adhered to the postoperative protocol for patellofemoral cartilage repair. Specifically, he ambulated with crutches and a knee brace locked in full extension (to avoid shear stress across the patellofemoral joint) for 6 weeks. The weightbearing progression involved nonweightbearing for 4 weeks, then partial weightbearing for 2 weeks, and full weightbearing after 6 weeks. ROM was limited as follows: 0° to 30° for 2 weeks, 0° to 90° for 2 weeks, and then 10° of additional flexion per week. At 6 months postoperatively, the patient was permitted to return to low-impact activities (including brisk walking and swimming). At 9 months, he was permitted to return to higher-impact activities (including jogging and then running).
At 1-year followup, the patient reported he was doing well in general. He experienced essentially no pain. The preoperative pain completely resolved. The knee was gliding well. The patient denied any swelling. He was running and hitting baseballs without difficulty. On physical examination, right knee ROM was 0° to 135°. There was no point tenderness. There was no appreciable effusion. Radiographs of the right knee demonstrated healing of the MFC OCD lesion, with the boundary between the progeny fragment and parent bone nearly nondistinct (Fig. 7). In addition, the radiodensity of the subchondral bone of the trochlear OCD was the same as the surrounding parent bone.
Fig. 7A–D.
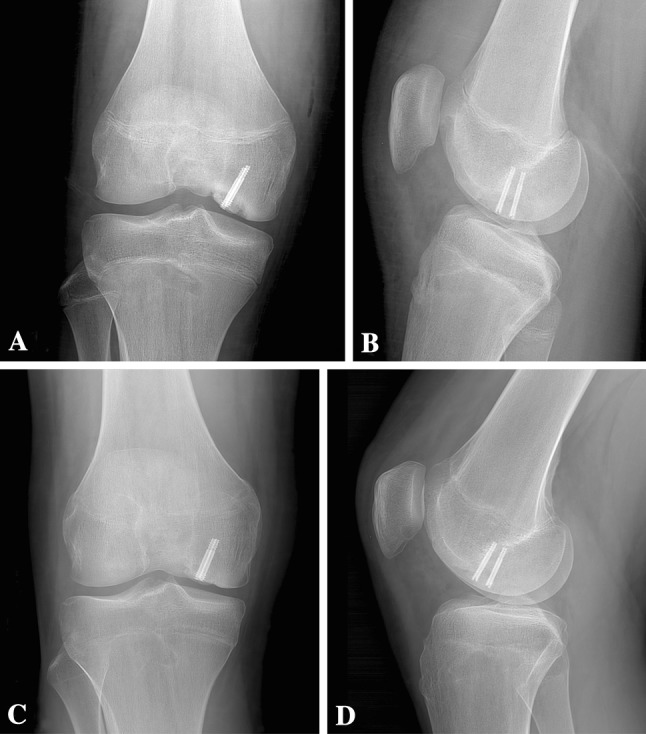
Radiographs at (A) age 15 years 9 months (immediately postoperatively) and (B) 1 week postoperatively confirm fragment fixation of the MFC OCD with the two variable-pitch screws. The trochlear OCD remains visible. Followup radiographs at (C) age 16 years 5 months and (D) 16 years 8 months demonstrate healing of the MFC OCD lesion, with the boundary between the progeny fragment and parent bone nearly nondistinct. In addition, the radiodensity of the subchondral bone of the trochlear OCD is the same as the surrounding parent bone.
Discussion
Approximately 85% of OCD cases in the knee manifest in the MFC, the main weightbearing surface [1]. The lateral aspect of the MFC, seen in 51% [4] to 74% [1] of cases, is thus referred to as the classical or extended classical position. Of the remaining patients, 13% experience lesions of the LFC and only 2% exhibit defects in the trochlear notch [1]. These much more uncommon lesions of the LFC are reportedly associated with the presence of an intact discoid meniscus, with the exact location varying with complete or incomplete discoid and any evidence of meniscal tears [5].
In 2008, Hanna et al. [7] described two patients with unilateral, bicondylar femoral OCD. Both patients were male; one was skeletally immature while the other was skeletally mature. Neither patient reported a specific history of trauma on initial presentation for knee pain. Both patients underwent autologous chondrocyte implantation of the LFC lesions; however, the MFC lesions were not treated operatively at the time of the report, yet the author noted repair would follow [7].
Identical twin studies have shown male pairs who developed OCD of the MFC, one set with matching lesions in the left knee [10] and the other set with bilateral lesions [11], indicating a strong genetic component. Previous studies have also shown what appears to be a genetic linkage with a Mendelian inheritance pattern [8, 12, 14]. Researchers have identified a mutation in the ACAN gene, which is important for cartilage function, as responsible for dominant familial OCD [17]. This dominant inheritance is easily seen in some cases, such as a family in which a mother and three of her four daughters all developed bilateral OCD [15]. Conversely, earlier studies such as that of Petrie [13] refute the idea that OCD is a familial inherited disorder but rather suggest it is a “…disorder produced by different etiologies.” Interestingly, the aforementioned studies provide a peculiar juxtaposition of the genetic component of OCD. Until larger, more robust pedigree analysis is performed, it is likely the repetitive microtrauma cause for OCD etiology will be favored.
This case of three OCD lesions developing unilaterally in one knee is a unique clinical presentation of OCD and has never been described previously. This case highlights the different treatment for each OCD lesion: nonoperative treatment for the lesion of the LFC; bone grafting and fixation for the salvageable lesion of the MFC; and resection of the nonfunctional progeny tissue, bone grafting, and drilling of the unsalvageable lesion of the trochlea. The subsequent development of a MFC OCD lesion after using a varus unloader brace is unique and lends itself to the hypothesis described by others [3, 6, 18] that repetitive stress or microtrauma to the knee may contribute to the development of an OCD lesion. Specifically, the partial redistribution of weight from the LFC to the MFC may have contributed to the development of the MFC OCD lesion that followed.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Vanderbilt University, Nashville, TN, USA.
References
- 1.Aichroth P. Osteochondritis dissecans of the knee: a clinical survey. J Bone Joint Surg Br. 1971;53:440–448. [PubMed] [Google Scholar]
- 2.Cahill BR. Osteochondritis dissecans of the knee: treatment of juvenile and adult forms. J Am Acad Orthop Surg. 1995;3:237–247. doi: 10.5435/00124635-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Chu PJ, Shih JT, Hou YT, Hung ST, Chen JK, Lee HM. Osteochondritis dissecans of the glenoid: a rare injury secondary to repetitive microtrauma. J Trauma. 2009;67:E62–E64. doi: 10.1097/TA.0b013e318047c02a. [DOI] [PubMed] [Google Scholar]
- 4.Crawford DC, Safran MR. Osteochondritis dissecans of the knee. J Am Acad Orthop Surg. 2006;14:90–100. doi: 10.5435/00124635-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Deie M, Ochi M, Sumen Y, Kawasaki K, Adachi N, Yasunaga Y, Ishida O. Relationship between osteochondritis dissecans of the lateral femoral condyle and lateral menisci types. J Pediatr Orthop. 2006;26:79–82. doi: 10.1097/01.bpo.0000191554.34197.fd. [DOI] [PubMed] [Google Scholar]
- 6.Fairbanks H. Osteo-chondritis dissecans. Br J Surg. 1933;21:67–82. doi: 10.1002/bjs.1800218108. [DOI] [Google Scholar]
- 7.Hanna SA, Aston WJ, Gikas PD, Briggs TW. Bicondylar osteochondritis dissecans in the knee: a report of two cases. J Bone Joint Surg Br. 2008;90:232–235. doi: 10.1302/0301-620X.90B2.19705. [DOI] [PubMed] [Google Scholar]
- 8.Kozlowski K, Middleton R. Familial osteochondritis dissecans: a dysplasia of articular cartilage? Skeletal Radiol. 1985;13:207–210. doi: 10.1007/BF00350575. [DOI] [PubMed] [Google Scholar]
- 9.Linden B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47:664–667. doi: 10.3109/17453677608988756. [DOI] [PubMed] [Google Scholar]
- 10.Mackie T, Wilkins RM. Case report: osteochondritis dissecans in twins: treatment with fresh osteochondral grafts. Clin Orthop Relat Res. 2010;468:893–897. doi: 10.1007/s11999-009-1017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei-Dan O, Mann G, Steinbacher G, Cugat RB, Alvarez PD. Bilateral osteochondritis dissecans of the knees in monozygotic twins: the genetic factor and review of the etiology. Am J Orthop (Belle Mead NJ). 2009;38:E152–E155. [PubMed] [Google Scholar]
- 12.Mubarak SJ, Carroll NC. Familial osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1979;140:131–136. [PubMed] [Google Scholar]
- 13.Petrie PW. Aetiology of osteochondritis dissecans: failure to establish a familial background. J Bone Joint Surg Br. 1977;59:366–367. doi: 10.1302/0301-620X.59B3.893517. [DOI] [PubMed] [Google Scholar]
- 14.Phillips HO, Grubb SA. Familial multiple osteochondritis dissecans: report of a kindred. J Bone Joint Surg Am. 1985;67:155–156. [PubMed] [Google Scholar]
- 15.Pick MP. Familial osteochondritis dissecans. J Bone Joint Surg Br. 1955;37:142–145. doi: 10.1302/0301-620X.37B1.142. [DOI] [PubMed] [Google Scholar]
- 16.Robertson W, Kelly BT, Green DW. Osteochondritis dissecans of the knee in children. Curr Opin Pediatr. 2003;15:38–44. doi: 10.1097/00008480-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Stattin EL, Wiklund F, Lindblom K, Onnerfjord P, Jonsson BA, Tegner Y, Sasaki T, Struglics A, Lohmander S, Dahl N, Heinegård D, Aspberg A. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet. 2010;86:126–137. doi: 10.1016/j.ajhg.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JN. A diagnostic sign in osteochondritis dissecans of the knee. J Bone Joint Surg Am. 1967;49:477–480. [PubMed] [Google Scholar]


