Abstract
Background
Failure of initial treatment for juvenile osteochondritis dissecans (OCD) may require further surgical intervention, including microfracture, autograft chondrocyte implantation, osteochondral autografting, and fresh osteochondral allografting. Although allografts and autografts will restore function in most adults, it is unclear whether fresh osteochondral allograft transplantations similarly restore function in skeletally immature patients who failed conventional treatment.
Questions/purposes
Therefore, we determined function in (1) daily activity; (2) sports participation; and (3) healing (by imaging) in children with juvenile OCD who failed conventional therapy and underwent fresh osteochondral allograft transplantation.
Methods
We retrospectively reviewed 11 children with OCD of the knee treated with a fresh stored osteochondral allograft between 2004 and 2009 (six males and five females). The average age of the children at the time of their allograft surgery was 15.2 years (range, 13–20 years). The clinical assessments included physical examination, radiography, MRI, and a modified Merle D’Aubigné-Postel score. The size of the allograft was an average of 5.11 cm2. The minimum followup was 12 months (average, 24 months; range, 12–41 months).
Results
All patients had returned to activities of daily living without difficulties at 6 months and returned to full sports activities between 9 and 12 months after surgery. The modified Merle D’Aubigné-Postel score improved from an average of 12.7 preoperatively to 16.3 at 24 months postoperatively. Followup radiographs at 2 years showed full graft incorporation and no demarcation between the host and graft bone.
Conclusions
Our observations suggested fresh osteochondral allografts restored short-term function in patients with juvenile OCD who failed standard treatments.
Level of Evidence
Level IV, case series. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteochondritis dissecans (OCD) occurs in children and adults. It typically has a high rate of healing with no operative managements [5, 19, 20] and therefore most authors suggest initial nonoperative treatment for juvenile OCD [2, 6, 7, 17–20, 23, 32, 33]. These same authors have also described indications for operative intervention when nonoperative treatment is not successful or when the symptoms are substantial enough to warrant. Operative intervention such as extraarticular and transarticular drilling, OCD fragment fixation with absorbable screws, metal screws, or autogenous osteochondral plugs, or even OCD fragment excision has been recommended [1, 4, 5, 24, 34]. However, OCD lesions can progress after unsuccessful initial treatment and present with large bone and cartilage defects, leading to substantial joint symptoms and long-term disability and are a precursor to osteoarthritis at a young age [6, 7, 20]. In these cases large osteochondral defects in children or adults should be considered for reconstructive treatment options, including allograft or autograft transplantation.
Patients with OCD should be evaluated initially with a thorough history and physical examination. Although symptoms are often the best markers for a patient’s problem and his or her recovery from treatment, radiologic evaluations play an important role in determining the extent of the disease process as well as the options for treatment. Radiographs taken serially are commonly used and usually document the progression of the disease. MR images can provide more information regarding the stability of the lesions and correlate better with their arthroscopic appearance and therefore are more heavily relied on for making decisions regarding treatment [10, 29]. Proper interpretation requires T1- and T2-weighted MRIs to be used [26]. In patients with a high T2 signal in addition to a breach in the articular cartilage on the T1-weighted images, correlation between the MRI and arthroscopic findings was found 85% of the time [26, 32]. These grading systems have been helpful but lack the detail needed to differentiate lesions often encountered in an exclusively pediatric population.
Most surgeons recommend surgery to stabilize and facilitate healing of the lesion [20, 23] when patients have persistent pain and/or mechanical symptoms along with MRI findings of lesion instability. Surgical techniques include lesional drilling [11, 19], internal fixation of the lesion [18, 20, 31], and débridement of the lesional interface with possible bone grafting [12, 14, 27]. Obviously, intact cartilage with good supporting bone is crucial to normal long-term joint function (full joint motion, activities, and pain relief). Lesion drilling involves the process of drilling small holes in the lesion in hopes of stimulating neovascularization into the necrotic area to enhance healing [31]. Some sources suggest the best indication for drilling is when the lesion remains still attached to the native bone base [1, 19]. In these cases, there is a fibrocartilage layer separating the underlying bone and OCD fragment. Drilling the lesion promoted neovascularization across the fibrocartilage layer between the lesion and the surrounding native bone. In one study of skeletally immature patients with OCD, drilling alone reduced pain within days and radiologic healing seen within 12 months in 10 of 11 patients [5]. It also resulted in a full ROM and union of the fragment to the femoral condyle at 1 to 5 years followup [5]. Another study reported that extraarticular and intraepiphyseal drilling provided the ability to return to activities at 2.8 months on average and resulted in radiographic healing at 11.9 months on average for 75% of OCD lesions [11]. These observations have led many surgeons to include some form of lesional drilling with a host of other surgical treatment options [20, 23]. Excision of a loose or displaced fragment leads to symptomatic improvement (increased function and less pain) and a likely return to daily activities [3]. However, in one report [34], 65% of patients had radiographic changes (joint-space narrowing or joint-space loss on the weightbearing radiograph), frequent periods of swelling, recurrent pain, and mild to moderate effects on activities of daily living at an average of 8.9 years (range, 4–15 years) after excision.
Although the understanding of OCD treatment has advanced substantially over the past decade allowing most patients to return to a high level of function, some patients have not had relief of symptoms despite previous surgical intervention. These unfortunate patients present difficult decisions regarding treatment. Authors have discussed the possibility of repeat drilling, repeat OCD fixation, microfracture, or autologous chondrocyte implantation (ACI) [16, 21, 25, 27, 28]. The use of osteochondral autografting (mosaicplasty) or allografting in these cases has also been discussed [8, 12, 13]. However, it is unclear and to what extent these approaches relieve pain, restore function, and provide durable healing.
We therefore (1) determined function in daily activity; (2) sports participation; and (3) radiographic and MR image healing in children with juvenile OCD and failed conventional treatments before and after fresh osteochondral allograft transplantation.
Patients and Methods
We retrospectively reviewed 11 children (13 lesions) who underwent fresh osteochondral allografting of the knee for severe juvenile OCD at two institutions (Children’s Hospital of Wisconsin and Connecticut Children’s Medical Center) between 2004 and 2009 (Table 1). During this time we treated a total of 127 patients with juvenile OCD. Patients in our study were indicated for osteochondral allografts because they each had previously undergone therapy for an OCD lesion that (1) failed to heal; (2) had a residual large osteochondral defect on a weightbearing area in the knee; or (3) had persistent pain for at least 6 months after initial treatment. The contraindications for fresh allografts were: (1) lesions deemed too large to be treated (> 8 cm2); (2) inability to find an allograft that matched the patient’s surface contour; (3) untreated ligamentous instability; (4) untreated mechanical malalignment of the lower extremity; and (5) patient’s unwillingness to comply with postoperative rehabilitation protocols. All patients were age 18 years or younger at the time of initial diagnosis and had failed treatment of an osteochondral lesion. The average age of the children at the initial diagnosis was 13.5 years (range, 11–18.6 years) and 15.2 years (range, 13–20.4 years) at the time of osteochondral allografting. There were six males and five females. The minimum followup was 12 months (mean, 24 months; range, 12–41 months). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. The institutional review boards at the Children’s Hospital of Wisconsin, Medical College of Wisconsin, and Connecticut Children’s Medical Center approved this study.
Table 1.
Patient information, surgical technique, and clinical assessments (mean and range)
| Clinical information and measurements | Results | ||
|---|---|---|---|
| Sex (male:female) | 6:5 | ||
| Average age at diagnosis (years) (range) | 13.5 (11–18.6) | ||
| Average age at allograft (years) (range) | 15.2 (13–20.4) | ||
| OCD location* | |||
| MFC | 4 | ||
| LFC | 7 | ||
| Patella | 1 | ||
| Trochlea | 1 | ||
| Average OCD size (cm2) (range) | 5.1 (1.8–8) | ||
| Allograft type | |||
| Plug | 8 | ||
| Shell | 4 | ||
| Fixation technique | |||
| Press-fit | 8 | ||
| Fixation | 4 | ||
| Pain (0–10) | |||
| Preoperative | 5.6 | ||
| Latest followup | 1.2 | ||
| Daily activity level | Full | Limited | None |
| Preoperative | 1 | 10 | 0 |
| Postoperative | 11 | 0 | 0 |
| Sport | Full | Limited | None |
| Preoperative | 0 | 7 | 4 |
| Postoperative | 9 | 1 | 1† |
| Average functional score (0–18) (range) | |||
| Preoperative | 12.7 (10–14) | ||
| Postoperative | 16.3 (10–18) | ||
| Average followup (months) (range) | 24 (12–41) | ||
* More than one OCD lesion per knee for two patients; †this represents a single patient who has chronic pain issues and had the lowest functional and highest pain scores by far although he had a complete allograft incorporation and no visible radiographic graft margins; OCD = osteochondritis dissecans; MFC = medial femoral condyle; LFC = lateral femoral condyle.
Before allografting, we obtained a weightbearing AP weightbearing notch view and lateral radiographs as well as a MR scan of the knee. The lesional area was determined using biplanar MR measurements: Stage 1, small change of signal without clear margins of fragment; Stage 2, osteochondral fragment with clear margins but without fluid between fragment and underlying bone; Stage 3, fluid is visible partially between fragment and underlying bone; Stage 4, fluid is completely surrounding the fragment but the fragment is still in situ; and Stage 5, fragment is completely detached and displaced (loose body) [12].
We noted any history of previous or subsequent extremity surgery. The patient’s current activity level, knee ROM, and functional result were graded using the modified D’Aubigné-Postel 18-point scale [9, 12, 15]. Pain level at rest and with daily and recreational/sporting activities was noted and whether it limited their activities. All patients had pain with activity and limitations in daily activities and sports. Preoperatively nine patients had at least mild joint effusion. The average preoperative modified Merle D’Aubigné-Postel score was 12.7, primarily because of high pain levels with and without activity (Table 1). The osteochondral lesions were reviewed radiographically according to Rodererdts and Gleissner [30] and Anderson and Pagnani [3]. This grading system uses radiographs to determine whether the lesion was showing signs of separation from the native bone and whether a sclerotic line was present at the lesion margin. This system is important in determining the need to proceed with an allograft because recontouring of the subchondral bone is believed to be important. The most common location for a lesion was the lateral femoral condyle (seven lesions), and there were four lesions in the medial femoral condyle, one lesion in the trochlea, and one lesion in the patella.
A fresh stored osteochondral allograft with a mean size of 5.1 cm2 (range, 1.8–8 cm2) was performed at the discretion of the senior surgeons (RL, CN). All were harvested fresh and stored until transplantation. All fresh stored allografts were transplanted between 14 and 21 days. Indications included substantial pain and limitation of function resulting from the pain and substantial bone loss at the site of the lesion when determined to be at a depth requiring augmentation. All knees had an open arthrotomy. The grafts either were round plugs or custom keyhole shell grafts, which were harvested from the corresponding area of the donor hemicondyle and its thickness is typically less than 1.0 cm. The shell grafts were selected based on lesion geometry, primarily for linear defects. Cylindrical plugs were used whenever possible. There were no limb alignment corrections. One child had two areas that required grafting: one on the femoral trochlea and one on the lateral femoral condyle. Supplementary fixation (either metal or bioabsorbable screws) was used in the four keyhole cases to improve graft stability.
Postoperatively, patients underwent an 8- to 12-week period of limited weightbearing on crutches with physical therapy (continuous passive motion device was not used routinely) and bracing prescribed for a minimum of 6 to 8 weeks. Patients were discouraged from sports for at least 6 months.
We saw patients at 6 weeks, 3 months, 6 months, 1 year, and then yearly. Patients rated their pain according to a 10-point visual analog scale (VAS) and were again graded with the modified D’Aubigné-Postel scale. Patients’ pain levels were determined both by asking what their current pain level was daily and what their pain level was while in the office, because we assess passive and active ROM and gait. Pain level was also graded on if the patient needed analgesics for relief. Gait is assessed by the patient’s ability to walk greater than 0.8 km, ability to walk up and down stairs, the use of any walking aids (crutches), and gait patterns looking for abnormalities such as a lack of normal swing or increased knee flexion at foot strike [12]. Sports participation was based on the patient’s prior involvement before the injury took place and was assessed by questioning about their ability to participate in school physical educational classes and desired level of sports involvement. Full sports participation was defined as the patient’s return to their prediagnosis level of sport involvement. Intensity of participation was elicited from the patient, whether they play recreationally or competitively. Preoperatively 10 of the 11 patients were limited in their activity as a result of their knee problems. The eleventh patient continued to participate despite pain and swelling.
At each visit we obtained knee radiographs; supine AP and lateral, notch, and sunrise radiographs were obtained as needed. Four of the patients had follow-up MRIs at least 2 years after allografting. Radiographs were specifically reviewed by the two senior surgeons (RL, CN, the treating surgeons) to independently determine graft incorporation and whether other changes from preoperative examinations had occurred. The radiographic criteria for graft incorporation are continuous cancellous bone between the graft and native bone and no radiolucency. We looked carefully for evidence of joint space narrowing, squaring of the condyles, presence of loose bodies, or changes on the corresponding surface of the tibia or patella.
Results
Pain level was reduced on average from 5.6 before allografting to 1.2 at most recent followup. Full knee motion was restored in all patients (minimum, 125°). The average modified D’Aubigné-Postel score improved to 14.8 at 6 months, 16 at 12 months, and 16.3 at 24 months followup postoperatively (Table 1).
All patients returned to activities of daily living without difficulties by 6 months and to full sports activities between 9 and 12 months after surgery, including sports and gym classes. The mean pain and functional scores were influenced by one child who reported pain at 10 both before and after allografting despite being able to do all activities at his most recent followup.
Radiographically, all grafted areas were well healed by 6 to 9 months but continued in some cases to have some altered subchondral bone contour relative to normal. Radiographs at last followup showed all grafts to have incorporated to the native bone. There were no signs of joint space narrowing, condylar squaring, or irregularities on the corresponding surface of the tibia or patella. In the four patients with MRIs, we observed intact articular surfaces but clear demarcations between the host and donor cartilage. These four patients had some minimal high signal intensities on T2 at the host/graft interface. A case example before and after allograft transplantation was presented with radiographs (Fig. 1A–C), MRIs (Fig. 2B), and intraoperative images (Fig. 3A–B).
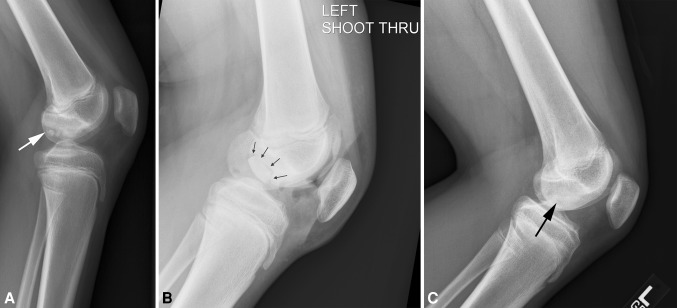
Fig. 1A–C.
(A) Lateral radiograph of a 13-year-old boy shows a large lateral condyle juvenile OCD lesion (arrow) 6 months after failed transarticular drilling and before allografting. (B) Lateral radiograph of the same patient’s knee at 6 weeks after allografting shows a clear demarcation of the 2.2 cm × 2.7-cm allograft (arrow). (C) Lateral radiograph of the same patient’s knee at 28 months postallografting shows complete allograft incorporation and no graft margins visible (arrow).
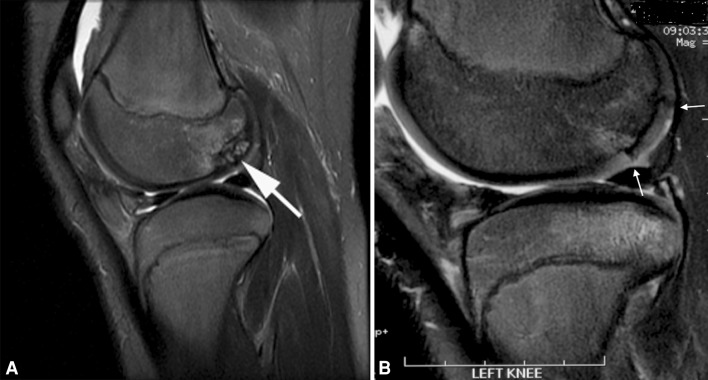
Fig. 2A–B.
(A) Corresponding T2 fat-saturated MR image in sagittal view shows a large OCD defect at the posterior portion of lateral condyle (arrow). (B) Corresponding T2 fat-saturated MR image in sagittal view 28 months postallografting shows allograft incorporating well with the host site without bony gap and persistent demarcation between allograft and native cartilage (arrow).
Fig. 3A–B.
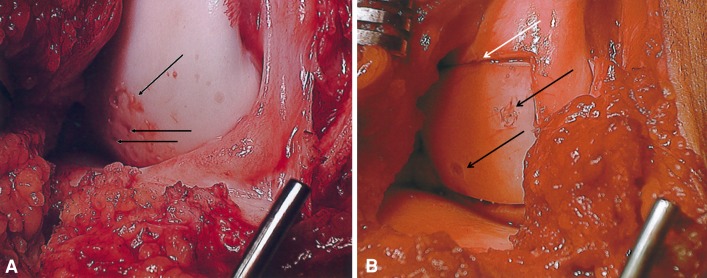
(A) Intraoperative photograph of corresponding lateral condylar OCD lesion before allografting shows multiple drilling holes with persistent loose cartilage (arrow). (B) Intraoperative photograph of completed fresh osteochondral allograft shows a well-positioned shell allograft with a slight margin gap (white arrow) and two bioabsorbable screws (black arrows).
Discussion
Juvenile OCDs occur most commonly in the knee. Their etiology is often unknown and some are discovered serendipitously when the knee is examined for other reasons. In most cases, when these lesions are discovered early, they can be treated successfully with standard treatment, either nonoperative or operative as described by several authors [1, 2, 4, 5, 18]. In the uncommon cases when these treatments are not successful, more aggressive and extensive treatments are often needed. This is especially the case when there is bone loss resulting in a loss of the normal subchondral boney contour at the site of the lesions. The use of osteochondral allografts in situations such as these has been proposed and used by others [4, 8, 12–15, 24]. Therefore, we determined function in (1) daily activity; (2) sports participation; and (3) healing (by imaging) in children with juvenile OCD and failed conventional treatments.
Although promising, our study does have some limitations. The first is the small number of patients. We hope to enroll patients in a prospective manner as we move forward in addition to following this current group for a longer duration. Second, our study was retrospective without a set protocol for evaluations. Although our evaluation of these patients is similar today as compared with their initial presentation, as we learn more about the use of osteochondral allografts, it may be appropriate for different aspects to be more fully evaluated. This will require a multicentered study, the foundation for which is currently being established. Third, our longest followup period of 41 months is too short to know whether osteochondral allografting in the pediatric population reduces the risk of subsequent osteoarthritis or the need for joint reconstruction in the long term. Although osteochondral allografting into an osteochondral defect is reportedly associated with a higher percentage of “successful” surgery in adults [14, 28], the reasons for the failures was not clear to these authors. Additionally, Williams et al. [33] noted that the average length of survival of an osteochondral graft in the adult population was 42 months. Authors have suggested multiple reasons for these failures including infection, cell-mediated immunity to the bony portion of the graft, and mechanical wear [22, 33]. Children may be excellent candidates for these grafts because they tend to have fewer comorbidities than their adult counterparts. It is our plan to follow this cohort of patients to determine the durability of the grafts in this population. Fourth, we could have used MRI for all patients postallografting as a more rigorous method to better determine the osteochondral graft/host healing and graft chondrocyte survival.
We used the modified D’Aubigné and Postel score system at the time of study [9, 12, 15]. One study used the modified D’Aubigné and Postel score to assess the functional outcome for patients who received fresh osteochondral allograft transplantation and found an increased mean score from 13.0 preoperatively to 16.4 at a mean followup of 7.7 years [12]. In our study the D’Aubigné-Postel score improved from an average of 12.7 preoperatively to 16.3 24 months postoperatively. Despite both studies demonstrating improved objective measurements after fresh osteochondral transplantation, including relieved pain, increased joint ROM as well as knee function, this 18-point scale has not been validated for the knee. The International Knee Documentation Committee will be used for future clinical research. Each of our patients had major pain before allografting. In each case, their pain improved with the overall average decreasing from 5.6 to 1.1 on a 10-point VAS. None of the patients after incorporation of the graft had pain at rest. Each of the patients who experienced a decrease in motion also improved. All patients had full extension and no patient had less than 125° of flexion after graft incorporation.
Before allografting, sports participation was severely limited as a result of the OCDs. Only one of the patients preoperatively could participate in sports although he did so despite pain and recurrent effusion. After graft incorporation, each of the patients was able to return to activities to the full extent they wished without limitations.
Success of grafting procedures needs to, at their core, achieve healing of the graft to the native bone. Reestablishing the solid subchondral foundation is essential for the surfaces to function normally. In our cases, as judged by radiographic evaluation, each healed fully between 6 and 9 months.
Although many studies are available on the efficacy of allografts and autografts in adults, little is documented in children about the clinical outcomes of allograft transplantation. Although some of the adults may have developed OCD before closure of the physis, it was not diagnosed until later, which would represent a subgroup of nonhealing juvenile OCD [20, 32]. The Miniaci and Tytherleigh-Strong [24] study does show promising results with all children who had an autograft fully healed after 1 year. We showed that fresh osteochondral allografting was used in 8.7% of our cases and this surgical technique provided this small group of patients with decreased pain, improved or maintained ROM, and improved functional ability. Although these patients represented only a small percentage of patients with juvenile OCD, hopefully the successful outcome seen in this short-term followup will be long-lasting as a result of the use of viable chondrocytes and the high rate of bone incorporation seen similar to the reports of fresh osteochondral allografts in adults with osteochondral defects [13].
There are alternatives to osteochondral allografting. Mosaicplasty is an option for more limited osteochondral defects [4, 24]; however, the drawbacks include (1) creating an additional defect in the knee; (2) results deteriorating with multiple plugs; (3) difficulty in matching surface contours for larger defects; and (4) the plug depth is typically greater than 10 mm. ACI is a viable alternative and has been effective in some situations [27, 28]. ACI does little to address the associated bony defects, which are often quite large. ACI also lacks the structural characteristics that the allograft provides such as the normal extracellular cartilage matrix and an intact cartilage-bone interface relying on the body’s ability to fill in the gaps [25]. Microfracture of the defect area is an alternative treatment but like ACI does not address the bony defect or any of the structural cartilage defects [20].
Our study cases demonstrate that osteochondral allografting is a reasonable alternative to larger osteochondral defects in children with open physes when traditional first-line surgical therapies fail. There were distinct advantages of this treatment over other tissue grafting techniques, including absence of donor site morbidity and restoration of normal tissue architecture. The main drawbacks to this treatment were the cost of the donor tissue, the need for a separate surgery because of limited available fresh grafts, possibility of immunologic consequences, and the potential for disease transmission. Progress continues to be made on techniques to minimize the likelihood of disease transmission and to increase the chondrocyte viability of the transplanted tissue [22]. Emmerson et al. [12] performed fresh osteochondral allografting for 64 adult patients with OCD in the femoral condyle. Of 10 patients who underwent reoperation, none were reportedly for disease transmission. Similarly, we did not find any case with disease transmission in our study.
Osteochondral allografts will likely remain a limited option for treating severe juvenile OCD that has failed standard treatment methods. We believe the data support continued use and monitoring of fresh osteochondral allografts in skeletally immature patients with OCD lesions.
Acknowledgments
We thank Mr Marquez-Barrientos and Mrs McKeon for assisting with data recording and photograph preparations.
Footnotes
Each author certifies that he or she, or a member of their immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI, USA; and Elite Sports Medicine, Connecticut Children’s Farmington, CT, USA.
References
- 1.Aglietti P, Buzzi R, Bassi PB, Fioriti M. Arthroscopic drilling in juvenile osteochoindritis dissecans of the medial femoral condyle. Arthroscopy. 1994;10:286–291. doi: 10.1016/S0749-8063(05)80113-6. [DOI] [PubMed] [Google Scholar]
- 2.Anderson AF, Anderson CN. Management of osteochoindritis dissecans of the knee. Techniques in Knee Surgery. 2005;4:23–35. doi: 10.1097/01.btk.0000154843.06650.6b. [DOI] [Google Scholar]
- 3.Anderson AF, Pagnani MJ. Osteochondritis dissecans of the femoral condyles long-term; results of excision of the fragment. Am J Sports Med. 1997;25:830–834. doi: 10.1177/036354659702500617. [DOI] [PubMed] [Google Scholar]
- 4.Berlet GC, Mascia A, Miniaci A. Treatment of unstable osteochoindritis dissecans lesions of the knee using autogenous osteochondral grafts (mosaicplasty) Arthroscopy. 1999;15:312–316. doi: 10.1016/S0749-8063(99)70041-1. [DOI] [PubMed] [Google Scholar]
- 5.Bradley J, Dandy DJ. Results of drilling osteochoindritis dissecans before skeletal maturity. J Bone Joint Surg Br.1989;71:642–644. [DOI] [PubMed]
- 6.Cahill BR. Osteochondritis dissecans of the knee: treatment of juvenile and adult forms. J Am Acad Orthop Surg. 1995;3:237–247. doi: 10.5435/00124635-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Cahill BR. Current concepts review. Osteochondritis dissecans. J Bone Joint Surg Am. 1997;79:471–472. [PubMed] [Google Scholar]
- 8.Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990;72:574–581. [PubMed] [Google Scholar]
- 9.D’Aubigne RM, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed] [Google Scholar]
- 10.De Smet AA, Ilahi OA, Graf BK. Untreated osteochoindritis dissecans of the femoral condyles: prediction of patient outcome using radiographic and MR findings. Skeletal Radiol. 1997;26:463–467. doi: 10.1007/s002560050267. [DOI] [PubMed] [Google Scholar]
- 11.Edmonds EW, Albright J, Bastrom T, Chambers HG. Outcomes of extra-articular, intra-epiphyseal drilling for OCD of the knee. J Pediatr Orthop. 2010;30:870–878. doi: 10.1097/BPO.0b013e3181f5a216. [DOI] [PubMed] [Google Scholar]
- 12.Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907–914. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 13.Garrett JC. Fresh osteochondral allografts for treatment of articular defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clin Orthop Relat Res. 1994;303:33–37. [PubMed] [Google Scholar]
- 14.Ghazavi M, Pritzker K, Davis A, Gross A. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br. 1997;79:1008–1013. doi: 10.1302/0301-620X.79B6.7534. [DOI] [PubMed] [Google Scholar]
- 15.Gortz S, Deyoung A, Bugbee W. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468:1269–1278. doi: 10.1007/s11999-010-1250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudas R, Simonaityte R, Cekanauskas E, Tamosiūnas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop. 2009;29:741–748. doi: 10.1097/BPO.0b013e3181b8f6c7. [DOI] [PubMed] [Google Scholar]
- 17.Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg Am. 1984;66:1340–1348. [PubMed] [Google Scholar]
- 18.Kocher MS, Czarnecki JJ, Anderson JS, Micheli LJ. Internal fixation of juvenile osteochondritis dissecans lesions of the knee. Am J Sports Med. 2007;35:712–718. doi: 10.1177/0363546506296608. [DOI] [PubMed] [Google Scholar]
- 19.Kocher MS, Micheli LJ, Yaniv M, Zurakowski D, Ames A, Adrignolo AA. Functional and radiographic outcome of juvenile osteochoindritis dissecans of the knee treated with transarticular arthroscopic drilling. Am J Sports Med. 2001;29:562–566. doi: 10.1177/03635465010290050701. [DOI] [PubMed] [Google Scholar]
- 20.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med. 2006;34:1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan SP, Skinner JA, Bartlett W, Carrington RWJ, Flanagan AM, Briggs TWR, Bentley G. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88:61–64. doi: 10.1302/0301-620X.88B1.16796. [DOI] [PubMed] [Google Scholar]
- 22.Langer F, Percy EC. Osteochoindritis dissecans and anomalous centres of ossification: a review of 80 lesions in 61 patients. Can J Surg. 1971;14:208–215. [PubMed] [Google Scholar]
- 23.Michael JW, Wurth A, Eysel P, Konig DP. Long-term results after operative treatment of OCD of the knee joint—30 year results. Int Orthop. 2008;32:217–221. doi: 10.1007/s00264-006-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miniaci A, Tytherleigh-Strong G. Fixation of unstable osteochoindritis dissecans lesions of the knee using arthroscopic autogenous osteochondral grafting (mosaicplasty) Arthroscopy. 2007;23:845–851. doi: 10.1016/j.arthro.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Moriya T, Wada Y, Watanabe A, Sasho T, Nakagawa K, Mainil-Varlet P, Moriya H. Evaluation of reparative cartilage after autologous chondrocyte implantation for osteochondritis dissecans: histology, biochemistry, and MR imaging. J Orthop Sci. 2007;12:265–273. doi: 10.1007/s00776-007-1111-8. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor MA, Palaniappan M, Khan N, Bruce CE. Osteochoindritis dissecans of the knee in children: a comparison of MRI and arthroscopic findings. J Bone Joint Surg Br. 2002;84:258–262. doi: 10.1302/0301-620X.84B2.11823. [DOI] [PubMed] [Google Scholar]
- 27.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85:17–24. doi: 10.1302/0301-620X.85B1.13948. [DOI] [PubMed] [Google Scholar]
- 28.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Pill S, Ganley T, Milam RA, Lou J, Meyer JS, Flynn J. Role of MRI and clinical criteria in predicting successful nonoperative treatment of osteochoindritis dissecans in children. J Pediatr Orthop. 2003;23:102–108. [PubMed] [Google Scholar]
- 30.Rodegerdts U, Gleissner B. [Long-term experience with the operative treatment of osteochondrosis dissecans of the knee joint] [in German] Orthop Praxis. 1979;8:612–622. [Google Scholar]
- 31.Smillie IS. Osteochondritis dissecans of the knee. Am J Orthop. 1964;6:236–241. [PubMed] [Google Scholar]
- 32.Wall E, Von Stein D. Juvenile osteochondritis dissecans. Orthop Clin N Am. 2003;34:341–353. doi: 10.1016/S0030-5898(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 33.Williams JS, Jr, Bush-Joseph CA, Bach BR., Jr Osteochoindritis dissecans of the knee. Am J Knee Surg. 1998;11:221–232. [PubMed] [Google Scholar]
- 34.Wright RW, McLean M, Matava MJ, Shively RA. Osteochoindritis dissecans of the knee: long-term results of excision of the fragment. Clin Orthop Relat Res. 2004;424:239–243. doi: 10.1097/01.blo.0000128216.10732.d8. [DOI] [PubMed] [Google Scholar]