Abstract
Background
Joint gaps and mediolateral (ML) soft tissue balance have long been known to affect clinical scores and patient function after TKA, but the relationship between gaps and soft tissue balance remain poorly defined. If specific relationships exist between soft tissue tension and patient function, then objective targets could be established to assist surgeons in achieving more consistent postoperative knee function.
Questions/purposes
By performing instrumented gap measurements during TKA, we sought to quantify the relationships between intraoperative soft tissue tension and clinical scores and patient function.
Methods
We prospectively followed 57 patients with 63 primary TKAs with posterior-stabilized prostheses. Joint gaps and ML soft tissue balance were measured intraoperatively from 0° to 135° with the patella reduced after independent bone cuts and soft tissue releases. We determined the relationships between these intraoperative measurements and postoperative ROM and Knee Society scores at minimum 2-year followup.
Results
Larger joint gaps at 120° and 135° flexion predicted larger postoperative knee flexion (r = 0.296 and r = 0.393, respectively), whereas larger gaps at 10° flexion predicted greater postoperative knee extension (r = 0.285). Knees with rectangular joint gaps did not show better ROM or Knee Society scores compared with knees with trapezoidal joint gaps. In the range of normal surgical variation, neither joint gaps nor gap asymmetry affected the incidence of postoperative instability.
Conclusions
Avoiding small joint gaps in extension and in deep flexion should allow patients who undergo TKAs to obtain maximum ROM.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
A TKA is one of the most effective methods to relieve pain and improve function in patients with arthritic knees [4, 5, 25, 29, 36]. Obtaining functional ROM after TKA is one of the most important goals for normal daily activities [1, 10, 22]. Preoperative ROM is reportedly the most important factor influencing postoperative ROM [9, 13, 26], but many other factors, including implant design [6, 23], mediolateral (ML) soft tissue balance [32], flexion-extension gap balance [11, 18], joint-line height [20], femoral posterior condylar offset [3, 8], and PCL tension [24, 39], also influence postoperative ROM.
Intraoperative joint gaps and ML soft tissue balance are potentially modifiable surgical variables affecting clinical outcomes after TKA [14, 30, 32]. Various devices to measure joint gaps and ML soft tissue balance have been reported, including lamina spreaders [7], tensors [19, 27, 38], and electronic instruments [33, 41]. Intraoperative joint gaps usually are measured with the patella everted or laterally shifted, which tightens lateral structures and reduces the effect of the extensor mechanism in deep flexion. This may preclude accurate evaluation of joint gaps and ML soft tissue balance [18, 19, 41]. In addition, intraoperative joint gaps generally are measured in extension and 90° flexion, which provides limited information in mid- and deep flexion. Measuring joint gaps with the patella reduced and in smaller flexion intervals will provide detailed characterization of intraoperative coronal joint laxity. Using a tensor to measure joint gaps over the ROM with the patella in place, Matsumoto et al. [18] showed a correlation between joint gap changes from 90° to 135° flexion and postoperative knee flexion. Despite these efforts, the causal relationships between joint gaps and tissue balance, and clinical function like ROM, remain unclear.
We therefore addressed four questions related to soft tissue procedures during TKA: (1) How do intraoperative joint gaps affect clinical results, especially ROM? (2) How does intraoperative ML soft tissue balance affect clinical results, especially ROM? (3) How do intraoperative joint gaps and ML soft tissue balance affect postoperative knee instability? (4) How does preoperative femorotibial angle (FTA) affect intraoperative gaps and ML balance?
Patients and Methods
We prospectively followed all 66 patients who underwent 72 primary TKAs with implantation of posterior-stabilized (PS) prostheses between January 2006 and August 2008. All patients requiring TKAs received PS prostheses during this period. All patients gave informed consent for this institutional review board-approved study. Joint gaps and ML soft tissue balance were recorded during each case. Two patients (two knees) with previous high tibial osteotomies were excluded; seven patients (seven knees) also were excluded from the study because unsatisfactory initial balance required additional soft tissue releases, and there was not enough time to conduct a second comprehensive measurement of joint gaps. These nine exclusions left 57 patients (63 knees). A sample of 52 knees was computed to produce 80% power (1-β) for correlating intraoperative joint gaps and postoperative ROM using an effect size of 0.33, or an r2 value explaining 10% of the data variance.
Subject age at the time of surgery averaged 72 years (range, 51–84 years). The study cohort included six men (10 knees) and 44 women (53 knees) with a preoperative diagnosis of osteoarthritis in 54 knees and rheumatoid arthritis in nine. Of the 63 knees, 22 (35%) had at least an AP instability (≥ 5 mm) or ML instability (≥ 5°) as defined by the Knee Society scoring system [12]. Preoperative measurements were obtained within 1 month before surgery by one observer (TW) (Table 1). FTA was measured using standing knee AP radiographs showing at least 15 cm of bone above and below the joint line. The minimum followup was 2 years (mean, 2.3 years; range, 2–3 years).
Table 1.
Preoperative and postoperative clinical values*
| Variables | Preoperative | Followup | p value (paired t-test) | p, r values (correlation) |
|---|---|---|---|---|
| Extension (°) | −7 ± 9 | −1 ± 3 | < 0.001 | < 0.001, r = 0.433 |
| Flexion (°) | 124 ± 21 | 126 ± 18 | 0.455 | < 0.001, r = 0.604 |
| Knee Society knee score | 38 ± 13 | 93 ± 6 | < 0.001 | 0.537 |
| Knee Society function score | 42 ± 17 | 65 ± 22 | < 0.001 | < 0.001, r = 0.638 |
| Instability (+/−) | 22/41 | 28/35 | 0.516 (chi square) | |
| Femorotibial angle (°) | 185 ± 8 (162–203) | 175 ± 2 (170–179) | < 0.001 | 0.695 |
* Data are shown as the mean ± SD (range); r = Pearson’s correlation coefficient.
One surgeon (TW) performed all TKAs using generally accepted techniques for minimally invasive surgery and the same prosthesis (NexGen® LPS Flex Mobile; Zimmer, Warsaw, IN, USA) without a navigation system. An air tourniquet was pressurized to 330 mm Hg during surgery. The mini-midvastus approach, with an independent bone-cutting technique, was used for all knees [35]. The distal femur was cut perpendicular to its mechanical axis, removing the amount of bone corresponding to the prosthetic femoral component thickness. The proximal tibia was cut perpendicular to its coronal mechanical axis and with a 7°-sagittal posterior tibial slope, removing bone on the intact side that corresponded to the prosthetic tibial component thickness. The posterior femoral condyles were cut with 3° external rotation from the posterior condylar line. Whiteside’s line was used as a femoral rotation reference in valgus knees [37]. The patella was not resurfaced in these knees.
Traditional gap balancing techniques, including osteophyte removal and soft tissue releases, were used to obtain rectangular joint gaps independent of the instrumented tensor. The PCL was sacrificed in all knees. The deep layer of the medial collateral ligament (MCL) was released in all knees with varus deformities. After osteophyte removal, the superficial layer of the MCL, posterior capsule, semimembranosus, and pes anserinus were released sequentially until adequate ML soft tissue balance was achieved. No soft tissue release of lateral structures was required to obtain acceptable ML soft tissue balance for two valgus knees with less than 170° FTA.
Joint gaps and ML soft tissue balance were measured after performing soft tissue releases. Femoral cuts were performed, and the femoral trial was put in place. A tensor (Offset Repo-Tensor®; Zimmer, Tokyo, Japan), developed by Kobe University [19], was placed between the tibial cut surface and the femoral trial. The tensor consisted of three parts: (1) an upper seesaw plate; (2) a lower platform plate; and (3) an extraarticular main body (Fig. 1A). The lower platform plate was fixed on the center of the tibial cut surface and held in place with small pins protruding from the bottom side of the plate. The seesaw plate had a post that fit into the intercondylar space and articulated with the cam of the femoral trial. This post-cam mechanism controlled the tibiofemoral translation in the coronal and sagittal planes over the entire knee flexion arc. Although the tibiofemoral articulation is flat, the kinematics between the tensor and the femoral trial are close to that between the tibial tray and the femoral implant. The main body connected the other two parts. The tensor was small enough to be used for minimally invasive procedures, and the offset arm allowed surgeons to use the device with the patella reduced. The tensor provided numerical measures of the joint gap at the center of the knee and the ML tilting angle from full extension to deep flexion. The patella was reduced to the original position and several sutures were used to maintain correct patellofemoral position throughout the ROM (Fig. 1B). A joint distraction force of 40 lb (18 kg) was applied by the tensor at each knee angle, and the central joint gaps and ML tilting angles were directly measured at 0°, 10°, 30°, 60°, 90°, 120°, and 135° flexion (Fig. 1C). Preliminary studies with this tensor indicated the joint gap, at full extension with 40 lb of joint distraction force, corresponded most closely to the appropriate tibial insert thickness [19]. One observer (TW) measured all cases with direct supervision over the surgical assistant who supported the thigh to maintain appropriate sagittal alignment and reduce the influence of thigh mass on knee forces. The accuracy of this measurement has been estimated to be ± 0.3 mm in the joint gap [19]. The original protocol assessed joint gaps at 0°, 30°, 60°, 90°, and 120° flexion, and this was augmented after the first 21 knees to include 10° (51 knees) and 135° (48 knees) flexion to better assess the terminal ranges of motion.
Fig. 1A–C.
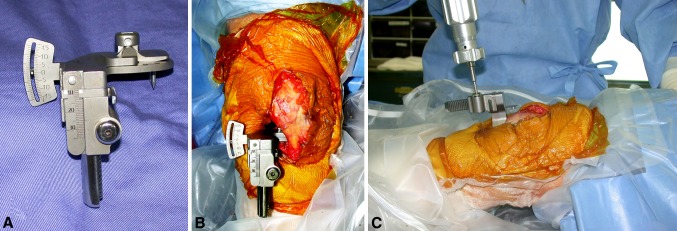
(A) The tensor used in this study consisted of an upper seesaw plate, a lower platform plate, and an extraarticular main body. The central joint gap and the ML soft tissue balance (tilting angle) could be measured though the entire ROM. (B) The tensor had an offset arm allowing measurement with the patella reduced during minimally invasive TKA (flexed left knee, the right side shows the proximal part of the leg). (C) A joint distraction force of 40 lb (18 kg) was applied to the tensor during gap measurements.
We defined the clinical gap as the measured joint gap minus the tibial construct thickness (tibial baseplate plus polyethylene insert implanted in each patient), which represents the postimplantation laxity at each angle. When the measured gap was smaller than the thickness of the implanted tibial construct, the clinical gap had a negative value. ML soft tissue balance was assessed by calculating the mean joint gap-tilting angle over all flexion angles for each patient.
Postoperatively, continuous epidural anesthesia was continued for 2 days. The day after surgery, quadriceps setting and active straight-leg raising were encouraged. Full weightbearing on the surgically treated knee was allowed. On the second day after surgery, the drainage tube and the epidural catheter were removed. ROM exercise was started, and gait exercise bearing full weight was encouraged. Continuous passive motion machines were used for all patients. The goal of rehabilitation was a stable gait with a single cane, although this was ascertained for each patient depending on the preoperative gait ability.
Patients were followed postoperatively at 1 month, 3 months, 6 months, 1 year, and every year thereafter. Physical examinations including Knee Society knee score and function score [12] were performed, and radiographs of the surgically treated knee were obtained before surgery and at each followup.
Based on the mean joint gap tilting angle, the 63 knees were classified into three groups: (1) knees with mean joint gap tilting less than −1.0° (lateral tight group, 17 knees); (2) knees with mean joint gap tilting between −1.0° and 1.0° (well-balanced group, 17 knees); and (3) knees with mean joint gap tilting greater than 1.0° (medial tight group, 29 knees); the clinical results were compared among these three groups. Joint instability at the final followup also was considered. The intraoperative measurements of knees with postoperative instability and those without instability were compared. Finally, we examined the relation between the preoperative FTA and intraoperative measurements. Correlations between clinical gaps and last followup values of ROM, Knee Society knee and function scores were evaluated using Pearson’s test. ANOVA with post hoc Tukey’s test was used for the last followup value comparisons among the three joint gap-tilting groups. An unpaired t-test was used for clinical gap comparisons between knees with and without postoperative instability. A chi-square test was used to compare postoperative instability among the three tilting groups. ANOVA was used to compare preoperative FTA among the three tilting groups. SPSS statistical software (IBM, Armonk, NY, USA) was used for these analyses.
Results
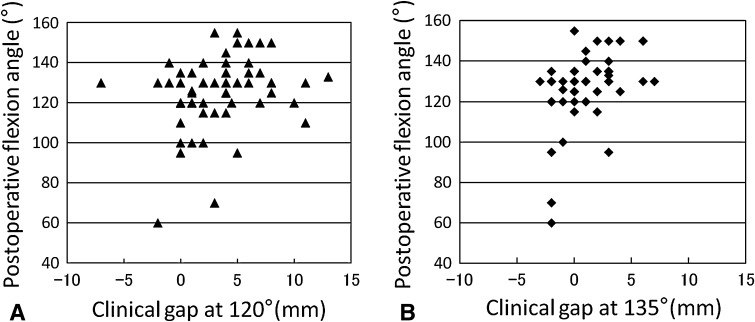
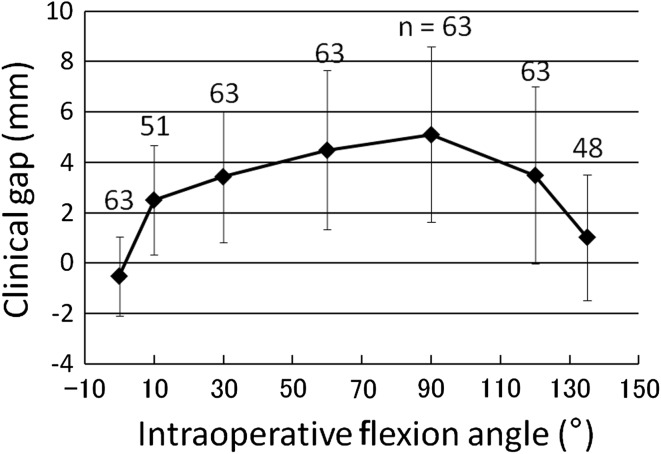
Clinical gaps at 120° and 135° correlated with postoperative knee flexion (r = 0.296, p = 0.018; r = 0.393, p = 0.006, respectively) (Fig. 2), and clinical gaps at 10° correlated with postoperative knee extension (r = 0.285, p = 0.043) (Table 2). Clinical gaps, however, did not correlate with Knee Society knee or function score (Table 2). Average clinical gaps were smallest at 0° knee flexion, increased over 2 mm at 10°, gradually increased an additional 2 mm up to 90°, and then progressively decreased at 120° and 135° (Fig. 3). Maximum clinical gaps of 5.1 mm were observed at 90° knee flexion.
Fig. 2A–B.
(A) A clinical gap at 120° had positive correlations with postoperative knee flexion angles (r = 0.296, p = 0.018, n = 63). (B) A clinical gap at 135° had positive correlations with postoperative knee flexion angles (r = 0.393, p = 0.006, n = 48).
Table 2.
Correlation between clinical gaps and clinical values
| Knee flexion | p, r values (correlation) | ||||
|---|---|---|---|---|---|
| Postoperative extension | Postoperative flexion | Postoperative KS | Postoperative FS | Preoperative FTA | |
| 0° (n = 63) | p = 0.434 | p = 0.930 | p = 0.684 | p = 0.472 | p = 0.462 |
| 10° (n = 51) | p = 0.043 | p = 0.477 | p = 0.111 | p = 0.934 | p = 0.043 |
| r = 0.285 | r = 0.285 | ||||
| 30° (n = 63) | p = 0.175 | p = 0.697 | p = 0.178 | p = 0.952 | p = 0.102 |
| 60° (n = 63) | p = 0.579 | p = 0.833 | p = 0.127 | p = 0.353 | p = 0.063 |
| 90° (n = 63) | p = 0.377 | p = 0.336 | p = 0.107 | p = 0.525 | p = 0.068 |
| 120° (n = 63) | p = 0.360 | p = 0.018 | p = 0.297 | p = 0.972 | p = 0.989 |
| r = 0.296 | |||||
| 135° (n = 48) | p = 0.371 | p = 0.006 | p = 0.227 | p = 0.596 | p = 0.427 |
| r = 0.393 | |||||
r = correlation coefficient; KS = Knee Society knee score; FS = Knee Society function score; FTA = femorotibial angle.
Fig. 3.
The average clinical gap, defined as the measured joint gap minus the combined thickness of the tibial component and polyethylene insert, increases with knee flexion until 90° and then decreases with further flexion. There were statistical differences between the clinical gaps at all flexion angles: between 0° and 10°, 30°, 60°, 120° (p < 0.001); between 10° and 60° (p = 0.004); 90° (p < 0.001); between 30° and 90° (p = 0.017); 135° (p < 0.001); between 60° and 135° (p < 0.001); between 90° and 120° (p = 0.023); 135° (p < 0.001); and between 120° and 135° (p < 0.001).
We found no differences among the three tilting groups in terms of postoperative knee extension angle, flexion angle, Knee Society knee or function score (Table 3). The mean joint gap-tilting angle of all knees averaged 0.6° (range, −9.3° to 9.0°).
Table 3.
Relationship between joint gap tilt and clinical results or preoperative FTA*
| Variables | Lateral tight (n = 17) | Well-balanced (n = 17) | Medial tight (n = 29) | p value |
|---|---|---|---|---|
| Tilting angle (°) | −4.0 ± 2.7 (−9.3 to −1.1) | 0.0 ± 0.7 (−1.0 to 1.0) | 3.6 ± 2.1 (1.3 to 9.0) | < 0.001 (ANOVA) |
| Extension (°) | −1 ± 2 | −2 ± 3 | −1 ± 2 | 0.575 (ANOVA) |
| Flexion (°) | 125 ± 13 | 124 ± 21 | 124 ± 20 | 0.987 (ANOVA) |
| Knee Society knee score | 95 ± 5 | 91 ± 5 | 93 ± 7 | 0.126 (ANOVA) |
| Knee Society function score | 66 ± 13 | 71 ± 22 | 62 ± 26 | 0.343 (ANOVA) |
| Instability (+/−) | 7/10 | 10/7 | 11/18 | 0.369 (chi square) |
| Preoperative FTA (°) | 188 ± 6 (179–203) | 183 ± 8 (162–195) | 185 ± 9 (165–203) | 0.254 (ANOVA) |
* Data are shown as the mean ± SD (range); FTA = femorotibial angle.
Clinical gaps did not affect postoperative stability. No clinical gaps differed between knees with postoperative instability and knees without instability (Table 4). Average tilting angles did not affect (p = 0.369) postoperative stability (Table 3). Of 28 knees (44%) with at least AP or ML instability at last followup, 22 had AP instability (≥ 5 mm), two had ML instability (≥ 5°), and four had AP and ML instability. These were mostly anterior laxities detected during manual examination of the patients’ knees with relaxed muscles, not clinical instabilities reported by patients. None of these knees had AP or ML instability greater than 10 mm nor did any knee have functional instability or require revision surgery.
Table 4.
Clinical gaps of knees with and without postoperative instability*
| Clinical gap | With postoperative instability (mm) | Without postoperative instability (mm) | p value (unpaired t-test) |
|---|---|---|---|
| 0° (n = 63) | −0.7 ± 1.3 | −0.4 ± 1.8 | 0.493 |
| 10° (n = 51) | 2.8 ± 1.9 | 2.3 ± 2.4 | 0.404 |
| 30° (n = 63) | 3.9 ± 2.4 | 3.0 ± 2.7 | 0.176 |
| 60° (n = 63) | 4.8 ± 3.2 | 4.2 ± 3.2 | 0.453 |
| 90° (n = 63) | 5.7 ± 3.8 | 4.6 ± 3.2 | 0.230 |
| 120° (n = 63) | 4.0 ± 3.2 | 3.1 ± 3.7 | 0.302 |
| 135° (n = 48) | 1.3 ± 2.7 | 0.7 ± 2.3 | 0.452 |
* Data are shown as the mean ± SD.
The preoperative FTA positively correlated with (r = 0.285, p = 0.043) the clinical gap at 10° flexion (Table 2). However, the preoperative FTA did not differ among the three joint gap-tilting groups (Table 3).
Discussion
It remains debatable how surgeons should tension soft tissues for knee arthroplasties. Tissues are manipulated most easily during surgery, so it is important to establish objectively the relationships between intraoperative tissue tension and postoperative clinical and functional outcomes. We, therefore, addressed four questions related to soft tissue procedures during TKA: (1) How do intraoperative joint gaps affect clinical results, especially ROM? (2) How does intraoperative ML soft tissue balance affect clinical results, especially ROM? (3) How do intraoperative joint gaps and ML soft tissue balance affect postoperative knee instability? (4) How does preoperative FTA affect intraoperative gaps and ML balance?
Several study limitations need to be acknowledged. First, our patient cohort was sufficient to address the primary study question but lacked statistical power to address the secondary questions. Larger subject cohorts are required to provide definitive findings for our Questions 2 through 4. Second, many factors affect knee arthroplasty outcomes, so one must take a narrow view to focus solely on the relationships between intraoperative laxity measurements and clinical results. Acknowledging this limitation, we believe a single-surgeon series is appropriate to assess these relationships. Third, our subject cohort consisted primarily of women with osteoarthritis. Our findings may not generalize to men or patients with rheumatoid arthritis.
Consistent with our expectation, clinical gaps at 120° and 135° had positive correlations with postoperative flexion angle, whereas clinical gaps at 10° showed a positive correlation with postoperative extension angle. Adequate joint spaces should contribute positively to the outcome of TKA [15, 16] (Table 5). Matsumoto et al. [18] used the same tensor with and without patella eversion and reported gap differences (135°–90°) with the patella reduced showed a positive correlation with postoperative knee flexion (Table 5). Asano et al. [2] observed that soft tissue tension in extension during surgery positively correlated with postoperative extension deficits (Table 5). In our study, clinical gaps in deep knee flexion showed positive correlations with postoperative knee flexion, whereas the clinical gap at 10° showed a positive correlation with postoperative knee extension. These results highlight the importance of adequate joint gaps greater than 90° for greater postoperative flexion and gaps at 10° knee flexion for full postoperative knee extension. Clinical gaps were greater in midflexion, but none of our observations suggest these knees had midflexion functional instability. Average clinical gaps in midflexion did not exceed the average gap at 90°, which was 5 mm (Fig. 3). Gaps greater than 5 mm appear to offer no additional benefit for knee ROM (Fig. 2).
Table 5.
Comparison of effect of joint gap or laxity on clinical results from past and current studies
| Study | Findings | Number of knees | Followup (years) |
|---|---|---|---|
| Kuster et al. [15] | Varus and valgus laxity between 4° and 8° on either side in 20° flexion improved patient satisfaction, ROM without deleterious short- to mid-term effects | 44 | 4.5 (range, 2–7) |
| Matsuda et al. [17] | Coronal laxity, especially balanced laxity, is important for achieving improved ROM in mobile-bearing TKAs | 80 | 1 |
| Matsumoto et al. [18] | Joint gap change value (135°–90°) by reducing patellofemoral joint showed positive correlation with postoperative knee flexion angle | 25 | 2 |
| Asano et al. [2] | The extension deficit became larger with an increase of soft tissue tension | 64 | 1 |
| Current study | Clinical gaps at 120° and 135° had positive correlations with postoperative flexion angle, whereas clinical gaps at 10° showed a positive correlation with postoperative extension angle; well-balanced knees did not show better clinical results | 63 | 2.3 (range, 2–3) |
We did not find well-balanced knees had better ROM or Knee Society scores. Knees with average tilting angles greater than 1° had comparable results to those with neutrally balanced knees. Several studies [14, 30, 32] report that ML soft tissue balance is an important factor for a successful TKA and that inadequate ML soft tissue balance is believed to result in poor outcomes. Some studies [17, 28] have suggested that intraoperative and postoperative ML soft tissue balances averaged 0° to 2° at extension and at 90° knee flexion. We found gap tilt averaged 0.6°, consistent with previous reports. Perfect balance is difficult to achieve [7], and it has been questioned whether rectangular gaps are the ideal goal because the normal joint gap is trapezoidal [31]. Our data did not show superior clinical outcomes in the most well-balanced knees. Our data suggested that as much as 4° gap tilt is consistent with good short-term results using a PS prosthesis.
Clinical gaps or tilting angles did not affect postoperative instability. Too much joint laxity is associated with persistent pain and poor long-term outcomes resulting from instability [12, 21, 34]. We suspected greater postoperative instability would result from greater joint gaps and/or imbalanced ML soft tissues. In our series, 44% of knees had a carefully detected small amount of AP or ML laxity. Neither of these intraoperative variables, however, considerably affected postoperative stability for the ranges we observed. Yamakado et al. [40] reported the mean AP and ML laxities of knees with cruciate-retaining prostheses as 9.7 mm and 10.6°, respectively. Mitts et al. [21] found 37% of knees with AP laxity greater than 5 mm and 78% of knees with ML laxity greater than 5° in their knee arthroplasties with cruciate-retaining prostheses. It appears a range of joint laxities is consistent with good short-term outcomes, and future work is required to determine the magnitude of laxity that tips the balance toward instability and poor clinical outcomes.
Preoperative FTA did not affect the average tilting angle after adequate soft tissue balancing. Some studies have reported an increase of varus deformity negatively affected the postoperative ROM [13, 26]. Our results did not exhibit a strong correlation between preoperative FTA and postoperative ROM. The preoperative FTA, however, showed a positive correlation with the clinical gap at 10°. Knees with severe varus deformity required a more medial soft tissue release to equalize tension with the elongated lateral structures, resulting in a larger gap near extension. The preoperative FTA did not differ among the three joint gap-tilting groups, indicating more aggressive soft tissue releases were performed in more severely deformed knees to achieve ML soft tissue balance.
Understanding the characteristics of intraoperative joint gaps in TKA using PS prostheses with the patella reduced makes it possible to predict postoperative ROM and provides surgeons objective guidance for soft tissue balancing during surgery. Intraoperative joint gaps at deep knee flexion affect postoperative knee flexion, whereas joint gaps near extension affect knee extension. We could not determine specific target ranges for ML soft tissue balance in TKAs with PS prostheses. Further investigation will be done to determine adequate ML soft tissue balance during surgery to achieve better postoperative knee function.
Acknowledgments
We thank Hirotsugu Muratsu MD, for advice on using the intraoperative joint gap tensor developed at Kobe University.
Footnotes
Each author certifies that he, or a member of his immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that Tsuchiura Kyodo General Hospital and the University of Florida approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Tsuchiura Kyodo General Hospital, Tsuchiura, Ibaraki, Japan.
References
- 1.Acker SM, Cockburn RA, Krevolin J, Li RM, Tarabichi S, Wyss UP. Knee kinematics of high-flexion activities of daily living performed by male Muslims in the Middle East. J Arthroplasty. 2011;26:319–327. doi: 10.1016/j.arth.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Asano H, Muneta T, Sekiya I. Soft tissue tension in extension in total knee arthroplasty affects postoperative knee extension and stability. Knee Surg Sports Traumatol Arthrosc. 2008;16:999–1003. doi: 10.1007/s00167-008-0591-1. [DOI] [PubMed] [Google Scholar]
- 3.Bellemans J, Banks S, Victor J, Vandenneucker H, Moemans A. Fluoroscopic analysis of the kinematics of deep flexion in total knee arthroplasty: influence of posterior condylar offset. J Bone Joint Surg Br. 2002;84:50–53. doi: 10.1302/0301-620X.84B1.12432. [DOI] [PubMed] [Google Scholar]
- 4.Chalidis BE, Sachinis NP, Papadopoulos P, Petsatodis E, Christodoulou AG, Petsatodis G. Long-term results of posterior-cruciate-retaining Genesis I total knee arthroplasty. J Orthop Sci. 2011;16:726–731. doi: 10.1007/s00776-011-0152-1. [DOI] [PubMed] [Google Scholar]
- 5.Dennis DA, Clayton ML, O’Donnell S, Mack RP, Stringer EA. Posterior cruciate condylar total knee arthroplasty: average 11-year follow-up evaluation. Clin Orthop Relat Res. 1992;281:168–176. [PubMed] [Google Scholar]
- 6.Dennis DA, Komistek RD, Stiehl JB, Walker SA, Dennis KN. Range of motion after total knee arthroplasty: the effect of implant design and weight-bearing conditions. J Arthroplasty. 1998;13:748–752. doi: 10.1016/S0883-5403(98)90025-0. [DOI] [PubMed] [Google Scholar]
- 7.Griffin FM, Insall JN, Scuderi GR. Accuracy of soft tissue balancing in total knee arthroplasty. J Arthroplasty. 2000;15:970–973. doi: 10.1054/arth.2000.6503. [DOI] [PubMed] [Google Scholar]
- 8.Hanratty BM, Thompson NW, Wilson RK, Beverland DE. The influence of posterior condylar offset on knee flexion after total knee replacement using a cruciate-sacrificing mobile-bearing implant. J Bone Joint Surg Br. 2007;89:915–918. doi: 10.1302/0301-620X.89B7.18920. [DOI] [PubMed] [Google Scholar]
- 9.Harvey IA, Barry K, Kirby SP, Johnson R, Elloy MA. Factors affecting the range of movement of total knee arthroplasty. J Bone Joint Surg Br. 1993;75:950–955. doi: 10.1302/0301-620X.75B6.8245090. [DOI] [PubMed] [Google Scholar]
- 10.Hemmerich A, Brown H, Smith S, Marthandam SS, Wyss UP. Hip, knee, and ankle kinematics of high range of motion activities of daily living. J Orthop Res. 2006;24:770–781. doi: 10.1002/jor.20114. [DOI] [PubMed] [Google Scholar]
- 11.Higuchi H, Hatayama K, Shimizu M, Kobayashi A, Kobayashi T, Takagishi K. Relationship between joint gap difference and range of motion in total knee arthroplasty: a prospective randomised study between different platforms. Int Orthop. 2009;33:997–1000. doi: 10.1007/s00264-009-0772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 13.Kawamura H, Bourne RB. Factors affecting range of flexion after total knee arthroplasty. J Orthop Sci. 2001;6:248–252. doi: 10.1007/s007760100043. [DOI] [PubMed] [Google Scholar]
- 14.Kurosaka M, Yoshiya S, Mizuno K, Yamamoto T. Maximizing flexion after total knee arthroplasty: the need and the pitfalls. J Arthroplasty. 2002;17(4 suppl 1):59–62. doi: 10.1054/arth.2002.32688. [DOI] [PubMed] [Google Scholar]
- 15.Kuster MS, Bitschnau B, Votruba T. Influence of collateral ligament laxity on patient satisfaction after total knee arthroplasty: a comparative bilateral study. Arch Orthop Trauma Surg. 2004;124:415–417. doi: 10.1007/s00402-004-0700-7. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda Y, Ishii Y, Noguchi H, Ishii R. Effect of flexion angle on coronal laxity in patients with mobile-bearing total knee arthroplasty prostheses. J Orthop Sci. 2005;10:37–41. doi: 10.1007/s00776-004-0863-7. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda Y, Ishii Y, Noguchi H, Ishii R. Varus-valgus balance and range of movement after total knee arthroplasty. J Bone Joint Surg Br. 2005;87:804–808. doi: 10.1302/0301-620X.87B6.15256. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto T, Mizuno K, Muratsu H, Tsumura N, Fukase N, Kubo S, Yoshiya S, Kurosaka M, Kuroda R. Influence of intra-operative joint gap on post-operative flexion angle in osteoarthritis patients undergoing posterior-stabilized total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15:1013–1018. doi: 10.1007/s00167-007-0331-y. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Muratsu H, Tsumura N, Mizuno K, Kuroda R, Yoshiya S, Kurosaka M. Joint gap kinematics in posterior-stabilized total knee arthroplasty measured by a new tensor with the navigation system. J Biomech Eng. 2006;128:867–871. doi: 10.1115/1.2354201. [DOI] [PubMed] [Google Scholar]
- 20.Meneghini RM, Ritter MA, Pierson JL, Meding JB, Berend ME, Faris PM. The effect of the Insall-Salvati ratio on outcome after total knee arthroplasty. J Arthroplasty. 2006;21(6 suppl 2):116–120. doi: 10.1016/j.arth.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Mitts K, Muldoon MP, Gladden M, Jr, Padgett DE. Instability after total knee arthroplasty with the Miller-Galante II total knee: 5- to 7-year follow-up. J Arthroplasty. 2001;16:422–427. doi: 10.1054/arth.2001.22250a. [DOI] [PubMed] [Google Scholar]
- 22.Mulholland SJ, Wyss UP. Activities of daily living in non-Western cultures: range of motion requirements for hip and knee joint implants. Int J Rehabil Res. 2001;24:191–198. doi: 10.1097/00004356-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls RL, Schirm AC, Jeffcote BO, Kuster MS. Tibiofemoral force following total knee arthroplasty: comparison of four prosthesis designs in vitro. J Orthop Res. 2007;25:1506–1512. doi: 10.1002/jor.20438. [DOI] [PubMed] [Google Scholar]
- 24.Parsley BS, Conditt MA, Bertolusso R, Noble PC. Posterior cruciate ligament substitution is not essential for excellent postoperative outcomes in total knee arthroplasty. J Arthroplasty. 2006;21(6 suppl 2):127–131. doi: 10.1016/j.arth.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Rand JA, Ilstrup DM. Survivorship analysis of total knee arthroplasty: cumulative rates of survival of 9200 total knee arthroplasties. J Bone Joint Surg Am. 1991;73:397–409. [PubMed] [Google Scholar]
- 26.Ritter MA, Harty LD, Davis KE, Meding JB, Berend ME. Predicting range of motion after total knee arthroplasty: clustering, log-linear regression, and regression tree analysis. J Bone Joint Surg Am. 2003;85:1278–1285. doi: 10.2106/00004623-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Sambatakakis A, Attfield SF, Newton G. Quantification of soft-tissue imbalance in condylar knee arthroplasty. J Biomed Eng. 1993;15:339–343. doi: 10.1016/0141-5425(93)90013-O. [DOI] [PubMed] [Google Scholar]
- 28.Sasanuma H, Sekiya H, Takatoku K, Takada H, Sugimoto N. Evaluation of soft-tissue balance during total knee arthroplasty. J Orthop Surg (Hong Kong). 2010;18:26–30. doi: 10.1177/230949901001800106. [DOI] [PubMed] [Google Scholar]
- 29.Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system: results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80:850–858. doi: 10.1302/0301-620X.80B5.8368. [DOI] [PubMed] [Google Scholar]
- 30.Scuderi GR. The stiff total knee arthroplasty: causality and solution. J Arthroplasty. 2005;20(4 suppl 2):23–26. doi: 10.1016/j.arth.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Tokuhara Y, Kadoya Y, Nakagawa S, Kobayashi A, Takaoka K. The flexion gap in normal knees: an MRI study. J Bone Joint Surg Br. 2004;86:1133–1136. doi: 10.1302/0301-620X.86B8.15246. [DOI] [PubMed] [Google Scholar]
- 32.Unitt L, Sambatakakis A, Johnstone D, Balancer Study Group Short-term outcome in total knee replacement after soft-tissue release and balancing. J Bone Joint Surg Br. 2008;90:159–165. doi: 10.1302/0301-620X.90B2.19327. [DOI] [PubMed] [Google Scholar]
- 33.Wallace AL, Harris ML, Walsh WR, Bruce WJ. Intraoperative assessment of tibiofemoral contact stresses in total knee arthroplasty. J Arthroplasty. 1998;13:923–927. doi: 10.1016/S0883-5403(98)90200-5. [DOI] [PubMed] [Google Scholar]
- 34.Waslewski GL, Marson BM, Benjamin JB. Early, incapacitating instability of posterior cruciate ligament-retaining total knee arthroplasty. J Arthroplasty. 1998;13:763–767. doi: 10.1016/S0883-5403(98)90027-4. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T, Muneta T, Ishizuki M. Is a minimally invasive approach superior to a conventional approach for total knee arthroplasty? Early outcome and 2- to 4-year follow-up. J Orthop Sci. 2009;14:589–595. doi: 10.1007/s00776-009-1383-2. [DOI] [PubMed] [Google Scholar]
- 36.Whiteside LA. Cementless total knee replacement: nine- to 11-year results and 10-year survivorship analysis. Clin Orthop Relat Res. 1994;309:185–192. [PubMed] [Google Scholar]
- 37.Whiteside LA, Arima J. The anteroposterior axis for femoral rotational alignment in valgus total knee arthroplasty. Clin Orthop Relat Res. 1995;321:168–172. [PubMed] [Google Scholar]
- 38.Winemaker MJ. Perfect balance in total knee arthroplasty: the elusive compromise. J Arthroplasty. 2002;17:2–10. doi: 10.1054/arth.2002.29321. [DOI] [PubMed] [Google Scholar]
- 39.Wyss T, Schuster AJ, Christen B, Wehrli U. Tension controlled ligament balanced total knee arthroplasty: 5-year results of a soft tissue orientated surgical technique. Arch Orthop Trauma Surg. 2008;128:129–135. doi: 10.1007/s00402-007-0541-2. [DOI] [PubMed] [Google Scholar]
- 40.Yamakado K, Kitaoka K, Yamada H, Hashiba K, Nakamura R, Tomita K. Influence of stability on range of motion after cruciate-retaining TKA. Arch Orthop Trauma Surg. 2003;123:1–4. doi: 10.1007/s00402-002-0453-0. [DOI] [PubMed] [Google Scholar]
- 41.Yoshino N, Watanabe N, Watanabe Y, Fukuda Y, Takai S. Measurement of joint gap load in patella everted and reset position during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2009;17:484–490. doi: 10.1007/s00167-008-0656-1. [DOI] [PubMed] [Google Scholar]