Abstract
Background
Recent advances have been made in using chondrocytes and other cell-based therapy to treat cartilage defects in adults. However, it is unclear whether these advances should be extended to the adolescent and young adult-aged patients.
Questions/purposes
We assessed cell-based surgical therapy for patellar osteochondritis dissecans (OCD) in adolescents and young adults by (1) determining function with the International Knee Documentation Committee (IKDC) subjective and Lysholm-Gillquist scores; and (2) evaluating activity level using the Tegner-Lysholm scale.
Methods
We retrospectively reviewed 23 patients between 12 and 21 years of age (mean 16.8 years) treated for OCD lesions involving the patella from 2001 to 2008. Twenty patients had autologous chondrocyte implantation and three patients had cultured bone marrow stem cell implantation. There were 19 males and four females. We obtained preoperative CT scans to assess patella subluxation, tilt, and congruence angle to determine choice of treatment. We obtained IKDC subjective knee evaluation scores, Tegner-Lysholm activity levels, and Lysholm-Gillquist knee scores preoperatively and at 6, 12, and 24 months postoperatively.
Results
Mean IKDC score, Tegner-Lysholm outcomes, and Lysholm-Gillquist scale improved from 45, 2.5, and 50, respectively, at surgery to 75, 4, and 70, respectively, at 24-month followup. Complications include periosteal hypertrophy observed in two patients.
Conclusion
Cell-based therapy was associated with short-term improvement in function in adolescents and young adults with patellar OCD.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteochondritis dissecans (OCD) is a chronic condition characterized by a limited lesion of subchondral bone necrosis, which progresses slowly toward the separation of a segment of articular cartilage and its underlying avascular subchondral bone from the surrounding cancellous bone [1, 28]. This condition affects twice as many males compared with females in the age group of 10 to 20 years [28]. OCD involving the patella is seen in less than 2% of all cases [4]. The primary cause of OCD remains inconclusive, although a widely accepted theory is a combination of repeated trauma and ischemia of the joint surfaces [6, 7, 22].
Treatment of patella articular cartilage lesions is challenging owing to the complexity of the patellofemoral joint and the limited capacity to heal. Historically, several techniques were used to stabilize the lesions and resurface the patellofemoral surface. Matava and Brown [20] described arthroscopic fixation with resorbable pins to stabilize patellar OCD and retain a congruent articulation until healing occurs. Marrow stimulation techniques using abrasion and/or drilling to penetrate the subchondral bone technique were described by Mandelbaum et al. [19], whereas Pridie [25] reported the use of microfracture techniques to stimulate the development of a fibrocartilaginous layer.
Cell-based therapy broadly encompasses autologous chondrocyte implantation (ACI) and bone marrow-derived mesenchymal stem cell (BMSC) implantation. ACI reportedly improves function in patients with full-thickness patellofemoral chondral defects [15]; in one article, chondroplasty increased the level of knee function in 86% of the patients at 2-year followup [14] and in another was associated with durable function for as long as 11 years [23]. Gobbi et al. [11] reported improvements in the International Knee Documentation Committee (IKDC) subjective scores (46.09 to 77.06), Tegner-Lysholm (2.56 to 4.94), and EuroQol Visual Analog Scale (56.76 to 78.23) at 2 years using chondrocyte grafts but a decline of the IKDC subjective scores and Tegner-Lysholm scores in patients with multiple and patellar lesions from 2 to 5 years followup. Using arthroscopy, Brittberg et al. [5] found transplants were level and had the same macroscopic appearance as the surrounding cartilage at 3 months posttransplant. Bentley et al. [2] also reported that second-look arthroscopy at 1 year demonstrated excellent or good functional outcomes (defined as a score of > 50) in 82% after ACI by the Cincinnati rating system and Stanmore functional rating system. In an animal study, Wakitani et al. [29] reported that uniform differentiation of osteochondral progenitor cells into chondrocytes throughout the defects took place as early as 2 weeks posttransplantation.
Wakitani et al. [30–32] advocated the use of BMSCs for cell-based cartilage repair. They reported better arthroscopic and histological grading in the cell-transplanted group and improvements in patients’ clinical symptoms [31, 32]. In vitro studies supported the feasibility of BMSCs in vitro by demonstrating that sufficient quality chondrocytes can be derived from mesenchymal stem cells [3, 16]. Immature porcine studies by Ho et al. [14] reported enhanced healing with seeded mesenchymal stem cells on an osteochondral implant in a biphasic construct. In the cartilage region, there was reduced incidence of fibrocartilage and improved glycosaminoglycan content. In the bone, a higher degree of mineralization that facilitated the functional restoration of the overlying cartilage was noted. However, this technique has not been studied for the treatment of patella OCD in adolescents and young adults.
We therefore (1) determined function using the IKDC subjective score and Lysholm-Gillquist score; and (2) evaluated activity level using the Tegner-Lysholm scale in adolescents and young adults treated with cell-based therapy for patellar OCD.
Patients and Methods
We reviewed all 23 patients between the ages of 12 and 21 years with OCD lesions in the patella treated by cell-based therapy from 2001 to 2008. Twenty patients had ACI, whereas three patients had BMSCs implanted. The indications for surgery were: (1) pain; (2) locking; and (3) instability. The contraindications included: (1) early osteoarthritic changes; (2) inflammatory diseases such as rheumatoid arthritis; (3) previous trauma or infection to the growth plate; and (4) underlying tibial femoral malalignment. For this study we included patients only with International Cartilage Repair Society (ICRS) Grade 3 or 4 patella OCD diagnosed clinically and radiographically. We excluded patients older than 21 years, those with concomitant inflammatory arthritis, or syndromic or osteochondral defects as a result of traumatic dislocations. Of the 23 patients, 19 (83%) were male and only four were female patients representing a male-to-female ratio of 5:1. The mean age of our patients was 16.8 years (range, 12–21 years). The majority were Chinese (61%), Malay (26%) followed by Indian (13%). Previous major or insidious trauma was reported in 83% of our patients. The minimum followup was 2 years (mean, 6 years; range, 2–11 years). No patients were lost to followup. Data were retrieved from an actively maintained database of patients obtained through prospective interviews preoperatively and at followup visits. The study protocol was approved by the National Healthcare Group Domain-Specific Review Board (NHG DSRB reference number D/00/814) and the University Hospital Ethic Committee.
Preoperatively all patients had plain radiographs of the knee, including AP, lateral, intercondylar, and skyline views. All patients underwent CT scans of tracking of the patella at 0°, 10°, and 20° of flexion to determine patella subluxation, tilt, and congruence angle. Depending on the degree of patella tilt and/or subluxation, concomitant distal realignment or lateral release surgeries were performed. The criteria for realignment surgery were (1) increased tibial tubercle-trochlear groove distance > 15 mm; and/or (2) increased patellar tilt > 20° guided by a clinical assessment of a patellar glide. Concomitant Elmslie-Trillat and Roux-Goldthwaite were performed in four and two patients, respectively.
All patients were treated by the senior author (JHH). For ACI, a small amount of cartilage tissue (1 cm × 0.5 cm) was taken from nonweightbearing areas that were deemed macroscopically healthy by arthroscopy. The harvested tissue was transferred into a specimen container filled with sterile saline (10 mL) and processed within 60 minutes. The sample was washed twice with phosphate-buffered saline (Gibco BRL, Grand Island, NY, USA) and then minced before being transferred aseptically into a tube with 5 mL collagenase NB6 (Sigma, St Louis, MO, USA) for overnight digestion at 37°C in a water bath. Digested chondrocytes were washed with Dulbecco modified Eagle medium (DMEM)/F12 (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL) to stop the enzymatic reaction. These cells were then cultured in T-75-cm2 flasks with DMEM/F12 containing 10% FBS and 50 mg/Ml L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma) in a humidified atmosphere of 5% CO2 at 37°C. Cells were seeded at a cell density of 5000 cells/cm2. We changed the initial medium change after 7 days, when adherent cells were recognized. We subsequently changed the medium two to three times a week until the preparation of cell sheets, which were formed in the presence of ascorbic acid (passage 1). For each surgery, at least four cell sheets were prepared and approximately 2 million cells/cm2 were applied.
For BMSCs, with the patient under local anesthesia, 30 mL of bone marrow was aspirated using a Jamshidi needle from the iliac crests of each patient into heparinized syringes and transferred into sterile containers. Seventy milliliters of each patient’s blood was collected as well. The bone marrow aspirate was processed within 60 minutes. The heparinized bone marrow aspirate was mixed with a one-fifth volume of 6% (w/v) dextran (molecular weight 100,000) (Sigma) and left standing at room temperature for 30 minutes to eliminate erythrocytes. The remaining cells were washed twice with DMEM. These cells were cultured in T-75 cm2 flasks with an initial culture medium consisting of DMEM containing 10% FBS, 50 mg/mL l-ascorbic acid 2-phosphate sesquimagnesium salt hydrate, and 1% antibiotic-antimycotic (penicillin 100 U/mL, streptomycin 0.1 mg/mL, amphotericin B 0.25 mg/mL) (Sigma) in a humidified atmosphere of 5% CO2 at 37°C. The cells were seeded at a density of 10,000 cells/cm2. We initially changed the medium after 5 days when adherent cells were recognized. Subsequently, culture media without antibiotics were used and changed two to three times a week. Sheets of cells were formed in the presence of ascorbic acid (passage 1) and for each surgery. A minimum of four cell sheets with approximately 2 million cells/cm2 was applied. Seventy milliliters of venous blood from each patient was transferred into two 50-mL tubes for overnight incubation at 4°C. After centrifuging the tube with slow acceleration, the serum was carefully aspirated and transferred to a new tube. Repeated centrifugation with slow acceleration for 3 minutes at 3000 rpm at ambient temperature was performed. The serum was aspirated into a syringe and filtered with a sterile 0.2-mm filter. The filtered serum was tested for sterility, antihuman immunodeficiency virus, and hepatitis B antigen, and then stored at a temperature of −20°C. Flow cytometry against CD90+, CD105+, CD14−, and CD34− was used to confirm that cultured cells were mesenchymal stem cells. Saline that was used for transporting the cartilage biopsy specimen to the laboratory, aspirated bone marrow, and culture media (without antibiotic) were tested for sterility and Mycoplasma hominis contamination. For each surgery, at least 10 to 15 million cells (with a viability rate of 96%) were returned for implantation.
Four to 5 weeks after the cells were harvested, surgery was performed following the guidelines outlined by Brittberg et al. [5]. The cell sheets were transported to the operating room in a sterile container within the patients’ own serum. The débrided chondral defect (without damaging subchondral bone) was measured after arthrotomy. Subsequently, we harvested a periosteal patch from the proximal part of the tibia or distal part of the femur according to the measured size. Next, the harvested periosteum was sutured precisely to the rim of the débrided defect(s). The cultured chondrocytes or BMSCs were implanted beneath the patch and very fine stitches (microsuture 7-0) were used to hold the periosteum to the defected site. To avoid cell leakage, we used fibrin glue to create a watertight seal.
To derive maximum benefit from the surgery, patients were advised to strictly follow the rehabilitation protocol. The rehabilitation protocol began on the day of surgery and included passive ROM and isometric muscle contractions. Patients were able to begin active motion and partial weightbearing at 6 weeks progressing to full weightbearing exercises. The rehabilitation protocol varies according to the patient age, previous activity level, and concomitant procedures performed. Rehabilitation focuses on four areas: walking/weightbearing, ROM, strength, and cardiovascular capacity.
Patients were evaluated preoperatively and at 6, 12, and 24 months postoperatively. Assessments were performed by our trained research staff using the ICRS Cartilage Injury Evaluation Package, which included questions from the IKDC subjective knee evaluation form, the knee scale reported by Lysholm and Gillquist [18], and the activity level scale reported by Tegner and Lysholm [27].
The MIXED effect model (with random intercept) was used to evaluate the effect of visit time on individual outcomes such as IKDC, Tegner-Lysholm, and Lysholm-Gillquist scores, respectively. This method of analysis appropriately accounts for the possible correlation between repeated measurements of an individual. Residual plots examining the effect of visit time on IKDC, Tegner-Lysholm, and Lysholm-Gillquist scores showed that the residuals were approximately normally distributed with a mean of 0. All statistical evaluations were made assuming a two-sided test using STATA Version 11 (StataCorp, College Station, TX, USA).
Results
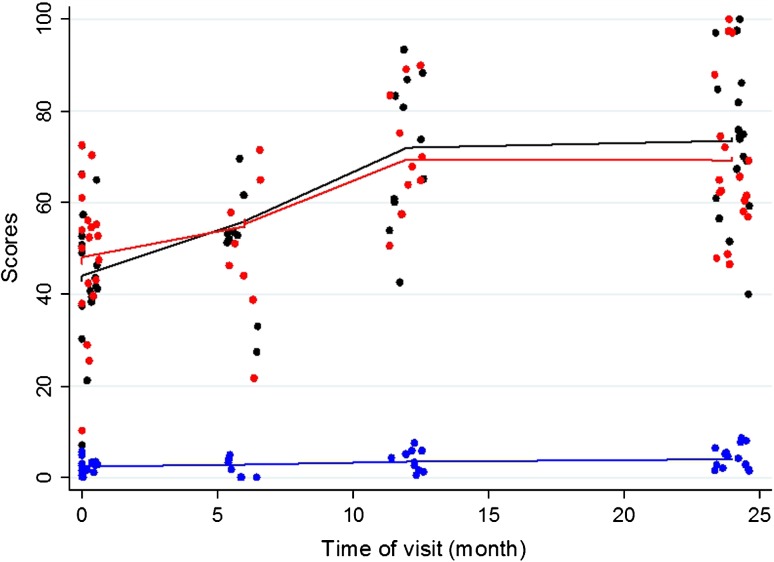
On average, IKDC scores increased by 1.27 (95% CI, 0.87–1.66; p < 0.001) for every subsequent month of visit during the 2-year followup period (Table 1). Improvements were seen as early as at the 6-month followup. Modest improvement was seen after the first year. We found improvements in performance over time throughout the entire followup period from a mean score of 45 (range, 5.8–63.0) at surgery to a mean score of 75 (range, 40.2–96.6) at 24-month followup. The average increase in the Lysholm-Gillquist score was 0.94 (95% CI, 0.56–1.32; p < 0.001) for every subsequent month of visit during the 2-year followup period (Table 1). The mean scores of our patients improved from 50 (range, 11–79) at surgery to 70 (range, 48–100) at 24-month followup (Fig. 1). Similar to the IKDC score, improvement of scores was mainly seen during the first 12 months postoperatively with modest improvement after the first year.
Table 1.
Effect of time on IKDC, Tegner, and Lysholm outcomes
| Scoring instrument | Estimate | 95% CI | p value |
|---|---|---|---|
| IKDC | 1.27 | 0.87–1.66 | < 0.001 |
| Tegner | 0.07 | 0.04–0.10 | < 0.001 |
| Lysholm | 0.94 | 0.56–1.32 | < 0.001 |
IKDC = International Knee Documentation Committee.
Fig. 1.
On average, IKDC scores increased by 1.27 (95% CI, 0.87–1.66; p < 0.001) for every subsequent month of visit. Similarly, the average increase in Tegner-Lysholm and Lysholm-Gillquist scores were 0.07 (95% CI, 0.04–0.10) and 0.94 (95% CI, 0.56–1.32), respectively. The plots for IKDC, Tegner-Lysholm, and Lysholm-Gillquist scores are marked black, blue, and red, respectively.
The average increase in the Tegner-Lysholm activity level score was 0.07 (95% CI, 0.04–0.10; p < 0.001) for every subsequent month of visit during the 2-year follow-up period (Table 1). Premorbidly, our patients reported a mean activity level of 7 (range, 4–9). This corresponds to being able to participate in high-demand recreational sports or low-demand competitive sports. At diagnosis, we noted an initial clustering of scores around the mean of 2.5 (range, 0–5), corresponding to an activity level of light walking on uneven ground (Fig. 1). The mean score at 24-month followup was 4 (range, 2–7). This corresponds to moderately heavy labor or light recreational sports.
We report two cases of particular interest. Case 6, a 16-year-old boy, had an ICRS Grade 3, 2 cm × 2-cm circular lesion on the lateral facet of the left patella and underwent ACI in January 2002. He had IKDC, Tegner-Lysholm, and Lysholm-Gillquist scores of 81.6, 3, and 70 at 2-year followup compared with 21.43, 4, and 42 at surgery, respectively. Case 16, a 17-year-old boy, had an ICRS Grade 3, 2 cm × 1.5-cm lesion on the apex of the left patella and underwent ACI on November 2006. He had IKDC, Tegner-Lysholm, and Lysholm-Gillquist scores of 82.8, 5, and 74 at 2-year followup compared with 5.75, 0, and 11 at surgery, respectively. On MRI, hypertrophy of the periosteum conforming to joint congruity was observed as early as at the 6-month followup (Fig. 2). Subsequent imaging studies demonstrated similar findings. In these two cases, both patients were asymptomatic. The findings and further options were explained to them. Both declined further surgical intervention because they had good functional recovery of the knee.
Fig. 2A–B.
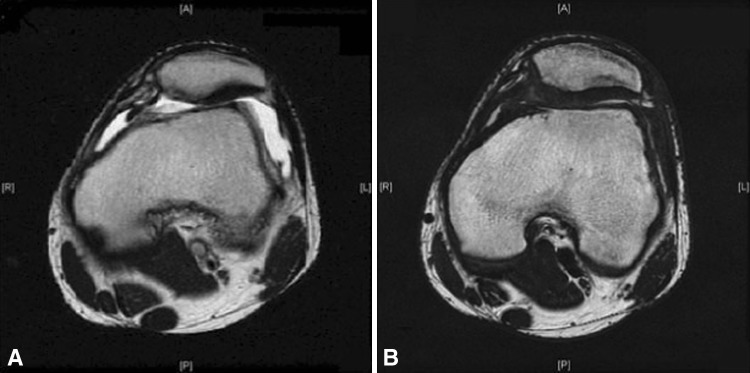
MRI scans of Case 16 (A) at surgery and (B) at 4 months postoperatively showing hypertrophy of periosteum.
Discussion
Recently a number of investigators have developed and used chondrocytes and other cell-based therapy to treat cartilage defects in adults with reported relief of pain and improved function. However, it is unclear whether these advances should be extended to the adolescent and young adult-aged patients. We therefore (1) determined clinical and functional outcomes using the IKDC subjective score and Lysholm-Gillquist score; and (2) evaluated the impact of surgery on activity level using the Tegner-Lysholm scale in assessing the relevance of cell-based therapy for patellar OCD in adolescents and young adults.
We note the following limitations to our study. First, we defined OCD earlier as a chronic condition characterized by a limited lesion of subchondral bone necrosis, which progresses slowly toward the separation of a segment of articular cartilage and its underlying avascular subchondral bone from the surrounding cancellous bone. In practice, it can be difficult to ascertain if the osteochondral defect is attributed to OCD or as a result of an unreported traumatic event. Trauma has been presumed a contributory factor in OCD [1, 17]. To reduce the heterogeneity of the cohort, we excluded patients with a history or signs of overt trauma, including dislocation of the patella and fractures of the other bones; we cannot exclude, however, the possibility of insidious trauma as a contributory factor in the remaining patients. Second, concomitant realignment procedures, a potential confounder, could have spuriously contributed to the functional improvement of our patients. In the literature, concomitant realignment procedures in patella lesions with abnormal tracking tend to have a better outcome [13]. Farr [9] contended that combined treatment of ACI and biomechanics-altering procedures may be a reasonable option for selected patients with coexisting patellofemoral lesions and mechanical disorders. Some of our patients had some form of realignment as a result of the degree of patellar instability. Third, we had a relatively small sample population. Our cohort was also mixed with patients receiving either chondrocytes or BMSCs. Certainly, higher evidence would arise from a large sample population and stratification into various treatment groups and statistical control of potentially confounding variables. Fourth, we focused on subjective patient-reported outcomes and did not look into objective assessment including MRI findings.
Historically, Schwarz et al. [26] concluded that patients who come to surgery for OCD of the patella have a guarded prognosis for full recovery of knee function: 62% had a fair or poor result on their rating scale and commonly had persistent pain and residual patellofemoral crepitus. Using the IKDC and Lysholm-Gillquist scores, we found the pretreatment quality of life is low. The IKDC scores of all our patients were within the 0 to 10th percentile measured on the instrument before surgery. When investigating the use of second-generation ACI in adults for full-thickness patellofemoral chondral defects, Gobbi et al. [11] found that in the eight patients who had second-look arthroscopy and biopsies, the repaired surface was nearly normal with biopsy samples characterized as hyaline-like in appearance. Peterson et al. [23] studied the durability of autologous chondrocyte transplantation of isolated cartilage defects on the femoral condyle or patella in the adult-aged group and concluded the technique is durable for 5 to 11 years. Based on our observations, we believe cell-based therapy remains a good option for treating children with OCD of the patella. It would be interesting to follow up on our group of patients and track their progress over the years.
Although we found improvements in the mean IKDC scores and Lysholm-Gillquist scores over a 2-year followup, not all of our patients returned to the level of activity anticipated for their age. As reported earlier, the mean activity level at 24-month followup was restricted to moderately heavy labor or light recreational sports. Furthermore, we noted an initial clustering of Tegner-Lysholm activity score at the mean of 2.5 at surgery. However, at 24-month followup, a dispersion of scores was observed. This suggests that although the majority of our patients reported functional improvement in terms of IKDC scores, less of them gain a proportionate increase in level of activity. This is of importance in our patients who are young and of school age. The reduction in activity in children can have a potential impact on their social, health, and psychological well-being.
We highlighted two interesting cases (Cases 6 and 16) earlier. In these two patients, the periosteum appeared to have overgrown the defect within limits of the anatomy of the patellofemoral joint. We believe cartilage hypertrophy and remodeling could have occurred in these two patients. Periosteal complications including fibrous overgrowth and periosteal delamination are well described in the literature. Peterson et al. [24] reported the incidence to be as high as 40% (26 of 65 joints) assessed by second-look arthroscopy. In patients requiring reoperation postimplantation, Henderson et al. [12] reported that the most common finding at surgery was periosteal hypertrophy or extrusion, which was painful in 19.2% of patients and tended to occur in the first 2 years from implantation. Certainly, more basic science evidence is needed to provide a good clinical correlation for a higher level of evidence.
ACI has some disadvantages: it requires regional or general anesthesia (for harvesting and implantation) for two knee procedures, there is difficulty in obtaining an adequate number of chondrocytes, the chondrocyte proliferation is slow, and patients may experience donor site morbidity [2, 5, 10]. Newer techniques such as matrix-associated autologous chondrocyte transplantation by using biomaterials seeded with chondrocytes as a scaffold instead of a periosteal patch lead to less surgical time and morbidity. Newer cell-based therapies including BMSCs have been suggested by various authors as a result of its superior proliferation rate and differentiation capacity [3, 16, 21]. Autologous matrix-induced chondrogenesis techniques combining the use of microfracture with matrix-based techniques using a collagen membrane to serve as a scaffold are increasingly studied for its potential to allow effective reconstruction of large fragments of a damaged cartilage surface without the need for a second intervention and complex laboratory preparation [8]. It would be interesting for future studies to compare the various cell-based therapy techniques based on the clinical and functional improvement seen in patients.
In summary, patellar OCD in adolescents may be debilitating. The use of cell-based therapy can improve short-term function, but more work is needed to assess the long-term durability of the procedures.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aichroth P. Osteochondritis dissecans of the knee. A clinical survey. J Bone Joint Surg Br. 1971;53:440–447. [PubMed] [Google Scholar]
- 2.Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–230. doi: 10.1302/0301-620X.85B2.13543. [DOI] [PubMed] [Google Scholar]
- 3.Biant LC, Bentley G. Stem cells and débrided waste: two alternative sources of cells for transplantation of cartilage. J Bone Joint Surg Br. 2007;89:1110–1114. doi: 10.1302/0301-620X.89B8.18911. [DOI] [PubMed] [Google Scholar]
- 4.Bradley J, Dandy DJ. Osteochondritis dissecans and other lesions of the femoral condyles. J Bone Joint Surg Br. 1989;71:518–522. doi: 10.1302/0301-620X.71B3.2722949. [DOI] [PubMed] [Google Scholar]
- 5.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 6.Clanton TO, DeLee JC. Osteochondritis dissecans. History, pathophysiology and current treatment concepts. Clin Orthop Relat Res. 1982;167:50–64. [PubMed] [Google Scholar]
- 7.Desai SS, Patel MR, Michelli LJ, Silver JW, Lidge RT. Osteochondritis dissecans of the patella. J Bone Joint Surg Br. 1987;69:320–325. doi: 10.1302/0301-620X.69B2.3818768. [DOI] [PubMed] [Google Scholar]
- 8.Dhollander AA, De Neve F, Almgvist KF, Verdonk R, Lambrecht S, Elewaut D, Verbruggen G, Verdonk PC. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2011;19:536–542. doi: 10.1007/s00167-010-1337-4. [DOI] [PubMed] [Google Scholar]
- 9.Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment outcomes. Clin Orthop Relat Res. 2007;463:187–194. [PubMed] [Google Scholar]
- 10.Fu FH, Zurakowski D, Browne JE, Mandelbaum B, Erggelet C, Moseley JB, Jr, Anderson AF, Micheli LJ. Autologous chondrocyte implantation versus debridement for treatment of full-thickness chondral defects of the knee: an observational cohort study with 3-year follow-up. Am J Sports Med. 2005;33:1658–1666. doi: 10.1177/0363546505275148. [DOI] [PubMed] [Google Scholar]
- 11.Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, Bathan L, Marcacci M. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37:1083–1092. doi: 10.1177/0363546509331419. [DOI] [PubMed] [Google Scholar]
- 12.Henderson IJ, Gui J, Lavigne P. Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy. 2006;22:1318–1324. doi: 10.1016/j.arthro.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 13.Henderson IJ, Lavigne P. Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee. 2006;13:274–279. doi: 10.1016/j.knee.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Ho ST, Hutmacher DW, Ekaputra AK, Hitendra D, Hui JH. The evaluation of a biphasic osteochondral implant coupled with an electrospun membrane in a large animal model. Tissue Eng Part A. 2010;16(4):1123–1141. doi: 10.1089/ten.tea.2009.0471. [DOI] [PubMed] [Google Scholar]
- 15.Jakob RP, Franz T, Gautier E, Mainil-Varlet P. Autologous osteochondral grafting in the knee: indication, results, and reflections. Clin Orthop Relat Res. 2002;401:170–184. doi: 10.1097/00003086-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 17.Livesley PJ, Milligan GF. Osteochondritis dissecans patellae. Is there a genetic predisposition? Int Orthop. 1992;16:126–129. doi: 10.1007/BF00180201. [DOI] [PubMed] [Google Scholar]
- 18.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 19.Mandelbaum BR, Browne JE, Fu F, Micheli L, Mosely JB, Jr, Erggelet C, Minas T, Peterson L. Articular cartilage lesions of the knee. Am J Sports Med. 1998;26:853–861. doi: 10.1177/03635465980260062201. [DOI] [PubMed] [Google Scholar]
- 20.Matava MJ, Brown CD. Osteochondritis dissecans of the patella: arthroscopic fixation with bioabsorbable pins. Arthroscopy. 1997;13:124–128. doi: 10.1016/S0749-8063(97)90222-X. [DOI] [PubMed] [Google Scholar]
- 21.Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 22.Peters TA, McLean ID. Osteochondritis dissecans of the patellofemoral joint. Am J Sports Med. 2000;28:63–67. doi: 10.1177/03635465000280012201. [DOI] [PubMed] [Google Scholar]
- 23.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 24.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Pridie K. A method of resurfacing knee joints. J Bone Joint Surg Br. 1959;41:618–619. [Google Scholar]
- 26.Schwarz C, Blazina ME, Sisto DJ, Hirsh LC. The results of operative treatment of osteochondritis dissecans of the patella. Am J Sports Med. 1988;16:522–529. doi: 10.1177/036354658801600516. [DOI] [PubMed] [Google Scholar]
- 27.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 28.Visona E, Chouteau J, Aldegheri R, Fessy MH, Moyen B. Patella osteochondritis dissecans end stage: the osteochondral mosaicplasty option. Orthop Traumatol Surg Res. 2010;96:543–548. doi: 10.1016/j.otsr.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 31.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13:595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 32.Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]