Abstract
Background
Mechanical autotransfusion systems for washed shed blood (WSB) were introduced to reduce the need for postoperative allogenic blood transfusions (ABTs). Although some authors have postulated decreased requirements for ABT by using autologous retransfusion devices, other trials, mostly evaluating retransfusion devices for unwashed shed blood (USB), verified a small or no benefit in reducing the need for postoperative ABT. Because of these contradictory findings it is still unclear whether autologous retransfusion systems for WSB can reduce transfusion requirements.
Questions/purposes
We therefore asked whether one such autologous transfusion system for WSB can reduce the requirements for postoperative ABT.
Methods
In a prospective, randomized, controlled study, we enrolled 151 patients undergoing TKA. In Group A (n = 76 patients), the autotransfusion system was used for a total of 6 hours (intraoperatively and postoperatively) and the WSB was retransfused after processing. In Control Group B (n = 75 patients), a regular drain without suction was used. We used signs of anemia and/or a hemoglobin value less than 8 g/dL as indications for transfusion. If necessary, we administered one or two units of allogenic blood.
Results
Twenty-three patients (33%) in Group A, who received an average of 283 mL (range, 160–406 mL) of salvaged blood, needed a mean of 2.1 units of allogenic blood, compared with 23 patients (33%) in Control Group B who needed a mean of 2.1 units of allogenic blood.
Conclusions
We found the use of an autotransfusion system did not reduce the rate of postoperative ABTs.
Level of Evidence
Level II, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
TKA may be associated with substantial perioperative and postoperative blood loss. Gombotz et al. reported rates of intra- and postoperative blood transfusions (autologous and allogenic) after TKA between 12% and 87% with a mean of approximately two units [9]. Because of the inherent risks [10, 33] such as transfusion-associated infections and immunomodulation [2] and economic considerations, one common goal in orthopaedic surgery is to reduce the need for allogenic blood transfusions (ABTs) through perioperative blood management [28]. Some studies support measures for reducing blood loss, which include preoperative blood donation [41], correction of preoperative anemia [37], pharmacologic agents such as tranexamic acid injection [29], and autologous retransfusion systems [5, 8, 26, 34, 36, 43].
Although the use of retransfusion systems is becoming more common in the orthopaedic community [15, 16, 37, 42], it remains unclear whether they reduce the requirement for ABTs. Although some authors [3, 6, 25] have found good-quality drainage blood with a hematocrit of 75% to 88% directly after collection, other studies [1, 14, 19, 31] could not verify a reduction in the requirements for postoperative ABTs by using these systems. However, it is difficult to compare the results of the different studies of retransfusion systems because of differences in techniques for processing shed blood (filtering, washing, and anticoagulation), criteria for transfusion, levels of evidence, and methods for determining blood loss.
In general, retransfusion devices can be divided in two major groups: systems for unwashed shed blood (USB) and systems for washed shed blood (WSB) [21, 23]. Owing to the higher related risk of a transfusion reaction to USB (compared with WSB), some authors [12, 35, 39, 40] advise washing the shed blood before retransfusion.
Because of lack of literature regarding WSB and contradictory results, there is no clear consensus regarding the utility of autotransfusion systems and its economic and clinical benefits to decrease the need for ABTs. Therefore, we performed a randomized, prospective, controlled trial comparing patients treated with an autologous transfusion system for WSB with a control group treated only with a regular drain without suction after TKA.
We determined (1) whether the use of an autologous transfusion system with WSB can reduce requirements for postoperative ABTs; (2) the effect of retransfused WSB on hemoglobin level by measuring hemoglobin values after WSB donation; and (3) whether there is a decreased postoperative infection rate with the use of an autologous transfusion system as postulated by del Trujillo et al. [7]. Our intention was to determine whether it is possible to reduce the need for postoperative ABTs and to lower the early postoperative infection rate.
Patients and Methods
We conducted a prospective, controlled, randomized study that included 151 patients treated with primary elective TKA for osteoarthritis from December 2007 to January 2009. During the study period, we treated a total of 223 patients with TKAs. For this study we included patients with primary osteoarthritis of the knee treated with primary nonconstraining TKA prostheses. We excluded 72 patients for the following reasons: (1) unwillingness to participate in the study (n = 53) and (2) revision arthroplasty (n = 19). The remaining 151 patients were randomized into two groups: Group A, in which the Orthopedic Perioperative Autotransfusion System (OrthoPAT®, Haemonetics Corp, Braintree, MA, USA) was used for intraoperative and postoperative blood salvage and retransfusions (n = 76 patients, 76 TKAs), and Control Group B, in which no retransfusion system was used (n = 75 patients, 75 TKAs). Randomization was accomplished by a blinded method performed by an independent secretary in the hospital’s administration office. Sealed envelopes were generated including an assigned patient code separating patients into two groups. Patients with even-numbered codes were allocated to Group A using OrthoPAT® for intraoperative and postoperative blood salvage and retransfusion. Patients with odd-numbered codes were assigned to Control Group B without using a retransfusion system. The envelopes were opened preoperatively in the operating room shortly before beginning surgery.
Because of missing data, the results of only 140 of the 151 patients were available: 70 patients (70 TKAs) in Group A and 70 patients (70 TKAs) in Control Group B. Six patients in Group A and five in Control Group B were removed from the study owing to (1) lack of data (six patients), (2) technical problems with the retransfusion system (four patients), and (3) acute intraoperative renal failure (one patient). We had prior approval of our institutional review board.
According to a preliminary power analysis that was based on a pilot study in our orthopaedic department, which included 40 patients (20 patients [20 TKAs] treated with OrthoPAT® versus 20 patients [20 TKAs] with no autologous retransfusion system), we found the proportions of 0.35 and 0.60 for ABT rate. A two-group Fisher’s exact test with a two-sided significance level of 0.05 had a power of 80% to detect a difference between the proportions 0.35 and 0.60 when the sample size in each group will be 70 patients. Owing to this a priori power analysis a difference of more than 17 ABTs would make a statistical difference.
We recorded demographic data, medical history (coronary artery disease, use of anticoagulants, and American Society of Anesthesiologists [ASA] classification [13]), preoperative and postoperative hemoglobin levels, duration of surgery, need for ABT, amount of retransfused WSB, and early complications (including allergic reactions, wound infections, minor and major bleeding, deep venous thrombosis, nerve injuries, pulmonary embolism) at the preoperative examination and during the hospital stay.
Preoperative data for cardiopathy, angiopathy, preoperative anemia, and anticoagulant treatment, demographic data (age, BMI, sex, ASA score), and mean duration of surgery showed no differences between Group A and Control Group B (Table 1). Preoperative anticoagulant and antiinflammatory medications were stopped 10 days before surgery. All patients received antithrombotic prophylaxis with 40 mg low-molecular-weight heparin 1 day before surgery and for 42 days postoperatively. If preexisting oral anticoagulation was necessary, treatment was changed to a standardized subcutaneous regime of low-molecular-weight heparin in therapeutic dosage.
Table 1.
Preoperative data
| Variable | Group A (n = 70) | Group B (n = 70) | SD | p value |
|---|---|---|---|---|
| Mean age (years) | 70 | 69 | 8 | 0.971 |
| Mean BMI (kg/m²) | 31 | 32 | 6 | 0.308 |
| Sex (number of patients) | Female: 49 Male: 29 |
Female: 49 Male: 29 |
≥ 0.999 | |
| Cardiopathy/angiopathy (number of patients) | Yes: 5 No: 65 |
Yes: 6 No: 64 |
≥ 0.999 | |
| Preoperative anticoagulant medication (number of patients) | Yes: 19 No: 51 |
Yes: 25 No: 45 |
0.363 | |
| ASA score | 1: 18.5% 2: 70% 3: 11.5% |
1: 18.5% 2: 63% 3: 18.5% |
0.477 | |
| Mean surgery time (minutes) | 100 (54–165) | 93 (46–140) | 21 | 0.075 |
ASA = American Society of Anesthesiologists.
All patients underwent a primary elective TKA without the use of an intraoperative tourniquet using a medial parapatellar surgical approach. The surgeries were performed in the same operating theaters with cemented, posterior-stabilized total knee prostheses with a rotating polyethylene inlay (NexGen® LPS-Flex Mobile; Zimmer, Inc, Warsaw, IN, USA) and a standardized postoperative rehabilitation regimen. The surgical procedures and anesthesia techniques were identical in both groups. Anesthesia technique (spinal or general) was selected according to preference and general state of the patient. Each patient received an intravenous perioperative infection prophylaxis of 1500 mg cefuroxime.
For postoperative pain management, patients of both groups received femoral nerve catheters with continuous infusions of ropivacaine (0.2%, 6 mL/hour) and single injections of ropivacaine (0.2%, 20 mL) via sciatic nerve catheters, if required. Starting at 72 postoperative hours, the pain was managed using NSAIDs and opiates, and the femoral and sciatic nerve pain catheters were removed.
In this study, we used a retransfusion system that processed the collected blood by completing anticoagulation, filtering, washing, and centrifugation steps. In Group A, the autotransfusion system was used for 6 hours total after skin incision (intraoperatively and postoperatively). Intraoperatively, the autotransfusion system was used with a negative pressure of 100 mm Hg; postoperatively, the pressure was at 50 mm Hg. The WSB was anticoagulated, filtered, washed with saline, and centrifuged to separate waste products. After retransfusion of the WSB, Group A continued with a closed drainage system without suction, similar to Control Group B for the same total of 48 hours postoperatively.
Postoperatively the patients were maintained at bedrest with continuous passive motion on the first postoperative day. Physical therapy was started on the second postoperative day twice a day for 45 minutes until the tenth postoperative day. Active exercise therapy with a walking assist three times a day and supervised autonomous training to improve ROM was added on the third postoperative day. After hospital treatment, all patients were transferred to a stationary rehabilitation facility for 3 more weeks. All patients were mobilized with a pair of full-weightbearing crutches for 6 weeks postoperatively.
The indications for transfusion were signs of anemia (vertigo, nausea, vomiting, hypotension [systolic blood pressure < 100 mm Hg], tachycardia [> 100 beats/minute]) or a hemoglobin level less than 8 g/dL. Hemoglobin levels were determined preoperatively, 2 hours after application of WSB, and on Postoperative Days 3 and 5 or if signs of anemia led to the need for ABT. At each administration of ABT, hemoglobin levels were recorded; patients with missing documentation were eliminated from the study.
To compare demographic and clinical data between the two groups, we performed several statistical tests. We determined differences in need for ABTs, sex, occurrence of cardiopathy/angiopathy, and need for preoperative anticoagulants using Fisher’s exact test. The t-test was used for evaluation of hemoglobin values, age, BMI, and surgery time. The Mann-Whitney U test was used for evaluation of the ASA score. We used SPSS® software (SPSS Version 19; SPSS Inc, Chicago, IL, USA) for statistical analyses.
Results
We found no difference (p = 0.999) in the need for ABT between the two groups: 23 of 70 patients (33%) in Group A received ABT versus 23 of 70 patients (33%) in Control Group B (Table 2). All of the allogenic blood units were transfused between Postoperative Days 2 and 5.
Table 2.
Postoperative data
| Variable | Group A (n = 70) | Group B (n = 70) | SD | p value |
|---|---|---|---|---|
| ABT rate (%) | 33 | 33 | ≥ 0.999 | |
| Hemoglobin value (g/dL) | ||||
| Preoperative | 13.9 | 14.0 | 1.3 | 0.583 |
| 2 hours after donation of WSB | 11.8 | 11.4 | 1.2 | 0.062 |
| Postoperative Day 3 | 10.2 | 9.9 | 1.6 | 0.221 |
| Postoperative Day 5 | 10.5 | 10.2 | 1.2 | 0.132 |
| At time of ABT | 8.8 | 8.8 | 6.0 | 0.799 |
| Erythrocyte concentrate (units) | < 2: 4.4% 2: 86.9% > 2: 8.7% |
< 2: 0.0% 2: 95.7% > 2: 4.3% |
||
ABT = allogenic blood transfusion; WSB = washed shed blood.
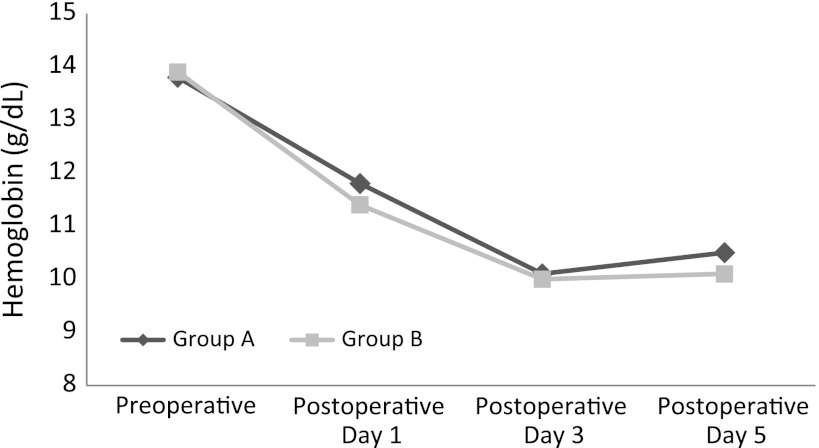
The mean hemoglobin value at the time of ABT was not different (p = 0.799) between the two groups (Table 2). In Group A, patients received a mean of 283 ± 122.6 mL WSB. The hemoglobin values of Group A, versus the values of Control Group B, tended to be higher (p = 0.062) 2 hours after the donation of the WSB but then decreased by Postoperative Days 3 (p = 0.221) and 5 (p = 0.132) (Fig. 1). We found no difference (p = 0.585) in the postoperative blood loss by Redon drainage between the two groups.
Fig. 1.
A graph shows hemoglobin values from preoperatively to 5 days postoperatively, with the low mark between Postoperative Days 3 and 5.
In one patient, we observed immediate hypotension, vomiting, and pyrexia after transfusion of the WSB. However, during the hospital stay (range, 9–14 postoperative days), we found no differences in early postoperative complications (allergic reactions, wound infections, deep venous thrombosis, minor and major bleeding, neural deficiencies, arterial embolism) or the number of erythrocyte units used in this patient because of acute anemia (Table 2).
Discussion
Orthopaedic surgeries, especially TKAs, frequently are associated with substantial perioperative and postoperative blood loss. Consequently, postoperative blood transfusions are common [9]. However, because of the well-documented risks of ABTs, such as transfusion-associated infections or immunomodulatory effects [2], a reduction in the use of ABT would be a great improvement. Although some authors reported the use of autologous retransfusion systems as a good opportunity to reduce perioperative blood loss [5, 26, 34, 36], there is no consensus regarding whether retransfusion systems also can reduce the use of postoperative ABTs. Therefore, we determined (1) whether the use of an autologous transfusion system with WSB can reduce requirements for postoperative ABTs; (2) the effect of retransfused WSB on hemoglobin level by measuring hemoglobin values after WSB donation; and (3) whether there is a decreased postoperative infection rate with the use of an autologous transfusion system as postulated by del Trujillo et al. [7].
Despite a prospective, randomized, controlled study design, this trial is restricted by some limitations. First, we could only use the results of 140 patients because of the exclusion criteria and incomplete documentation of the data. Second, we did not determine blood loss and the additional blood loss owing to the vacuum activity of the OrthoPAT® was not included in this evaluation. An accurate measurement of intraoperative blood loss is time intensive and needs the complex cooperation of different departments (orthopaedic surgeons, anesthetists, and nursing staff), which was not possible in our setting.
We found no benefit in using OrthoPAT® as a retransfusion system for WSB. Our results verified no reduction in the need for postoperative ABT by using OrthoPAT® as a retransfusion device for WSB. This is in contrast to some studies [7, 11, 20, 22, 24, 34] reporting a decreased requirement for ABT with a lower risk of transfusion-associated complications and economic benefit using one of several autologous retransfusion devices (OrthoPAT®; ABT Trans® [Surgical Innovations Ltd, Leeds, UK], Bellovac ABT® [AstraTech AB, Mölndal, Sweden]). In contradiction to our data, del Trujillo et al. postulated a lower ABT rate for patients undergoing THAs who were treated with the OrthoPAT® retransfusion device [7]. However, because we evaluated the OrthoPAT® for TKA a direct comparison with our results is difficult. Moonen et al. [20] reported a lower ABT rate in the retransfused group using the Bellovac ABT® for THAs and TKAs. This contrasts with the report of Amin et al. [1], who found no benefit of using Bellovac ABT® for only TKAs. Grosvenor et al. [11], Munoz et al. [22], and Smith et al. [34] suggested a decreased ABT rate for patients treated with autologous retransfusion systems. However, these studies used different retransfusion devices for TKAs and THAs. Therefore a reliable comparison with our results is not possible (Table 3). In addition, the retrospective study design [11], the comparison of a prospective study group with a retrospective control group [22], or the missing randomization procedure in a prospective published trial [7], all postulating a lower ABT rate for the reinfusion group, are critical limitations and therefore not directly comparable to our prospective, randomized study. Some previous studies that have noted only a small or no benefit in reducing the need for postoperative ABT evaluated a retransfusion device for USB [17, 18, 23, 30]. Even when trials reported a lower ABT rate for the autologous retransfusion group, they either found no difference in administered ABT units per patient [7, 20,34] or the ABT number per patient transfused was not analyzed [11, 22, 34] (Table 3). We believe patient blood management of individual patients would be more likely to reduce the number of administered ABT units in the retransfused and control groups than using an autologous retransfusion device. The vacuum activity of the retransfusion device caused additional blood loss, which could explain the absence of any benefit of the retransfusion system [18].
Table 3.
Comparison of current study results with literature
| Study | Group | Surgery | Number of patients | Device used | Shed blood | Mean retransfused salvaged blood (mL) | ABT rate (%) | Units/patients transfused | Mean hemoglobin preoperative (g/dL) | Mean hemoglobin postoperative (g/dL) | Followup postoperative hemoglobin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amin et al. [1] | Reinfusion group | TKA | 92 | Bellovac ABT® | WSB | 481 | 13 | 1.8 | 13.2 | 11.0 | 3rd postoperative day |
| Control group | TKA | 86 | 15 | 2 | 13.4 | 10.8 | |||||
| p values | 0.439 | > 0.05 | 0.864 | 0.968 | |||||||
| del Trujillo et al. [7] | Reinfusion group | THA | 60 | OrthoPAT® | WSB | 205 | 15 | 1.7 | 14.3 | 10.7 | 4th postoperative day |
| Control group | THA | 48 | 48 | 2.2 | 14.1 | 10.2 | |||||
| p values | 0.001* | 0.187 | 0.474 | 0.095 | |||||||
| Grosvenor et al. [11] | Reinfusion group | THA | 82 | ConstaVac CBC | USB | 349 | 10 | NA | NA | NA | NA |
| Control group | THA | 74 | 23 | NA | NA | NA | |||||
| p values | < 0.05* | NA | NA | NA | |||||||
| Moonen et al. [20] | Reinfusion group | THA/TKA | 80 | Bellovac ABT® | WSB | 308 | 6 | 2.2 | 14.0 | 9.9 | 3rd postoperative day |
| Control group | THA/TKA | 80 | 19 | 1.5 | 14.0 | 9.6 | |||||
| p values | 0.015* | > 0.05 | > 0.05 | > 0.05 | |||||||
| Munoz et al. [22] | Reinfusion group | TKA | 200 | ConstaVac CBC II | USB | NA | 11 | NA | 13.5 | 10.4 | 24–48 hours |
| Control group | TKA | 100 | 48 | NA | 13.6 | 10.4 | |||||
| p-values | < 0.01* | NA | > 0.05 | > 0.05 | |||||||
| Smith et al. [34] | Reinfusion group | THA | 76 | ABTrans® | WSB | 252 | 8 | 2.3 | 13.6 | 10.8 | Immediately postoperative |
| Control group | THA | 82 | 21 | 2.6 | 13.6 | 10.6 | |||||
| p values | 0.022* | NA | 0.859 | 0.324 | |||||||
| Current study | Reinfusion group | TKA | 76 | OrthoPAT® | WSB | 283 | 33 | 2.1 | 13.9 | 10.2 | 3rd postoperative day |
| Control group | TKA | 75 | 33 | 2.1 | 14.0 | 9.9 | |||||
| p values | ≥ 0.999 | > 0.05 | 0.583 | 0.221 |
WSB = washed shed blood; USB = unwashed shed blood; NA = not analyzed; * = significant difference.
The hemoglobin values tended to be higher in Group A 2 hours after WSB donation, which was not the case in the subsequent determinations on the third and fifth postoperative days. Although several articles have suggested a high hematocrit level of collected drainage blood [3, 6, 25], use of WSB did not result in a substantially higher hemoglobin value in the reinfusion group at all measurement points. The same conclusions have been proposed by others [1, 7, 20, 22], suggesting a small and insignificant elevation of the hemoglobin value after autologous blood retransfusion 3 to 4 days postoperatively. Smith et al. [34] measured hemoglobin values immediately after surgery. Grosvenor et al. [11] provided no postoperative hemoglobin levels for the reinfusion and control groups. Therefore a reliable comparison to our results was not possible (Table 3). However, our findings were similar to those in the literature since all published studies reporting preoperative hemoglobin levels between the retransfusion and control groups found no differences [1, 7, 20, 22, 34] (Table 3).
Although we found no differences in postoperative complications and occurrence of transfusion reactions between our two groups, del Trujillo et al. [7] reported a trend toward lower postoperative infection rates for patients treated with the OrthoPAT® autologous retransfusion system. However, we can confirm there is no higher incidence of early postoperative infections and the OrthoPAT® device is easy to use. Although we have used this device for several years, technical problems have occurred in just two patients during the study period, during which the autologous transfusion system was out of order.
Comparison of our results with those of other trials is difficult owing to the use of different autologous transfusion systems for TKAs and THAs. Our study is the only trial evaluating the OrthoPAT® for TKA. We found no differences in reducing the number of required ABTs or hemoglobin levels during followup. In one patient, we observed an allergic reaction during retransfusion of the WSB. The cost savings achieved by eliminating the autotransfusion system can be used to enhance the patient’s individual blood management as a key way to reduce blood transfusions [32, 38]. As a result of our findings, we now collect blood 8 weeks before the operation to determine the hemoglobin value and, if necessary, correct it (intravenous iron, erythropoietin). Additionally, intraoperatively we administer a single shot of tranexamic acid (if there is no contraindication) and check hemoglobin levels 6 hours postoperatively. If the levels are lower than 9.5 g/dL, they are monitored daily; otherwise, they are checked on Postoperative Days 3 and 5 or at any sign of anemia. If the hemoglobin level is lower than 8 g/dL, we try to limit the number of allogenic blood units by administering only one, in accordance with the clinical state and medical history. It is also our intention to reduce the need for ABTs by improving individual blood management for each patient [4, 27] and to find a more exact personal limit of the hemoglobin level.
Acknowledgments
We thank R. Germann Assoc. Prof. MD and G. Pfanner MD, Department of Anesthesia, University Teaching Hospital Feldkirch, Medical University of Innsbruck, Feldkirch, Austria, and A. von Strempel Assoc. Prof., MD, DEng, Department of Orthopaedic Surgery, University Teaching Hospital, Medical University of Innsbruck, Feldkirch, Austria, for scientific assistance and editorial review.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Department of Orthopaedic Surgery, Academic Teaching Hospital, Medical University of Innsbruck, Feldkirch, Austria.
References
- 1.Amin A, Watson A, Mangwani J, Nawabi D, Ahluwalia R, Loeffler M. A prospective randomised controlled trial of autologous retransfusion in total knee replacement. J Bone Joint Surg Br. 2008;90:451–454. doi: 10.2106/JBJS.G.00857. [DOI] [PubMed] [Google Scholar]
- 2.Biedler AE, Schneider SO, Seyfert U, Rensing H, Grenner S, Girndt M, Bauer I, Bauer M. Impact of alloantigens and storage-associated factors on stimulated cytokine response in an in vitro model of blood transfusion. Anesthesiology. 2002;97:1102–1109. doi: 10.1097/00000542-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Buchta C, Hanslik-Schnabel B, Weigl R, List J, Körmöczi GF, Macher M, Heinzl H, Höcker P, Wanivenhaus A, Kurz M. Quality of drainage blood: survival of red cells after re-transfusion and content of free hemoglobin and potassium. Int J Surg. 2005;3:250–253. doi: 10.1016/j.ijsu.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Clark CR. Perioperative blood management in total hip arthroplasty. Instr Course Lect. 2009;58:167–172. [PubMed] [Google Scholar]
- 5.Clark CR, Spratt KF, Blondin M, Craig S, Fink L. Perioperative autotransfusion in total hip and knee arthroplasty. J Arthroplasty. 2006;21:23–35. doi: 10.1016/j.arth.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Colwell CW, Jr, Beutler E, West C, Hardwick ME, Morris BA. Erythrocyte viability in blood salvaged during total joint arthroplasty with cement. J Bone Joint Surg Am. 2002;84:23–25. doi: 10.1302/0301-620X.84B1.12034. [DOI] [PubMed] [Google Scholar]
- 7.del Trujillo MM, Carrero A, Muñoz M. The utility of the perioperative autologous transfusion system OrthoPAT® in total hip replacement surgery: a prospective study. Arch Orthop Trauma Surg. 2008;128:1031–1038. doi: 10.1007/s00402-007-0440-6. [DOI] [PubMed] [Google Scholar]
- 8.Garvin KL, Feschuk CA, Sekundiak TD, Lyden ER. Blood salvage and allogenic transfusion needs in revision hip arthroplasty. Clin Orthop Relat Res. 2005;441:205–209. doi: 10.1097/01.blo.0000192033.50316.bf. [DOI] [PubMed] [Google Scholar]
- 9.Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47:1468–1480. doi: 10.1111/j.1537-2995.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodnough LT. Risks of blood transfusion. Anesthesiol Clin North America. 2005;23:241–252, v. [DOI] [PubMed]
- 11.Grosvenor D, Goyal V, Goodman S. Efficacy of postoperative blood salvage following total hip arthroplasty in patients with and without deposited autologous units. J Bone Joint Surg Am. 2000;82:951–954. doi: 10.2106/00004623-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Hansen E, Pawlik M. Reasons against the retransfusion of unwashed wound blood. Transfusion. 2004;44:45S–53S. doi: 10.1111/j.0041-1132.2004.04179.x. [DOI] [PubMed] [Google Scholar]
- 13.Harling DW. Consistency of ASA grading. Anaesthesia. 1995;50:659. [PubMed] [Google Scholar]
- 14.Hazarika S, Bhattacharya R, Bhavikatti M, Dawson M. A comparison of post-op haemoglobin levels and allogeneic blood transfusion rates following total knee arthroplasty without drainage or with reinfusion drains. Acta Orthop Belg. 2010;76:74–78. [PubMed] [Google Scholar]
- 15.Hendrych J. [Use of post-operative drainage and auto-transfusion sets in total knee arthroplasty] [in Czech] Acta Chir Orthop Traumatol Cech. 2006;73:34–38. [PubMed] [Google Scholar]
- 16.Jones HW, Savage L, White C, Goddard R, Lumley H, Kashif F, Gurusany K. Postoperative autologous blood salvage drains are they useful in primary uncemented hip and knee arthroplasty? A prospective study of 186 cases. Acta Orthop Belg. 2004;70:466–473. [PubMed] [Google Scholar]
- 17.Lakshmanan P, Purushothaman B, Sharma A. Impact of reinfusion drains on hemoglobin level in total knee arthroplasty. Am J Orthop (Belle Mead NJ). 2010;39:70–74. [PubMed] [Google Scholar]
- 18.Martin A, von Strempel A. Transfusion of autologous blood from reinfusion systems in total knee arthroplasty. Int Orthop. 2006;30:541–544. doi: 10.1007/s00264-006-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauerhan DR, Nussman D, Mokris JG, Beaver WB. Effect of postoperative reinfusion systems on hemoglobin levels in primary total hip and total knee arthroplasties: a prospective randomized study. J Arthroplasty. 1993;8:523–527. doi: 10.1016/S0883-5403(06)80218-4. [DOI] [PubMed] [Google Scholar]
- 20.Moonen AF, Knoors NT, van Os JJ, Verburg AD, Pilot P. Retransfusion of filtered shed blood in primary total hip and knee arthroplasty: a prospective randomized clinical trial. Transfusion. 2007;47:379–384. doi: 10.1111/j.1537-2995.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- 21.Moonen AF, Neal TD, Pilot P. Peri-operative blood management in elective orthopaedic surgery: a critical review of the literature. Injury. 2006;37:S11–S16. doi: 10.1016/S0020-1383(07)70006-2. [DOI] [PubMed] [Google Scholar]
- 22.Muñoz M, Ariza D, Garcerán MJ, Gómez A, Campos A. Benefits of postoperative shed blood reinfusion in patients undergoing unilateral total knee replacement. Arch Orthop Trauma Surg. 2005;125:385–389. doi: 10.1007/s00402-005-0817-3. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz M, García-Vallejo JJ, Ruiz MD, Romero R, Olalla E, Sebastián C. Transfusion of post-operative shed blood: laboratory characteristics and clinical utility. Eur Spine J. 2004;13(suppl 1):S107–S113. doi: 10.1007/s00586-004-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz M, Kühlmorgen B, Ariza D, Haro E, Marroquí A, Ramirez G. Which patients are more likely to benefit from postoperative shed blood salvage after unilateral total knee replacement? An analysis of 581 consecutive procedures. Vox Sang. 2007;92:136–141. doi: 10.1111/j.1423-0410.2006.00868.x. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz Gómez M, Ariza Villanueva D, Romero Ruiz A, Muñoz Morán E, Prat Arrojo I, Gómez Luque A. [Evaluation of the OrthoPAT autologous transfusion system by experimental models simulating intra- and postoperative blood salvage][in Spanish]. Rev Esp Anestesiol Reanim. 2005;52:321–327. [PubMed]
- 26.Newman JH, Bowers M, Murphy J. The clinical advantages of autologous transfusion: a randomized, controlled study after knee replacement. J Bone Joint Surg Br. 1997;79:630–632. doi: 10.1302/0301-620X.79B4.7272. [DOI] [PubMed] [Google Scholar]
- 27.Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86:1512–1518. doi: 10.2106/00004623-200407000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Pola E, Papaleo P, Santoliquido A, Gasparini G, Aulisa L, De Santis E. Clinical factors associated with an increased risk of perioperative blood transfusion in nonanemic patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2004;86:57–61. doi: 10.2106/00004623-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Ralley FE, Berta D, Binns V, Howard J, Naudie DD. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468:1905–1911. doi: 10.1007/s11999-009-1217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reize P, Endele D, Rudert M, Wülker N. [Postoperative autologous transfusion from blood drainage after total hip joint arthroplasty: how much value is really there?] [in German] Z Orthop Ihre Grenzgeb. 2006;144:400–404. doi: 10.1055/s-2006-942173. [DOI] [PubMed] [Google Scholar]
- 31.Rollo VJ, Hozack WJ, Rothman RH, Chao W, Eng KO. Prospective randomized evaluation of blood salvage techniques for primary total hip arthroplasty. J Arthroplasty. 1995;10:532–539. doi: 10.1016/S0883-5403(05)80157-3. [DOI] [PubMed] [Google Scholar]
- 32.Rosencher N, Kerkkamp HE, Macheras G, Munuera LM, Menichella G, Barton DM, Cremers S, OSTHEO Investigation Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–469. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections: the Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 34.Smith LK, Williams DH, Langkamer VG. Post-operative blood salvage with autologous retransfusion in primary total hip replacement. J Bone Joint Surg Br. 2007;89:1092–1097. doi: 10.2106/JBJS.F.00615. [DOI] [PubMed] [Google Scholar]
- 35.Southern EP, Huo MH, Mehta JR, Keggi KJ. Unwashed wound drainage blood: what are we giving our patients? Clin Orthop Relat Res. 1995;320:235–246. [PubMed] [Google Scholar]
- 36.Steinberg EL, Ben-Galim P, Yaniv Y, Dekel S, Menahem A. Comparative analysis of the benefits of autotransfusion of blood by a shed blood collector after total knee replacement. Arch Orthop Trauma Surg. 2004;124:114–118. doi: 10.1007/s00402-003-0629-2. [DOI] [PubMed] [Google Scholar]
- 37.Strümper D, Weber EW, Gielen-Wijffels S, Van Drumpt R, Bulstra S, Slappendel R, Durieux ME, Marcus MA. Clinical efficacy of postoperative autologous transfusion of filtered shed blood in hip and knee arthroplasty. Transfusion. 2004;44:1567–1571. doi: 10.1111/j.1537-2995.2004.03233.x. [DOI] [PubMed] [Google Scholar]
- 38.Tobias JD. Strategies for minimizing blood loss in orthopedic surgery. Semin Hematol. 2004;41(1 suppl 1):145–156. doi: 10.1053/j.seminhematol.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Warner C. The use of the orthopaedic perioperative autotransfusion (OrthoPAT) system in total joint replacement surgery. Orthop Nurs. 2001;20:29–32. doi: 10.1097/00006416-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Waters JH, Dyga RM. Postoperative blood salvage: outside the controlled world of the blood bank. Transfusion. 2007;47:362–365. doi: 10.1111/j.1537-2995.2007.01152.x. [DOI] [PubMed] [Google Scholar]
- 41.Woolson ST, Wall WW. Autologous blood transfusion after total knee arthroplasty: a randomized, prospective study comparing predonated and postoperative salvage blood. J Arthroplasty. 2003;18:243–249. doi: 10.1054/arth.2003.50058. [DOI] [PubMed] [Google Scholar]
- 42.Zacharopoulos A, Apostolopoulos A, Kyriakidis A. The effectiveness of reinfusion after total knee replacement: a prospective randomised controlled study. Int Orthop. 2007;31:303–308. doi: 10.1007/s00264-006-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarin J, Grosvenor D, Schurman D, Goodman S. Efficacy of intraoperative blood collection and reinfusion in revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85:2147–2151. doi: 10.2106/00004623-200311000-00013. [DOI] [PubMed] [Google Scholar]