Abstract
The signal transduction pathway by which insulin stimulates glucose transport is largely unknown, but a role for tyrosine and serine/threonine kinases has been proposed. Since mitogen-activated protein (MAP) kinase is activated by insulin through phosphorylation on both tyrosine and threonine residues, we investigated whether MAP kinase and its upstream regulator, p21ras, are involved in insulin-mediated glucose transport. We did this by examining the time- and dose-dependent stimulation of glucose uptake in relation to the activation of Ras-GTP formation and MAP kinase by thrombin, epidermal growth factor (EGF), and insulin in 3T3-L1 adipocytes. Ras-GTP formation was stimulated transiently by all three agonists, with a peak at 5 to 10 min. Thrombin induced a second peak at approximately 30 min. The activation of p21ras was paralleled by both the phosphorylation and the activation of MAP kinase: transient for insulin and EGF and biphasic for thrombin. However, despite the strong activation of Ras-GTP formation and MAP kinase by EGF and thrombin, glucose uptake was not stimulated by these agonists, in contrast to the eightfold stimulation of 2-deoxy-D-[14C]glucose uptake by insulin. In addition, insulin-mediated glucose transport was not potentiated by thrombin or EGF. Although these results cannot exclude the possibility that p21ras and/or MAP kinase is needed in conjunction with other signaling molecules that are activated by insulin and not by thrombin or EGF, they show that the Ras/MAP kinase signaling pathway alone is not sufficient to induce insulin-mediated glucose transport.
Full text
PDF

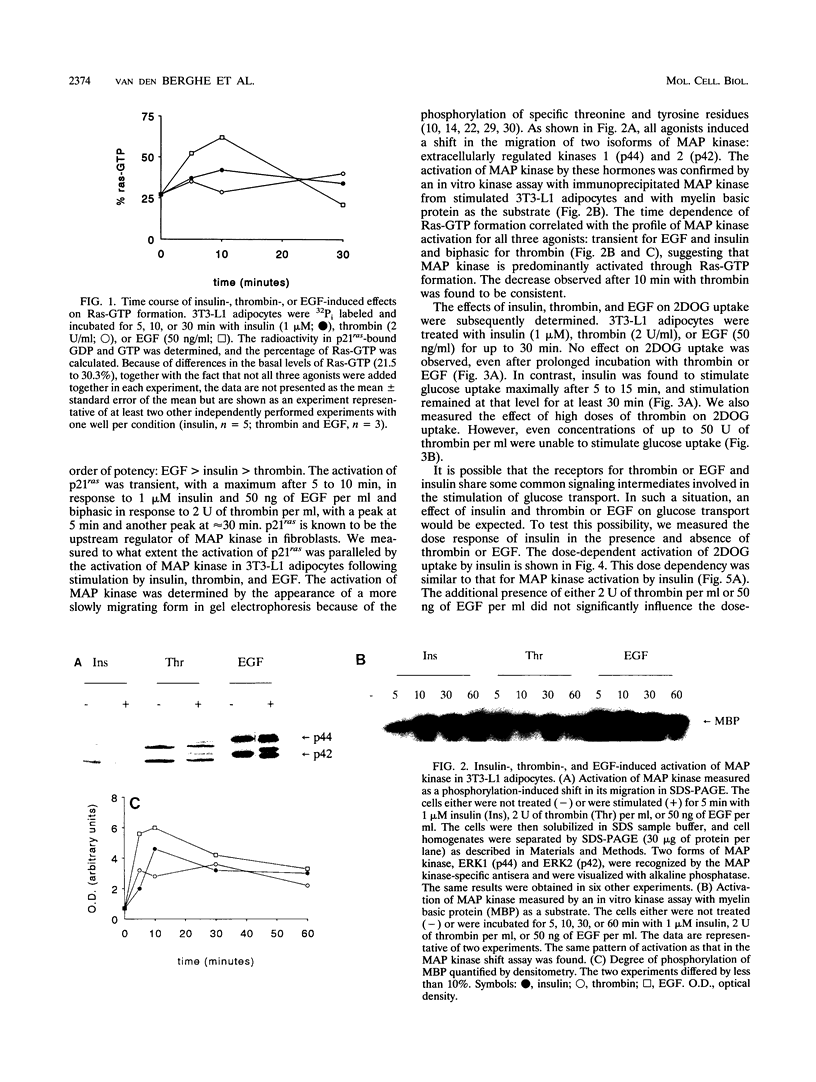
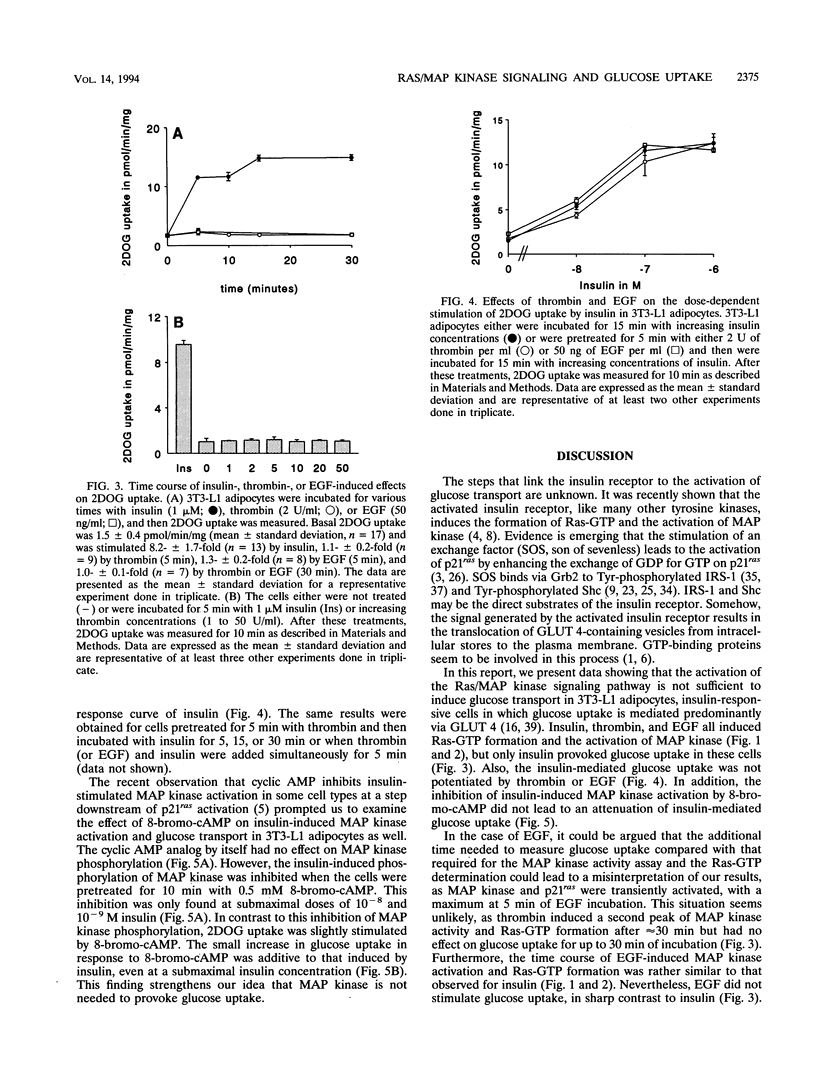
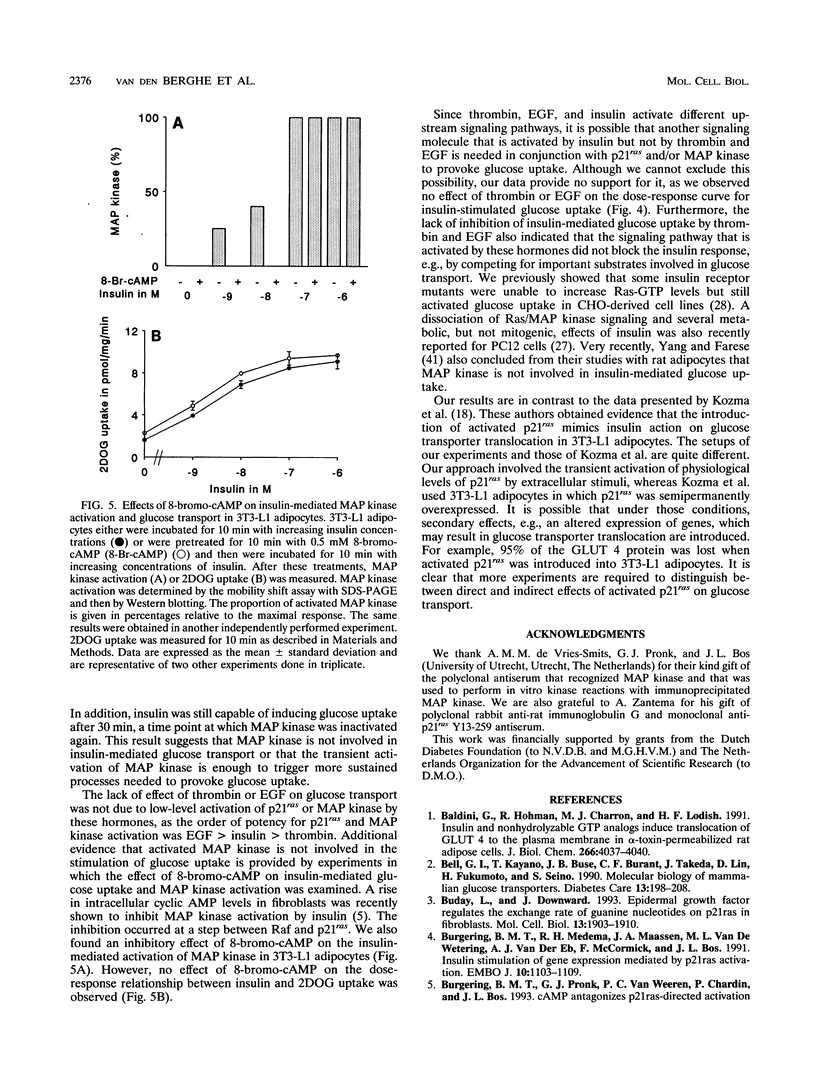

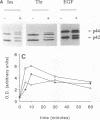
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini G., Hohman R., Charron M. J., Lodish H. F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT 4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J Biol Chem. 1991 Mar 5;266(7):4037–4040. [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates the exchange rate of guanine nucleotides on p21ras in fibroblasts. Mol Cell Biol. 1993 Mar;13(3):1903–1910. doi: 10.1128/mcb.13.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering B. M., Medema R. H., Maassen J. A., van de Wetering M. L., van der Eb A. J., McCormick F., Bos J. L. Insulin stimulation of gene expression mediated by p21ras activation. EMBO J. 1991 May;10(5):1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormont M., Tanti J. F., Grémeaux T., Van Obberghen E., Le Marchand-Brustel Y. Subcellular distribution of low molecular weight guanosine triphosphate-binding proteins in adipocytes: colocalization with the glucose transporter Glut 4. Endocrinology. 1991 Dec;129(6):3343–3350. doi: 10.1210/endo-129-6-3343. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Alessandrini A., Erikson R. L. Erks: their fifteen minutes has arrived. Cell Growth Differ. 1992 Feb;3(2):135–142. [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Sanghera J. S., Stewart J., Pelech S. L. Selective activation of p42 mitogen-activated protein (MAP) kinase in murine B lymphoma cell lines by membrane immunoglobulin cross-linking. Evidence for protein kinase C-independent and -dependent mechanisms of activation. Biochem J. 1992 Oct 1;287(Pt 1):269–276. doi: 10.1042/bj2870269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Sakai H. Okadaic acid activates microtubule-associated protein kinase in quiescent fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):671–674. doi: 10.1111/j.1432-1033.1990.tb19385.x. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- James D. E., Brown R., Navarro J., Pilch P. F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988 May 12;333(6169):183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Izumi T., Tobe K., Shiba T., Momomura K., Tashiro-Hashimoto Y., Kadowaki T. Substrates for insulin-receptor kinase. Diabetes Care. 1990 Mar;13(3):317–326. doi: 10.2337/diacare.13.3.317. [DOI] [PubMed] [Google Scholar]
- Kozma L., Baltensperger K., Klarlund J., Porras A., Santos E., Czech M. P. The ras signaling pathway mimics insulin action on glucose transporter translocation. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4460–4464. doi: 10.1073/pnas.90.10.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane M. D., Flores-Riveros J. R., Hresko R. C., Kaestner K. H., Liao K., Janicot M., Hoffman R. D., McLenithan J. C., Kastelic T., Christy R. J. Insulin-receptor tyrosine kinase and glucose transport. Diabetes Care. 1990 Jun;13(6):565–575. doi: 10.2337/diacare.13.6.565. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Hiken J. F., James D. E. Stimulation of glucose transport and glucose transporter phosphorylation by okadaic acid in rat adipocytes. J Biol Chem. 1990 Nov 15;265(32):19768–19776. [PubMed] [Google Scholar]
- Leevers S. J., Marshall C. J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992 Feb;11(2):569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Slot J. W., James D. E., Mueckler M. M. How cells absorb glucose. Sci Am. 1992 Jan;266(1):86–91. doi: 10.1038/scientificamerican0192-86. [DOI] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Medema R. H., de Vries-Smits A. M., van der Zon G. C., Maassen J. A., Bos J. L. Ras activation by insulin and epidermal growth factor through enhanced exchange of guanine nucleotides on p21ras. Mol Cell Biol. 1993 Jan;13(1):155–162. doi: 10.1128/mcb.13.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmichi M., Pang L., Ribon V., Saltiel A. R. Divergence of signaling pathways for insulin in PC-12 pheochromocytoma cells. Endocrinology. 1993 Jul;133(1):46–56. doi: 10.1210/endo.133.1.7686484. [DOI] [PubMed] [Google Scholar]
- Osterop A. P., Medema R. H., Bos J. L., vd Zon G. C., Moller D. E., Flier J. S., Möller W., Maassen J. A. Relation between the insulin receptor number in cells, autophosphorylation and insulin-stimulated Ras.GTP formation. J Biol Chem. 1992 Jul 25;267(21):14647–14653. [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Molecular signal integration. Interplay between serine, threonine, and tyrosine phosphorylation. Mol Biol Cell. 1992 Jun;3(6):583–592. doi: 10.1091/mbc.3.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Pronk G. J., de Vries-Smits A. M., Ellis C., Bos J. L. Complex formation between the p21ras GTPase-activating protein and phosphoproteins p62 and p190 is independent of p21ras signalling. Oncogene. 1993 Oct;8(10):2773–2780. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M., Chang H., Hoffman B. B., Azhar S. Resistance to insulin-stimulated glucose uptake in adipocytes isolated from spontaneously hypertensive rats. Diabetes. 1989 Sep;38(9):1155–1160. doi: 10.2337/diab.38.9.1155. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Lee C. H., Batzer A., Vicentini L. M., Zhou M., Daly R., Myers M. J., Jr, Backer J. M., Ullrich A., White M. F. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993 May;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. I., Cama A., Accili D., Barbetti F., Imano E., Kadowaki H., Kadowaki T. Genetic basis of endocrine disease. 1. Molecular genetics of insulin resistant diabetes mellitus. J Clin Endocrinol Metab. 1991 Dec;73(6):1158–1163. doi: 10.1210/jcem-73-6-1158. [DOI] [PubMed] [Google Scholar]
- Tobe K., Matuoka K., Tamemoto H., Ueki K., Kaburagi Y., Asai S., Noguchi T., Matsuda M., Tanaka S., Hattori S. Insulin stimulates association of insulin receptor substrate-1 with the protein abundant Src homology/growth factor receptor-bound protein 2. J Biol Chem. 1993 May 25;268(15):11167–11171. [PubMed] [Google Scholar]
- Weiland M., Schürmann A., Schmidt W. E., Joost H. G. Development of the hormone-sensitive glucose transport activity in differentiating 3T3-L1 murine fibroblasts. Role of the two transporter species and their subcellular localization. Biochem J. 1990 Sep 1;270(2):331–336. doi: 10.1042/bj2700331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Yang Y., Farese R. V. Insulin activates myelin basic protein (p42 MAP) kinase by a protein kinase C-independent pathway in rat adipocytes. Dissociation from glucose transport. FEBS Lett. 1993 Nov 1;333(3):287–290. doi: 10.1016/0014-5793(93)80672-h. [DOI] [PubMed] [Google Scholar]
- de Vries-Smits A. M., Burgering B. M., Leevers S. J., Marshall C. J., Bos J. L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992 Jun 18;357(6379):602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]
- van den Berghe N., Vaandrager A. B., Bot A. G., Parker P. J., de Jonge H. R. Dual role for protein kinase C alpha as a regulator of ion secretion in the HT29cl.19A human colonic cell line. Biochem J. 1992 Jul 15;285(Pt 2):673–679. doi: 10.1042/bj2850673. [DOI] [PMC free article] [PubMed] [Google Scholar]