Abstract
Background
Severe osteochondritis dissecans (OCD) in children and adolescents often necessitates surgical interventions (ie, drilling, excision, or débridement). Since extracorporeal shock wave therapy (ESWT) enhances healing of long-bone nonunion fractures, we speculated ESWT would reactivate the healing process in OCD lesions.
Questions/purposes
We asked whether ESWT would enhance articular cartilage quality, bone and cartilage density, and histopathology of osteochondral lesions compared to nontreated controls in an OCD rabbit model.
Methods
We harvested a 4-mm-diameter plug of the weightbearing osteochondral surface on the medial femoral condyle of each knee in 20 skeletally immature (8-week-old) female rabbits. We placed a piece of acellular collagen-glycosaminoglycan matrix into the cavity and then replaced the plug. Two weeks after surgery, we sedated each rabbit and treated the right knee in a single setting with shock waves: 4000 impulses at 4 Hz and 18 kV. The left knee was a sham control. Ten weeks after surgery, we assessed cartilage morphology of the lesion using a modified Outerbridge Grading System, bone and cartilage density using histologic imaging, bone and cartilage morphology using the histopathology assessment system, and radiographic bone density and union and compared these parameters between ESWT-treated and control knees.
Results
Histologically, we observed more mature bone formation and better healing (1.1 versus 3.4) and density of the cartilage (60 versus 49) on the treated side. Radiographically, we noted an increase in bony density (154 versus 138) after ESWT.
Conclusions
ESWT accelerated the healing rate and improved cartilage and subchondral bone quality in the OCD rabbit model.
Clinical Relevance
This therapeutic modality may be applicable in OCD treatment in the pediatric population. Future research will be necessary to determine whether it may play a role in healing of human osteochondral defects.
Introduction
Osteochondritis dissecans (OCD) is a condition where a segment of articular cartilage with its underlying subchondral bone gradually separates from the surrounding osteocartilaginous tissue. Nondisplaced stable lesions can be treated nonoperatively [1, 16], including activity restriction, immobilization, and nonweightbearing ambulation of the affected limb. Surgery often remains the only reasonable option in the treatment of unstable OCD lesions. However, according to Wright et al. [24], based on a 4- to 15-year followup study that used the Hughston grading scale [24], only six of 17 patients (35%) demonstrated either no symptoms, no activity limitations, and normal radiographs (two patients) or mild symptoms, mild activity limitations, and early radiographic changes (four patients) [24]. In a rabbit model of OCD using periosteal transplantation, osteochondral autografts, and autologous chondrocyte transplantation, Hui et al. [3] found all three methods enhanced the repair of chondral defects in OCD. Despite surgical treatments resulting in limited improvement of symptoms, functional activities, and joint space appearance, these modalities were invasive and had definite drawbacks.
Extracorporeal shock wave therapy (ESWT) was developed to treat various tendinopathies, such as calcific tendonitis of the shoulder, epicondylitis, and plantar fasciitis [3, 7, 8, 13, 18]. Its role was expanded to treat other conditions, such as femoral head necrosis, delayed unions, and nonunions of fractures [2, 4–6, 14, 15, 20]. Some studies have reported ESWT stimulates the healing or reactivates the growth process in bone, tendon, and surrounding tissue [7, 13, 18, 22]. This occurs when microdisruption is induced into both avascular and minimally vascular tissue to stimulate revascularization. This revascularization then proceeds to recruit growth factors and, possibly, stem cells necessary for normal healing [7, 21]. However, one study of low-energy level ESWT on bone in rabbits reported it had no side effects on joint cartilage [19]. The effects of moderate-energy level ESWT on the articular cartilage have been unclear.
Using a rabbit OCD model, we therefore assessed (1) articular cartilage morphology using a modified Outerbridge Grading System, (2) bone and cartilage density and morphology using histologic imaging and the histopathology assessment system, and (3) radiographic bone density and union in OCD lesions and compared these parameters between ESWT-treated and control knees.
Materials and Methods
We created a model of OCD in 20 skeletally immature (8-week-old) female New Zealand White rabbits. We first obtained a 4-mm-diameter plug from the weightbearing osteochondral surface harvested on the medial femoral condyle of each knee. We then placed a piece of acellular collagen-glycosaminoglycan matrix into the cavity to replace the plug. Two weeks after surgery, we sedated each rabbit and treated the right knee with ESWT. We used the left knee for a sham-operated control. Multiple radiographs in AP view were taken for both knees. Ten weeks after ESWT, we sacrificed all rabbits and subsequently performed a gross anatomy study and histologic review. The Institutional Animal Care and Use Committee (IACUC) approved this study.
The OCD model in the rabbit knee was as follows. Thirty minutes before surgery, we gave each rabbit a weight-dependent, subcutaneously administered anesthetic mixture of butorphanol (0.5 mg/kg), acepromazine (0.5 mg/kg), and buprenorphine (0.05 mg/kg) to induce anesthesia before surgery. All rabbits were placed on a warm-water-circulating heating pad. During the surgery, each rabbit was administered an inhalation of 2% to 3% isoflurane. We shaved both legs of each rabbit between the upper thigh and upper ankle. The rabbits were placed in a supine position with the knee flexed at 90°. Using a Number 15 scalpel blade, we made an anterior incision approximately 2 cm long on each knee. We cut the skin, fascia, and lateral quadriceps muscle in the proximal to distal direction, and after retraction, the knee was flexed 90°, exposing the medial condyle. We used a 4-mm round dermatologic biopsy punch to make a 4-mm (diameter) by 2-mm (depth) bone and cartilage extraction from the medial femoral condylar region of each knee. Before each cartilage and bone extraction, we made a measured mark on the biopsy punch to maintain depth consistency. A 3- by 3-mm precut piece of Surgisis® Gold Hernia Repair Graft (Cook® Biotech Inc, West Lafayette, IN, USA) was placed into each plug hole and gently pressed into place. Surgisis® is an acellular resorbable Type I collagen-glycosaminoglycan matrix derived from the porcine intestinal submucosa. We then placed the bone and cartilage extraction back into the biopsy cavity, covering the Surgisis® graft. The knee was straightened and a 2–0 Coated VICRYL™ (Polyglactin 910) suture (Ethicon Inc, Somerville, NJ, USA) was used to close the incision. After completion of the operation, we returned all rabbits to their cages and allowed them to move freely. We placed a membrane between the subchondral bone and femoral bone to further delay their union, thereby simulating a typical OCD lesion.
One day after surgery, we subcutaneously injected buprenorphine (0.05 mg/kg) two times daily for 2 days to provide postoperative pain relief. However, one rabbit experienced respiratory arrest. In two rabbits bone and cartilage plugs appeared dislodged on radiographs. We euthanized these three rabbits and removed them from the study. Beuthanasia®-D (1 mL/kg; Intervet/Schering-Plough Animal Health Corp, Summit, NJ, USA) was administered intravenously for euthanasia. Thus, 17 rabbits successfully completed the surgery and were used for the ESWT application. Two weeks after surgery, we anesthetized each rabbit using the methods mentioned above. Fifteen minutes after sedation, we placed each rabbit in a supine position with the right knee flexed at 90°. A coupling gel was applied to the skin surrounding the right knee and the focal point of the OssaTron® (Sanuwave Inc, Alpharetta, GA, USA) shock wave generator was placed 2 to 3 mm from the marked medial edge of the right femoral distal condyle (Fig. 1). Each knee was treated with 4000 impulses at a setting of 4 Hz and 18 kV (completed in 17 minutes), corresponding to an energy density of 0.24 mJ/mm2. The left femoral distal condyle on each rabbit was sham treated to mimic the treatment to the right leg and served as the control.
Fig. 1.
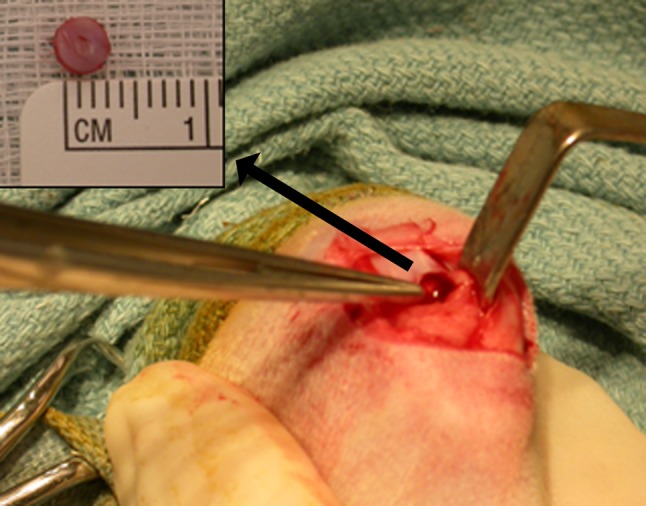
The photograph shows the bone and cartilage plug created using a 4-mm round dermatologic biopsy punch in the rabbit OCD model.
We obtained radiographic films of both the treatment and control groups at 1, 2, 5, and 10 weeks after ESWT. Each rabbit was sedated using weight-dependent subcutaneously administered acepromazine and placed in the supine position, whereupon AP and lateral radiographs of each knee were collected using a GE 2000 x-ray machine (General Electric, Waukesha, WI, USA) with 18- by 24-cm fine-resolution radiograph films. Bone mineral density was calculated for both the treated and control knee using a grayscale level ranging from 0 (white) to 256 (black) on a radiograph film [9, 17].
Ten weeks after ESWT, we euthanized all 17 remaining rabbits in the study as described above. After the rabbits were euthanized, we removed both the left and right knees from each rabbit, rinsed them with a solution of 0.9% normal saline, and photographed them with a digital camera. Three observers (MK, XCL, AP) independently examined the control and treated knees and classified the gross morphology of the articular cartilage using a modified Outerbridge Grading System [10] as follows: Grade 0, tissue completely healed with no evidence of prior injury; Grade 1, tissue displayed softening and swelling; Grade 2, tissue displayed fragmentation and fissuring in a small area; Grade 3, tissue displayed prominent and larger fragmentation and fissuring; and Grade 4, any tissue with major cartilage erosion down to bone. The average agreement among the three reviewers for all cases was 84% (range, 68%–100%).
After completion of gross analysis, we placed each specimen in a 10% zinc formalin solution, removed them after less than 2 hours, and began histologic processing. After 6 hours of decalcification, we collected histologic samples by placing each distal femur into a cutting chamber and performed a sagittal (lateral) cut. The sections we collected were the lateral, middle, and medial regions of the medial condyle. We stained sections using hematoxylin and eosin. Two authors (MK, XCL) independently evaluated each section using the histopathology assessment system [12] and one pathologist verified the final histologic results. The histopathology assessment ranged from Grades 0 (normal articular cartilage and subchondral bone) to 6 (loss of cartilage tissue and microfracture replaced with fibrocartilage). The average agreement between the two reviewers for all cases was 75% (range, 50%–100%).
Additionally, both bone and cartilage density around the OCD lesion were measured using an Olympus microscope (Olympus America, Inc, Center Valley, PA, USA) and imaging software (MicroSuite™; Olympus America, Inc). Using the Window®-based software, we identified the region of interests, calculated the density with a grayscale level, and performed statistical analysis at the area. Three coronal sections of the medial femoral condyle with the OCD plug were provided for histologic evaluation. Each slide included the entire bone and cartilage plug and its surrounding area. Three regions of interests were evaluated, including the medial plug border, midplug, and lateral plug border. In each region, the same-sized square was selected and density was calculated. Averaged densities for cartilage and bone were automatically measured for three regions in the computer [23].
We performed a Wilcoxon signed-rank test (SPSS®; SPSS Inc, Chicago, IL, USA; MATLAB®; The MathWorks Inc, Natick, MA, USA) to determine differences between the treatment and control knee in the following ordinal variables: gross morphology for the articular cartilage measured by a modified Outerbridge Grading System, radiographic bone density, histologic cartilage and bone density, and histopathology assessment score.
Results
Gross anatomic analysis of both the control (Fig. 2A) and treatment (Fig. 2B) groups 10 weeks after ESWT revealed a noticeable difference, with the knees from the treatment group exhibiting smoother cartilage surface, normal color, and no softening or swelling of the cartilage. The mean modified Outerbridge Grading System score was lower (p = 0.001) for the treated group (1.4 ± 0.2) than for the untreated group (2.4 ± 0.3) (Table 1).
Fig. 2A–B.
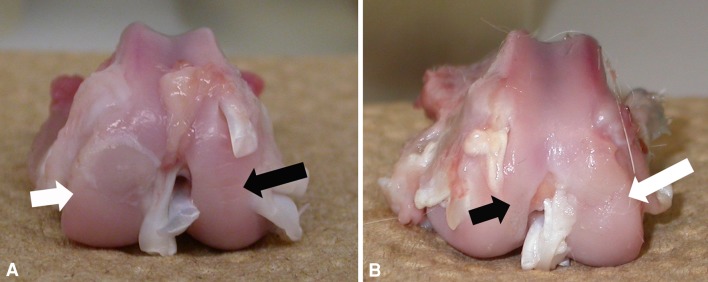
Photographs compare articular cartilage appearance around the OCD lesion area after 10 weeks in (A) control and (B) ESWT-treated knees. (A) The control knee shows mild swelling and softening of the cartilage with obvious fissuring (white arrow), whereas (B) the treated knee shows no swelling of the cartilage and slight fissuring adjacent to the normal host bone site (white arrow). Intact cartilage shows smooth surface and normal color on the contralateral side (black arrows).
Table 1.
Differences in gross anatomic assessment and histology around the OCD lesion at 10 weeks
| Method | ESWT-treated limb | Untreated limb | p value* |
|---|---|---|---|
| Modified Outerbridge Grading System [12] | 1.4 ± 0.2 | 2.4 ± 0.3 | 0.001 |
| Histopathology Assessment System [10] | 1.1 ± 1.0 | 3.4 ± 1.7 | < 0.0001 |
| Density (grayscale level) | |||
| Cartilage | 60 ± 15 | 49 ± 11 | 0.04 |
| Bone | 96 ± 17 | 94 ± 19 | 0.57 |
Values are expressed as mean ± SD; * Wilcoxon signed-rank test; OCD = osteochondritis dissecans; ESWT = extracorporeal shock wave therapy.
Radiographically, in the control group, the knees either appeared to still be in the healing process or showed evidence of incomplete healing (Fig. 3A), whereas in the ESWT-treated group, bone healing appeared to be progressing at a quicker rate at the 2-, 5-, and 10-week marks, with the 10-week radiograph showing either complete or almost complete healing in all knees (Fig. 3B). We observed lower (p = 0.02) mean bone density in ESWT-treated knees (114 ± 38 at Week 1) as compared to control knees (131 ± 29 at Week 1). There was higher (p = 0.004; p = 0.001) mean bone density in ESWT-treated knees (149 ± 22 at Week 3; 154 ± 21 at Week 5) as compared to control knees (143 ± 21 at Week 3; 138 ± 29 at Week 5), while there was no difference (p = 0.76) in bone density around the OCD lesion at 10 weeks between ESWT-treated (190 ± 12) and control (190 ± 17) knees (Table 2).
Fig. 3A–B.
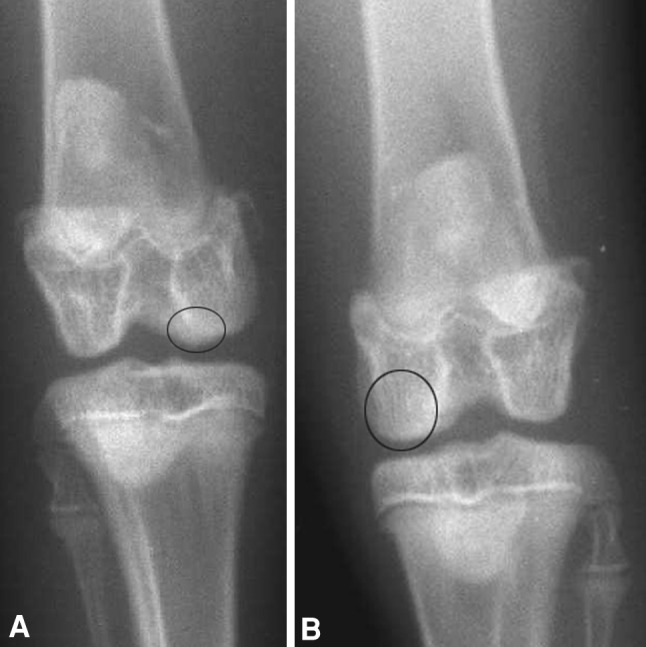
AP radiographs compare bone fusion on the OCD lesion area at 5 weeks in (A) control and (B) ESWT-treated knees. (A) The control knee shows an incompletely healed bone lesion (circle), whereas (B) the treated knee shows a completely healed bone lesion with remodeling (circle).
Table 2.
Difference in the bone density around the OCD lesion in radiographs over time
| Weeks | Bone density (grayscale level) | ||
|---|---|---|---|
| ESWT-treated group | Untreated group | p value* | |
| 1 | 114 ± 38 | 131 ± 29 | 0.02 |
| 3 | 149 ± 22 | 143 ± 21 | 0.004 |
| 5 | 154 ± 21 | 138 ± 29 | 0.001 |
| 10 | 190 ± 12 | 190 ± 17 | 0.76 |
Values are expressed as mean ± SD; * Wilcoxon signed-rank test; OCD = osteochondritis dissecans; ESWT = extracorporeal shock wave therapy.
The average histopathology assessment system grade was lower (p < 0.001) for the treatment group than for the control group: 1.1 ± 0.98 versus 3.4 ± 1.7, respectively. At 10 weeks, the cartilage adjacent to the OCD lesion in the control group exhibited deep fissuring and fibrillation (Fig. 4A); in a few cases, the cartilage had reduced thickness and was uneven with superficial fibrillation. However, the cartilage in the ESWT group often showed a smooth and even surface with organized chondrocyte distribution (Fig. 4B); the cartilage structure was close to normal (Fig. 4C), with mild fissuring and normal subchondral bone healing and fusion. The histomorphologic analysis demonstrated the cartilage density was greater (p = 0.04) in the ESWT-treated knees in control knees (Table 1). The bone density for ESWT-treated knees (96 ± 17) was similar (p = 0.57) to that for the control knees (94 ± 19) at Week 10 (Table 1).
Fig. 4A–C.
Images compare (A) nontreated cartilage, (B) ESWT-treated cartilage, and (C) intact cartilage at 10 weeks (stain, hematoxylin and eosin; original magnification, ×10). (A) The control knee shows larger denudation, few chondrocytes, increased fibrocartilage surface, and less mineralized cartilage (arrow). (B) The ESWT-treated knee shows close to normal cartilage structure with mild fissuring adjacent to the normal host bone site (white arrows) and normal subchondral bone healing and fusion (black arrow). (C) Intact cartilage shows normal-thickness cartilage with zones and subchondral bone structure (arrow).
Discussion
ESWT is reportedly effective in treating many orthopaedic conditions in humans and animal models [2–4, 6–8, 10, 11, 13–15, 17, 18, 20, 22]. Evidence supports bone response to ESWT with improved fracture healing; resolution of fracture nonunion and delayed union; increased bone mineral content, callus formation, and strength; and augmented femoral length and width. A systematic review of 1737 patients documented higher healing success rates, 62% versus 83%, for delayed union and nonunion of fractures using an electrohydraulic device [19]. Since OCD lesions involve both articular cartilage and its underlying subchondral bone, we used a rabbit OCD model to determine whether there were improvements in osteochondral repair based on a modified Outerbridge Grading System, cartilage and bone density based on histologic imaging, cartilage and bone morphology based on the histopathology assessment system, and radiographic bone density and union. ESWT for OCD lesions has potential benefits for treating OCD: it is noninvasive, relatively low-cost, and has minimal risks. Even if used to supplement conventional treatment, it has the potential to reduce healing time, improve outcomes, and lessen the need for rehabilitation and activity restrictions. ESWT could be started earlier than more invasive surgical treatments and may prevent further cartilage degeneration. This could result in reduced numbers of unstable lesions and lower the need for lesion excision and débridement, leading to better long-term outcomes.
We note limitations to our study. First, this was a pilot study and we assessed a single dose of ESWT. We cannot say whether other regimens with different energy levels, including multiple applications of ESWT, might not produce different findings. In an in vivo study with 24 immature rabbits receiving ESWT with 2000 shocks waves of 1.2 mJ/mm2, Ozturk et al. [11] reported no changes in chondrocytes on intact growth plates and no leg length differences at 0, 3, 12, and 24 weeks after treatment. However, our ESWT regimen used 4000 impulses of 0.24 mJ/mm2 and was associated with an improvement of the cartilage and subchondral bone structure. We presumed ESWT was more effective in the treatment of pathologic cartilage conditions with inflammatory or degenerative chondrocytes. Another study [1] found ESWT with 0.16 mJ/mm2 and 500 impulses augmented extracellular signal-regulated kinase and p38 kinase, which are highly related to bone formation as osteogenic factors. Second, the model lacks subchondral bone necrosis and articular cartilage degeneration in a progressive fashion since it was caused by an acute injury rather than by a chronic inflammatory condition. Therefore, the healing process of the osteochondral plug in the surrounding host site was faster when compared to chronic pathologic tissues. Another cartilage model used in rabbits simply created full-thickness articular cartilage defects for comparison of different cell transplantations [3]. However, isolated articular cartilage transplantation behavior is likely fundamentally different from the composite osteochondral tissue seen in human OCD and in this study. Third, using the Surgisis® as a barrier to healing of the osteochondral plug may not functionally represent the chronic fibrosis tissue found in human OCD. The histologic results of the treated OCD lesions showed dissolution of the Surgisis® graft in the majority of cases and replacement by bone and cartilage growth. However, in untreated OCD lesions, some acellular collagen tissues still existed with less bone growth and cartilage coverage. Our study also suggests the Surgisis® graft itself is nonactive acellular tissue that it is unlikely to play an active role in the healing process.
Radiographic assessment showed increased bone density in ESWT-treated knees compared to control knees. ESWT had an effect on both the healing rate and bone quality in the rabbit OCD model. The acceleration of bone remodeling was higher at 5 weeks after the ESWT. By 10 weeks, bone mineral density between the ESWT-treated side and control side showed no difference. An increased cartilage density measured by the histopathology assessment system indicated an improved quality of the cartilage layers on the articular surface in ESWT-treated OCD lesions at 10 weeks. There was less fissuring, no erosion, no matrix fibrillation in the deep zone, and some hypercellular tissue. Both reductions in macroscopic and microscopic scores indicated additional improvement in the cartilage quality and bone-healing process in the rabbit OCD model.
In conclusion, ESWT at a moderate dosage accelerated the healing rate and improved the quality of cartilage and subchondral bone remodeling process in this rabbit OCD model at the early stage. This therapeutic modality may be applicable to the treatment of OCD in the pediatric population. It also may prevent progression of a stable OCD lesion to an unstable OCD lesion.
Acknowledgments
We thank Dr. Vladimir Osipov, pathologist in the Froedtert Hospital, Medical College of Wisconsin, for his reviewing and scoring the histology. We extend our thanks to Mr. Angelo, research assistant, for help with animal care and the study.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was conducted at the Clement J. Zablocki Milwaukee VA Medical Center, Milwaukee, WI, USA, and Children’s Hospital of Wisconsin, Milwaukee, WI, USA.
References
- 1.Anderson AF, Anderson CN. Management of osteochondritis dissecans of the knee. Tech Knee Surg. 2005;4:23–35. doi: 10.1097/01.btk.0000154843.06650.6b. [DOI] [Google Scholar]
- 2.Gerdesmeyer L, Wagenpfeil S, Haake M, Maier M, Loew M, Wörtler K, Lampe R, Seil R, Handle G, Gassel S, Rompe JD. Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff. JAMA. 2003;290:2573–2580. doi: 10.1001/jama.290.19.2573. [DOI] [PubMed] [Google Scholar]
- 3.Hui JH, Chen F, Thambyah A, Lee EH. Treatment of chondral lesions in advanced osteochondritis dissecans. J Pediatr Orthop. 2004;24:427–433. doi: 10.1097/01241398-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current concepts review. Am J Sports Med. 2006;34:1181–1191. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig J, Lauber S, Lauber HS, Dreisilker U, Raedel R, Hotzinger H. High-energy shock wave treatment of femoral head necrosis in adults. Clin Orthop Relat Res. 2001;387:119–126. doi: 10.1097/00003086-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Norman D, Reis D, Zinman C, Misselevich I, Boss JH. Vascular deprivation-induced necrosis of the femoral head of the rat: an experimental model of avascular osteonecrosis in the skeletally immature individual or Legg-Perthes disease. Int J Exp Pathol. 1998;79:173–179. doi: 10.1046/j.1365-2613.1998.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden JA, Alvarez RG, Levitt R, Marlow M. Shock wave therapy (orthotripsy) in musculoskeletal disorders. Clin Orthop Relat Res. 2001;387:22–40. doi: 10.1097/00003086-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ogden JA, Alvarez RG, Levitt RL, Johnson JE, Marlow ME. Electrohydraulic high-energy shock-wave treatment for chronic plantar fasciitis. J Bone Joint Surg Am. 2004;86:2216–2228. doi: 10.2106/00004623-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Ohki M, Okano T, Nakamura T. Factors determining the diagnostic accuracy of digitized conventional intraoral radiographs. Dentomaxillofac Radiol. 1994;23:77–82. doi: 10.1259/dmfr.23.2.7835507. [DOI] [PubMed] [Google Scholar]
- 10.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 11.Ozturk H, Bulut O, Oztemur Z, Kaloglu C, Kol I. Effect of high-energy extracorporeal shock waves on the immature epiphysis in a rabbit model. Arch Orthop Trauma Surg. 2008;128:627–631. doi: 10.1007/s00402-007-0388-6. [DOI] [PubMed] [Google Scholar]
- 12.Pritzker KP, Ostergaard K, Salter DM. Towards standardization of osteoarthritis histopathology: terminology, topology and technology. Osteoarthritis Cartilage. 2000;8(suppl B):IP010. [Google Scholar]
- 13.Rompe JD, Kirkpatrick CJ, Kullmer K, Schwitalle M, Krischek O. Dose-related effects of shock waves on rabbit tendo Achillis. J Bone Joint Surg Br. 1998;80:546–552. doi: 10.1302/0301-620X.80B3.8434. [DOI] [PubMed] [Google Scholar]
- 14.Saisu T, Takahashi K, Kamegaya M, Mitsuhashi S, Wada Y, Moriya H. Effects of extracorporeal shock waves on immature rabbit femurs. J Pediatr Orthop B. 2004;13:176–183. doi: 10.1097/00009957-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Sauer ST, Marymont JV, Mizel MS. What’s new in foot and ankle surgery. J Bone Joint Surg Am. 2004;86:878–886. doi: 10.2106/00004623-200404000-00045. [DOI] [PubMed] [Google Scholar]
- 16.Sgaglione NA, Abrutyn DA. Update on the treatment of osteochondral fractures and osteochondritis dissecans of the knee. Sports Med Arthrosc Rev. 2003;11:222–235. doi: 10.1097/00132585-200311040-00003. [DOI] [Google Scholar]
- 17.Tagger M, Katz A. Radiopacity of endodontic sealers: development of a new method for direct measurement. J Endod. 2003;29:751–755. doi: 10.1097/00004770-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Thiel M. Application of shock waves in medicine. Clin Orthop Relat Res. 2001;387:18–21. doi: 10.1097/00003086-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Vaterlein N, Lussenhop S, Hahn M, Delling G, Meiss AL. The effect of extracorporeal shock waves on joint cartilage—an in vivo study in rabbits. Arch Orthop Trauma Surg. 2000;120:403–406. doi: 10.1007/PL00013770. [DOI] [PubMed] [Google Scholar]
- 20.Wang CJ, Huang HY, Chen HH, Pai CH, Yang KD. Effect of shock wave therapy on acute fractures of the tibia. Clin Orthop Relat Res. 2001;387:112–118. doi: 10.1097/00003086-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Wang CJ, Wang FS, Yang KD, Weng LH, Hsu CC, Huang CS, Yang LC. Shock wave therapy induces neovascularization at the tendon-bone junction. J Orthop Res. 2003;21:984–989. doi: 10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 22.Wang CJ, Yang KD, Wang FS, Hsu CC, Chen HH. Shock wave treatment shows dose-dependent enhancement of bone mass and bone strength after fracture of the femur. Bone. 2004;34:225–230. doi: 10.1016/j.bone.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 23.William KP. Digital Image Processing. New York, NY: John Wiley & Sons; 1991. [Google Scholar]
- 24.Wright RW, McLean M, Matava MJ, Shively RA. Osteochondritis dissecans of the knee. Clin Orthop Relat Res. 2004;424:239–243. doi: 10.1097/01.blo.0000128216.10732.d8. [DOI] [PubMed] [Google Scholar]



