Abstract
Introduction
Neonatal care has been considered as one of the first priorities for improving quality of life in children. In 2010, 10% of babies were born prematurely influencing national healthcare policies, economic action plans and political decisions. The use of complementary medicine has been applied to the care of newborns. One previous study documented the positive effect of osteopathic manipulative treatment (OMT) in reducing newborns’ length of stay (LOS). Aim of this multicentre randomised controlled trial is to examine the association between OMT and LOS across three neonatal intensive care units (NICUs).
Methods and analysis
690 preterm infants will be recruited from three secondary and tertiary NICUs from north and central Italy and allocated into two groups, using permuted-block randomisation.
The two groups will receive standard medical care and OMT will be applied, twice a week, to the experimental group only. Outcome assessors will be blinded of study design and group allocation. The primary outcome is the mean difference in days between discharge and entry. Secondary outcomes are difference in daily weight gain, number of episodes of vomit, regurgitation, stooling, use of enema, time to full enteral feeding and NICU costs. Statistical analyses will take into account the intention-to-treat method. Missing data will be handled using last observation carried forward (LOCF) imputation technique.
Ethics and dissemination
Written informed consent will be obtained from parents or legal guardians at study enrolment. The trial has been approved by the ethical committee of Macerata hospital (n°22/int./CEI/27239) and it is under review by the other regional ethics committees.
Results
Dissemination of results from this trial will be through scientific medical journals and conferences.
Trial registration
This trial has been registered at http://www.clinicaltrials.org (identifier NCT01645137).
Keywords: Complementary Medicine, Paediatrics, Preventive Medicine
Article summary.
Article focus
Osteopathic treatment as a complementary and coadjuvant therapy in neonatal intensive care unit (NICU).
Effectiveness of osteopathic procedures in reducing the newborns’ length of stay.
Osteopathy as a means to reduce NICU costs.
Key messages
Beneficial effects of osteopathic treatment on newborns’ health.
Cost-effectiveness of osteopathic procedures in NICU settings.
Strengths and limitations of the study
Robust study design based on multicentre nationwide randomised control trial.
Single blinding.
Introduction
Neonatal care has been one of the major focuses of the global health system policies, in terms of services delivered, to reduce neonatal mortality and morbidity. The last report of the WHO showed that more than 1 in 10 infants are born prematurely, resulting in 15 million premature infants worldwide in 2010.1 In spite of expensive neonatal intensive care units (NICUs), structural changes in the health care system have led to evidence-based guidelines that reduce preterm infants’ hospitalisation and deaths. A large rate of US paediatric hospital stays is secondary to neonatal conditions that rank among the most expensive items in the list of services provided for children.2 The highest average cost per infant is for preterm newborns with gestational age (GA) between 24 and 31 weeks, followed by those between 32 and 36 weeks, as opposed to the general population.3 Costs per surviving infant generally decrease with increasing GA. In the USA, preterm/low birth weight (LBW) infants account for half the hospitalisation costs of all newborns and one-quarter of overall paediatric costs.4 In Italy, the cost per infant per day ranged between €200 and €500 according to infants’ health conditions.5
Length of stay (LOS) in NICUs is strongly associated with GA and birth weight.6 Infants delivered at the earliest GA have the longest hospital stays, partly because of the higher incidence of medical complications in very LBW infants. The Italian healthcare institute reported an average LOS per different diagnostic categories ranging from 4 to 34 days.5
However, compared to term infants, premature infants are unique in their need to attain not only medical stability but also physiological maturity, including adequate temperature control, cessation of apnoea and bradycardia and adequate feeding behaviour, before they are safely discharged home.7 8
Patterns of hospitalisation of preterm infants are also associated with the presence of clinical symptoms of abnormal gastrointestinal function such as vomiting, regurgitation, gastric residuals and functional constipation.9–11
Osteopathy is a form of drug-free non-invasive manual medicine, designated as complementary and alternative medicine. It relies on manual contact for diagnosis and treatment.12 It respects the relationship of body, mind and spirit in health and disease; it lays emphasis on the structural and functional integrity of the body and the body’s intrinsic tendency for self-healing. Osteopathic practitioners use a wide variety of therapeutic manual techniques to improve physiological function and/or support homeostasis that has been altered by somatic (body framework) dysfunction (ICD-10-CM Diagnosis Code M99.00-09), that is, impaired or altered function of related components of the somatic system; skeletal, arthrodial and myofascial structures; and related vascular, lymphatic and neural elements.13
Osteopathic practitioners use their understanding of the relationship between structure and function to optimise the body's self-regulating, self-healing capabilities. This holistic approach to patient care and healing is based on the concept that a human being is a dynamic functional unit, in which all parts are interrelated possess self-regulatory and self-healing mechanisms. Two essential components of osteopathic health care are the structural evaluation of the patient for diagnosis and an array of manipulative techniques for treatment.12
Aim of the structural examination is to locate somatic dysfunctions, while the array of manipulative techniques is used to relieve tissues’ tensions, improve blood flow and lymphatic drainage.
Although several studies document the effect of osteopathic manipulative treatment (OMT) in a population of paediatric patients,14–16 the medical literature lacks information about any potential benefits of the use of OMT in preterm infants. The only study published demonstrated a potential positive effect of OMT in reducing the likelihood of excessive LOS and gastrointestinal symptoms.17
Aim of this multicentre randomised controlled trial is to examine the association between OMT and LOS in a larger population.
Methods and analysis
Aim of the study
Primary outcome is to evaluate the effectiveness of OMT in reducing LOS in a sample of premature infants.
Secondary outcome of the study is to evaluate the difference in daily weight gain, number of episodes of vomit and regurgitation, stooling, use of enema, time to full enteral feeding (breastfeeding and/or bottle) and NICU costs.
Study type
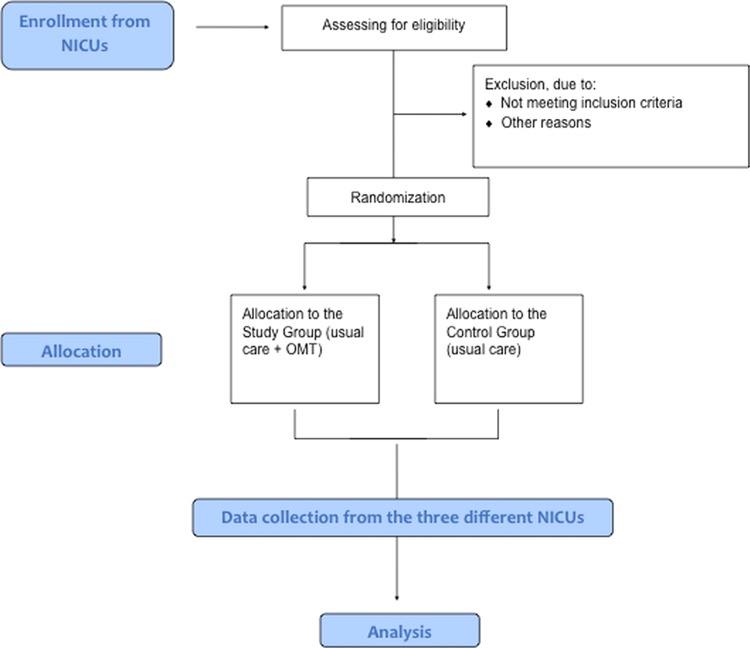
Interventional, multicentre nationwide single-blinded randomised controlled trial. Preterm infants entering the trial will either receive routine medical care plus osteopathic evaluation and treatment or routine medical care plus osteopathic evaluation only (figure 1).
Figure 1.
Flow chart describing, recruitment randomisation data collection and analysis of the study.
Participating NICUs
Patients will be recruited from three Italian secondary and tertiary NICUs at the public hospital in Pescara, Macerata and Monza.
The three participating NICUs are located throughout Italy in urban regions with catchment areas of about 40 000–150 000 inhabitants. Principal investigators of the participating NICUs are neonatologists and osteopaths with at least 5 years of experience in osteopathic treatments in NICU and specialised neonatal osteopathic education.
Population
Participants who meet the following inclusion criteria are considered eligible for the trial: male and female preterm infants entering the NICUs in the period between 1 July 2012 and 30 June 2013, preterms born in the same hospital of the referred NICU and free of medical complications and parents’ or legal guardians’ written informed consent.
Subjects presenting the following clinical conditions are excluded:
Gestational age <29 weeks
Gestational age >37 weeks
First OMT performed after 14 days from birth
Genetic disorders
Congenital disorders
Cardiovascular abnormalities
Proven or suspected necrotised enterocolitis with or without gastrointestinal perforation
Proven or suspected abdominal obstruction
Pre/post surgery patients
Pneumoperitoneum
Atelectasis
Newborn from an HIV seropositive/drug-addicted mother
Respiratory disorders
Transferred to/from other hospital
Admitted for preterminal comfort care (defined as neither intubation nor cardiorespiratory resuscitation)
Randomisation process
All patients entering the study are sequentially allocated to the experimental and control arms using R (R core team, Vienna, Austria), an open source statistical software, as computer random number generator.18
The type of randomisation procedure is permuted-block (ratio 1:1).
The process of randomisation is performed in the coordinating centre.
Research investigators
Investigators will be grouped into three:
1. Consultant performing the randomisation
2. Osteopaths performing the evaluation
3. Osteopaths performing the evaluation and treatment
Consultant performing the randomisation
An information technology consultant will be responsible for randomisation prior to the arrival of the osteopaths to the NICU.
Osteopaths performing the evaluation
Osteopaths in this group will perform the osteopathic evaluation in all infants entering the trial, with no knowledge about patients’ allocation.
The osteopathic evaluation will be performed in the absence of the ‘osteopaths performing the evaluation and treatment’.
Osteopaths performing the evaluation and treatment
‘Osteopaths performing the evaluation and treatment’ will perform an osteopathic evaluation and treatment of preterm infants from group ‘A’.
Intervention provided to the trial
Patients from experimental and control groups will receive routine medical care.
After study enrollment, all patients are sampled in group A and group B.
Group A (OMT): patients under usual medical care plus osteopathic treatment
Patients from this group will receive osteopathic care as follows: two treatments weekly for the entire LOS in the unit. Patients from group A will also receive an osteopathic evaluation from the ‘osteopaths performing the evaluation’.
Osteopathic treatments will only be applied to patients from group A and will be performed only by the ‘osteopaths performing the evaluation and treatment’, not involved in the study design, data entry and statistical analysis.
Each OMT session involve the structural examination and specific manipulative procedures.
In newborns the structural exam is usually performed with the child lying down in the open crib or incubator. Diagnostic criteria for somatic dysfunction are focused on tissue texture abnormalities, areas of asymmetry and misalignment of bony landmarks and the quality of motion, its balance and organisation.
The second part of the OMT session is characterised by the use of a variety of therapeutic manual techniques, addressed to increase range of motion and resolve the somatic dysfunctions diagnosed.
Techniques to be used are in line with the benchmarks for osteopathic treatment available in the medical literature and are limited to myofascial release, balanced ligamentous/membranous tension, indirect fluidic and v-spread. The whole session will last 30 min, 10 min for evaluation and 20 min for treatment.
Group B (no OMT): control group
Following the same schedule as for group A, patients from group B will receive usual medical care and osteopathic evaluation only. The osteopathic evaluation will last for 10 min. To maintain blinding of NICU personnel to patients’ allocation, the following 20 min osteopaths will keep their position close to the incubator or bed without touching the infant.
Osteopathic service will be provided twice a week, on Tuesdays and Fridays.
In case of critical preterm health conditions (ie, acute infections, peracute emergency care) or supplemental neonatal medical screening during the osteopathic service, the aforementioned intervention can be temporally stopped for a given trial participant. The infant will neither be evaluated or treated. This is in line with hospital safety procedures and primary care priority intervention.
Allocation concealment and blinding
NICU staffs are unaware of the study design and outcomes.
NICU staffs are blinded to patients’ allocation, since all infants will be touched by osteopaths from group A and B.
Data entering and data export
Data collection will be performed using an ad hoc locally developed software called EBOM-GCCN.
EBOM-GCCN dataset is an informatics tool that improves the efficiency and accuracy of data and has been developed to assist neonatologists, nurses and osteopaths in daily patients’ management.
The software consists of three sections:
Section 1: Intended for use by neonatologists and nurses for recording patients’ general details and all clinical information;
Section 2: Intended for use by ‘osteopaths performing the evaluation and treatment’;
Section 3: Intended for use by ‘osteopaths performing the evaluation’.
Records in sections 1, 2 and 3 are exclusively and respectively accessible to NICU staff, ‘osteopaths performing the evaluation and treatment’ and ‘osteopaths performing the evaluation’.
Nursing and medical records will be collected daily by the NICU staff, from the time the infants enter the unit to the time of discharge.
Osteopathic records will be collected twice weekly when the osteopathic service will be provided. ‘Osteopaths performing the evaluation and treatment’ will collect data in relation to the structural examination and the techniques applied, while the ‘osteopaths performing the evaluation’ will only collect data for the structural examination.
Data export will take place at the end of the study by the statistician from the coordinating centre, European Institute for Evidence-Based Osteopathic Medicine (EBOM).
Measurements
To evaluate the effect of the treatment, standard measurements will be recorded. Data will be collected at the baseline (entry time, T0), every time the osteopathic service is provided and at the end of the stay in the unit (discharge time, T1). An expected average period of 4 weeks has been considered.
The following measurements will be included for the primary and secondary outcomes.
Primary outcome: LOS
LOS will be used as primary outcome and is measured as the mean difference in days between T1 and T0.
According to international guidelines, the following physiological conditions are required for discharge: maintenance of body heat at room temperature, coordinated sucking, swallowing and breathing while feeding; sustained pattern of weight gain; stability of cardiorespiratory function (no episodes of apnoea/bradycardia for 2–5 days, free of supplemental oxygen support).8
Secondary outcomes
Secondary outcome measurements include the following parameters:
Daily weight gain, referred to as the net weight variation per day expressed in grams;
Episodes of vomit, the number of vomits per day;
Episodes of regurgitation, the number of regurgitations per day;
Episodes of stooling, the number of stools per day;
Use of enema, the number of enemas used per day;
Time to reach full enteral feeding, the number of days before autonomous feeding is achieved;
NICU costs, calculated as NICU daily newborn expenses, according to local authorities, multiplied by the newborn's LOS. Costs will be estimated in terms of Euros per day;
Side effects of treatment (osteopathy and clinical procedures).
In addition to these measurements, sociodemographic and clinical data will be collected apart from the following data:
1. Newborn's data: gender, gestational age, weight at birth and at entry, height, head circumference at birth, route, type and length of delivery, diagnosis at T0 and T1 and associated pathologies;
2. Mother's data: age, ethnicity, body mass index (BMI), nationality, number of previous pregnancies, clinical medical condition during pregnancy and concurrent pathologies;
3. Father's data: age, ethnicity, BMI, nationality, concurrent pathologies.
Statistical analyses
All calculations will be performed at the coordinating centre, EBOM.
Statistical analyses will take into account the intention-to-treat analysis. Missing data will be handled using last observation carried forward (LOCF) imputation technique. Arithmetic means and standard deviation will be used for the general characteristics of the study population. Univariate statistical tests will be performed to compare the experimental group and control group at the baseline. A generalised linear model, linear regression, will be considered to study the independent effect of OMT on primary endpoint and secondary endpoints, taking into account all possible confounders. The significance level will be at α=0.01. Differences between the groups will be presented as mean with 95% CI or in categories with OR for categorical data.
The statistical programme in use for randomisation and data analyses is R.18
Sample size
Sample size calculation used an effective size of 0.3 calculated from previous studies, considering a mean difference of 4 days between experimental and control group and an SD of 14. The statistical power is set at 0.90 and an α-level equal to 0.01. This produces a sample size of 333 per group. To prevent loss of power, the sample size is increased up to 345 subjects per group. The whole sample (N=690) is then divided, with a final result of N=230 preterm infants for each NICU. A longer period of study enrollment has been considered a strategy to achieve the estimated sample size.
Coordinating centre
The coordinating centre EBOM consists of an executive division, President and Vice President, an administration office, a group of researchers, an information technology consultant and a senior biostatistician.
The coordinating centre collaborates with the A.I.O.T.—Accademia Italiana Osteopatia Tradizionale.
Ethics and dissemination
During the routine initial postdelivery consultation, the neonatologist approaches parents or legal guardians explaining the patient information sheet. Written informed consent will then be obtained from the aforementioned guardians at study enrollment. Participation is voluntary and data collected will be sent to the coordinating centre using an anonymous identification for each patient.
The trail is approved by the ethical committee of Macerata hospital (n°22/int./CEI/27239) and it is under review by the other regional ethics committees. Moreover, the trial has been registered on clinicaltrial.gov (identifier NCT01645137).
The expected disadvantages for the intervention provided in the trial are null as well as no potential side effects owing to osteopathic care are predicted as shown by the recent osteopathic literature.19 However, any side effect will be recorded during the study period and appropriately discussed in the final paper.
Publication policy
The results of the trial will be published in peer-reviewed journals and presented at relevant congresses. The trial will be reported in accordance with the CONSORT recommendations.
Discussion
The purpose of this multicentre single-blinded RCT is to confirm the benefits of OMT in the care of preterm infants. In this trial, osteopathic care is not based on a predetermined protocol but on needs-based approach. Thus subjects can potentially be exposed to different techniques, leading to intersubjects and intrasubjects OMT variability. Nevertheless, the objective is to provide evidence of the impact of osteopathic approach rather than the association with a given manual protocol.
On the basis of quantitative data, the scope is to estimate the effect of OMT across different centres, proving generalisability on the effectiveness of osteopathy in NICUs. Well-designed researches are scarce in the osteopathic area, thus the present study will provide the most convincing evidence of relationship between exposure and effect.
To the best of our knowledge, this will be the first study in osteopathy applied to infants using gold-standards methods that include a randomised multicentre approach, a single hub computer-based data collection and a large sample size stratified by risk factors. In addition to this, the statistics that will be used will be aimed to consider different sources of variability, to allow reliable results for the effectiveness of OMT. Finally, the expected benefits for the intervention provided in the trial are shorter period of hospitalisation and improvements in the secondary outcomes, leading to enhanced quality of health service delivered and cost reductions.
Supplementary Material
Acknowledgments
Authors would like to thank Accorsi Alessandro, Cozzolino Vincenzo, D'Orazio Marianna, Gualdi Stefano, Lupacchini Mariacristina and Marinelli Benedetta for their precious contributions.
Footnotes
Contributors: FC, GP and GB conceptualised, designed and wrote the protocol. CR, CD'I, PF, PFP and LT reviewed the protocol for important intellectual content. All authors read and approved the final manuscript.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: Italian ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.World Health Organization, March of Dimes, PMNCH, Save the Children Born too soon: the global action report on preterm birth. In: Howson CP, Kinney MV, Lawn JE, eds. Geneva: World Health Organization, 2012 [Google Scholar]
- 2.Owens PL, Thompson J, Elixhauser Aet al. Care of children and adolescents in U.S. hospitals. In: 4 RHFBN, eds. AHRQ Publication 04–0004. Rockville, MD: Agency for Healthcare Research and Quality, 2003:35–8 [Google Scholar]
- 3.Clements KM, Barfield WD, Ayadi MF, et al. Preterm birth-associated cost of early intervention services: an analysis by gestational age. Pediatrics 2007;119:e866–74 [DOI] [PubMed] [Google Scholar]
- 4.Russell RB, Green NS, Steiner CA, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics 2007;120:e1–9 [DOI] [PubMed] [Google Scholar]
- 5.Italian Ministry of Health Rapporto annuale sull'attività di ricovero ospedaliero—dati SDO 2010. Roma: Ministero della salute, 2011 [Google Scholar]
- 6.Bakewell-Sachs S, Medoff-Cooper B, Escobar GJ, et al. Infant functional status: the timing of physiologic maturation of premature infants. Pediatrics 2009;123:e878–86 [DOI] [PubMed] [Google Scholar]
- 7.Eichenwald EC, Blackwell M, Lloyd JS, et al. Inter-neonatal intensive care unit variation in discharge timing: influence of apnea and feeding management. Pediatrics 2001;108:928–33 [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics. Committee on Fetus and Newborn Hospital discharge of the high-risk neonate—proposed guidelines. Pediatrics 1998;102(2 Pt 1):411–17 [PubMed] [Google Scholar]
- 9.Mezzacappa MA, Rosa AC. Clinical predictors of abnormal esophageal pH monitoring in preterm infants. Arq Gastroenterol 2008;45:234–8 [DOI] [PubMed] [Google Scholar]
- 10.Mihatsch WA, von Schoenaich P, Fahnenstich H, et al. The significance of gastric residuals in the early enteral feeding advancement of extremely low birth weight infants. Pediatrics 2002;109:457–9 [DOI] [PubMed] [Google Scholar]
- 11.Duman N, Utkutan S, Ozkan H, et al. Are the stool characteristics of preterm infants affected by infant formulas? Turk J Pediatr 2000;42:138–44 [PubMed] [Google Scholar]
- 12.World Osteopathic Health Organization. WOHO Osteopathic glossary, 2011
- 13.American Association of Colleges of Osteopathic Medicine Glossary of Osteopathic Terminology, 2002
- 14.Hayden C, Mullinger B. A preliminary assessment of the impact of cranial osteopathy for the relief of infantile colic. Complement Ther Clin Pract 2006;12:83–90 [DOI] [PubMed] [Google Scholar]
- 15.Vandenplas Y, Denayer E, Vandenbossche T, et al. Osteopathy may decrease obstructive apnea in infants: a pilot study. Osteopath Med Prim Care 2008;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philippi H, Faldum A, Schleupen A, et al. Infantile postural asymmetry and osteopathic treatment: a randomized therapeutic trial. Dev Med Child Neurol 2006;48:5–9; discussion 4 [DOI] [PubMed] [Google Scholar]
- 17.Pizzolorusso G, Turi P, Barlafante G, et al. Effect of osteopathic manipulative treatment on gastrointestinal function and length of stay of preterm infants: an exploratory study. Chiropr Man Ther 2011;19:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Development Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2010 [Google Scholar]
- 19.Hayes NM, Bezilla TA. Incidence of iatrogenesis associated with osteopathic manipulative treatment of pediatric patients. J Am Osteopath Assoc 2006;106:605–8 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.