Abstract
Objective
Some patients with the phenotype of severe sepsis may have no overt source of infection or identified pathogen. We investigated whether a procalcitonin-based algorithm influenced antibiotic use in patients with non-microbiologically proven apparent severe sepsis.
Design
This multicentre, randomised, controlled, single-blind trial was performed in two parallel groups.
Setting
Eight intensive care units in France.
Participants
Adults with the phenotype of severe sepsis and no overt source of infection, negative microbial cultures from multiple matrices and no antibiotic exposure shortly before intensive care unit admission.
Intervention
The initiation and duration of antibiotic therapy was based on procalcitonin levels in the experimental arm and on the intensive care unit physicians’ clinical judgement without reference to procalcitonin values in the control arm.
Main outcome measure
The primary outcome was the proportion of patients on antibiotics on day 5 postrandomisation.
Results
Over a 3-year period, 62/1250 screened patients were eligible for the study, of whom 31 were randomised to each arm; 4 later withdrew their consent. At day 5, 18/27 (67%) survivors were on antibiotics in the experimental arm, versus 21/26 (81%) controls (p=0.24; relative risk=0.83, 95% CI: 0.60 to 1.14). Only 8/58 patients (13%) had baseline procalcitonin <0.25 µg/l; in these patients, physician complied poorly with the algorithm.
Conclusions
In intensive care unit patients with the phenotype of severe sepsis or septic shock and without an overt source of infection or a known pathogen, the current study was unable to confirm that a procalcitonin-based algorithm may influence antibiotic exposure. However, the premature termination of the trial may not allow definitive conclusions.
Article summary.
Article focus
Non-microbiologically proven severe sepsis may be a common clinical problem.
The role of procalcitonin to guide antibiotic therapy in this population has not been investigated so far.
This study was registered at clinicaltrials.gov under the identifier NCT01025180.
Key messages
In the intensive care unit, the incidence of microbiologically unproven severe sepsis is low.
Procalcitonin levels are persistently elevated in this population.
Procalcitonin levels are unhelpful toin reducing antibiotic exposure in these patients.
Strengths and limitations of this study
This was the first multicentre, randomised trial investigating the role of procalcitonin levels to reduce antibiotic exposure in microbiologically unproven severe sepsis.
The main limitation was the premature termination of the trial owing to the very low incidence of this population.
Background
Severe sepsis is commonly defined as acute dysfunction of one or more organs secondary to an infection.1 The lung, abdomen and urogenital tract account for roughly 70% of severe sepsis cases.1 2 Gram-positive bacteria are involved in 30–50% of cases and Gram-negative bacteria in 25–30%,1 with some regional variations.2 Nevertheless, recent clinical trials included 5–30% of participants with a phenotype of septic shock and negative microbiological cultures.3–6 Except in patients with clinically obvious infection such as purpura fulminans or cellulitis, verification of a bacterial aetiology and identification of responsible pathogens is of paramount importance to confirm sepsis and optimise the anti-infective strategy.1 So far, there is no biomarker to confirm the bacterial aetiology of apparent sepsis in routine practice.1
In patients with suspected infection, the measurement of plasma procalcitonin (PCT) levels to aid decision-making regarding starting and stopping antibiotic therapy has become popular in the past decade. First of all, procalcitonin has been suggested to be a specific marker of bacterial infection.1 Second, large prospective, randomised, controlled multicentre interventional trials demonstrated that a PCT-based algorithm allowed safe reduction of antibiotic therapy duration in patients with severe lower respiratory tract infections admitted to the emergency room,7 and in sepsis patients in the intensive care unit (ICU).8 9 A recent systematic review and meta-analysis of seven trials found a weighted mean antibiotic exposure reduction of 3.15 days (95% CI 1.45 to 4.35) with a PCT-based algorithm relative to standard non-PCT-based practice, without increased incidence of adverse events.10
We previously proposed ruling out septic shock in patients with a clinically doubtful infection, negative bacterial cultures or other potential causes of acute organ dysfunction, and PCT levels <0.25 µg/l.1 We hypothesised that a PCT-based algorithm might be useful in guiding antibiotic therapy in ICU patients with apparent septic shock and no clear source of infection and no known pathogen and therefore conducted the present trial.
Methods
This was a multicentre, randomised, single-blind, controlled trial, performed on two parallel groups at eight centres in France. The study protocol (CCP #06005) was approved by the Comité de Protection des Personnes Île de France XI (Saint-Germain-en-Laye, France) on 19 January 2006. Written informed consent was obtained from patients or their closest relatives prior to randomisation and on a deferred basis when patients were incapable of consenting at that time. The study was evaluated by an independent data safety and monitoring board, which met on 3 January 2007. This study was registered at clinicaltrials.gov under the identifier NCT01025180.
Patients
All consecutive adults admitted to a participating ICU were eligible if they, for <48 h, had (1) the systemic inflammatory response syndrome, (2) acute dysfunction of at least one organ, (3) absence of indisputable clinical infection and (4) negative microbial cultures.1 These criteria are defined in online supplementary appendix 1. Exclusion criteria were pregnancy, burns over ≥15% of body surface area, trauma, outpatient or inpatient cardiac arrest, postorthopaedic surgery status, drug-related neutropenia, withdrawal of life-supportive therapies or a decision to withhold them, indisputable clinical infection or antibiotic exposure ≥48 h during the time shortly before ICU admission.
Interventions
In the control arm, the decision to start or stop antibiotic therapy was at the discretion of the patient's physician, without knowledge of the patient's PCT concentrations. In the experimental arm, both initiation and discontinuation of antibiotics were guided by a PCT-based algorithm (see online supplementary appendix 2),11 applied at 6 h and on day 3 and day 5 postrandomisation. Briefly, antibiotic therapy was not to be started or was to be halted when PCT was <0.25 µg/l, was strongly discouraged when PCT was ≥0.25 to <0.5 µg/l, was recommended when PCT was ≥0.5 to <5 µg/l and was strongly recommended when PCT was ≥5 µg/l. Owing to the fact that surgery may increase PCT levels,12 for patients enrolled in the 48-hour postoperative period, the respective PCT cut-offs were <4 µg/l, ≥4 to <9 µg/l and ≥9 µg/l. Investigators were strongly asked not to over-rule the algorithm every day up to the study day 5.
Randomisation and blinding
Patients were randomised in a 1:1 ratio according to a computer-generated list. Randomisation was centralised through a secured website and performed by an independent statistician, and was stratified by the centre and according to whether or not patients underwent surgery in the past 48 h, using permutation blocks, the size of which remained unknown to the investigators. Masking of antibiotic therapy was not feasible in this study. In the control arm, patients, physicians, nurses, investigators, study coordinators, the statistician and the sponsor remained blinded to PCT levels throughout the study.
Data collection and follow-up
At baseline, we systematically recorded (1) demographic and anthropometric data, (2) location prior to ICU admission, (3) type of admission: medical versus surgical, (4) previous health status including McCabe class13 and acute physiology and chronic health evaluation Knaus class,14 (5) severity of illness as assessed by vital signs, the Simplified Acute Physiology Score II score,15 the Sepsis-related Organ Failure Assessment (SOFA)16 score and arterial lactates levels, (6) standard laboratory test results, (7) C reactive protein (CRP) and PCT levels (measured with the BRAHMS PCT-sensitive KRYPTOR assay), (8) chest x-ray or CT results, (9) whole-body CT results (procedure performed as clinically indicated), (10) Gram stain and culture of blood, urine, bronchoalveolar lavage fluids, samples from any venous and arterial catheters, cerebrospinal fluid in patients with altered mental status, any fluid from a normally sterile cavity (obtained if clinically indicated), any wound from surgery or any other source (if present) and (11) any anti-infective drug(s) and any other relevant therapy including mechanical ventilation, vasopressors, renal replacement therapy, corticosteroids or any other immunomodulating drug. Vital signs, standard laboratory tests, anti-infective therapy and life-supportive interventions were recorded daily until hospital discharge or 30 days postrandomisation, whichever came first. Imaging was repeated as clinically indicated during the patient's ICU stay. Gram stain and microbial cultures were repeated on day 3, day 5 and at ICU discharge, and on any additional days as clinically indicated. Plasma CRP and PCT concentrations were measured at 6 h and on day 3 and day 5 postrandomisation. SOFA scores were computed on day 3 and day 5 postrandomisation.
Study outcomes
The primary outcome was the proportion of patients receiving antibiotics at day 5 postrandomisation. Patients who died before day 5 were assigned to the antibiotic therapy status as of their death date.
Secondary outcomes included death at day 5, at ICU discharge and at hospital discharge, the proportion of patients started on antibiotics postrandomisation, the duration of antibiotic exposure, the SOFA score at day 3 and day 5, the proportion of patients with infection acquired between randomisation and day 3, day 5 and ICU discharge, and ICU and hospital length-of-stay.
Sample size
Based on a previous study,11 we estimated that on day 5, ∼85% of control patients would be on antibiotics. Thus, we calculated that 57 patients in each arm would be needed to detect in a two-sided test with an 80% probability and a 0.05 type-I error, a 25% absolute reduction in the proportion of antibiotic-treated patients on day 5. We also estimated that 20% of patients would eventually be withdrawn from study after showing indisputable infection. Thus, 140 patients in total (70 in each arm) would be needed. Owing to the uncertainty surrounding the incidence of eligible patients, and the proportion of patients who would be diagnosed postrandomisation with indisputable sepsis, we planned an interim analysis after the enrolment of approximately 70 patients. The analysis could either lead to sample size adjustment17 or trial discontinuation if (1) the in-hospital death rate in the experimental arm exceeded by ≥10% that of controls or (2) the recruitment rate was unacceptably low.
Statistical analysis
The analysis was performed according to the intention-to-treat principle. Quantitative variables are expressed as means and SDs or medians and IQR whenever appropriate, and qualitative variables as numbers and percentages. The primary outcome was analysed using the χ² test. For secondary outcomes, the distribution of qualitative variables was compared between groups using χ² tests, and that of continuous variables using analysis of variance. Whenever a non-normal distribution was detected, between-group comparisons used non-parametric tests. Distributions for time-to-event variables were described using Kaplan-Meier methods and compared using log-rank tests, followed by Cox regression analysis incorporating any relevant prognostic factor, that is, variables associated with death in univariate analysis. All tests were two-sided. A p value of 0.05 was considered statistically significant. The only comparisons that were performed are those reported in the article, all of which were specified in the protocol, and none of which were post hoc. All statistical analyses were performed using the SAS V.9.3 (SAS Institute Inc, Cary, North Carolina, USA) and R (2.14.1) (http://www.R-project.org) software packages.
Results
Study discontinuation
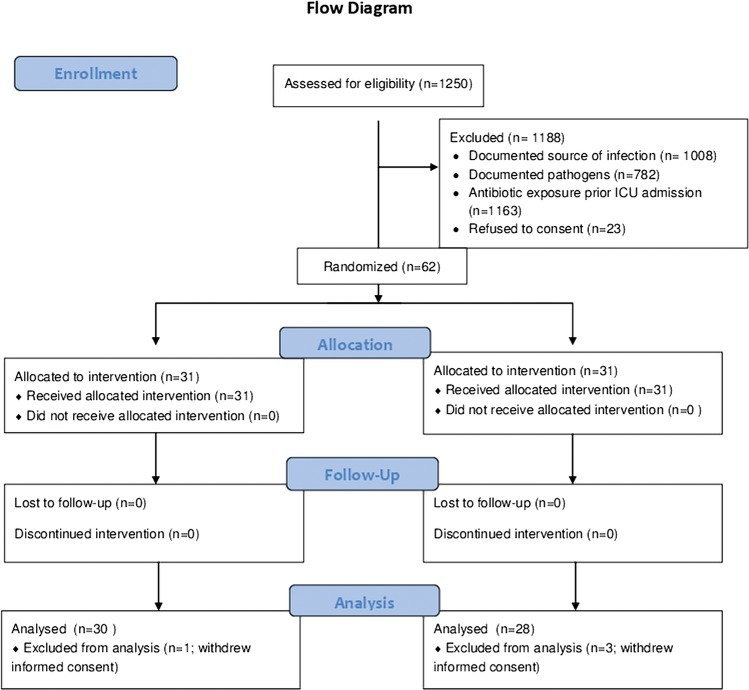
At the planned interim analysis in December 2009, the principal investigator, the sponsor and the data safety and monitoring board decided to stop the trial prematurely owing to the low incidence of eligible patients. From December 2006 to December 2009, of 1250 patients with the phenotype of severe sepsis or septic shock screened in the eight participating centres, only 62 patients were eligible (figure 1). Eighty-two per cent of screened patients were ineligible owing to a documented source of infection, documented pathogens or both, and ∼93% owing to previous antibiotic exposure. Of the 62 were randomised patients, 31 to each arm, 4 (3 in the control arm and 1 in the experimental arm) later withdrew their consent. According to French regulations, vital status being public information, mortality data are given for all randomised patients.
Figure 1.
Trial flow chart.
Study population
Table 1 summarises patient characteristics by study arm. The two groups were well balanced for demographic and anthropometric characteristics, the prevalence and severity of comorbidities and the severity of acute illness. Patients were predominantly men, with medical acute illness and requiring mechanical ventilation. None had indisputable infection. All patients remained corticosteroid-free during their ICU stay. At the time of inclusion, one patient in the experimental arm had received one dose of cephalosporin in the previous 36 h, and two in each group had received β-lactamins and quinolones in the previous 24 h.
Table 1.
Baseline characteristics by study arm of the 58 patients who were randomised and did not withdraw consent*
| Variable-median (IQR) | Control arm (n=28) | PCT-based algorithm arm (n=30) |
|---|---|---|
| Age, years | 54 (46–73) | 59 (40–67) |
| Female gender, n (%) | 9 (32.1%) | 6 (20%) |
| Body mass index, kg/m2 | 27.6 (22.6–30.9) | 22.9 (19.5–26.1) |
| Time to ICU admission, days | 0 (0–0) | 0 (0–0) |
| Time to randomisation, days | 3 (2–8) | 3 (2–6) |
| Location prior to ICU admission, n (%) | ||
| Community | 14 (50%) | 22 (73.3%) |
| Hospital ward | 13 (46.4%) | 8 (26.6%) |
| Long-term care facility | 1 (3.6%) | 0 (0%) |
| Type of admission, n (%) | ||
| Surgical | 1 (3.6%) | 1 (3.3%) |
| Medical | 27 (96.4%) | 29 (96.7%) |
| McCabe class, n (%) | ||
| 0: no life-threatening underlying disease | 18 (69.2%) | 26 (86.7%) |
| 1: life expectancy ≤5 years | 7 (26.9%) | 4 (13.3%) |
| 2: life expectancy <1 year | 1 (3.9%) | 0 (0%) |
| Knaus class, n (%)† | N=26 | N=29 |
| A (%) | 9 (34.6%) | 10 (34.5%) |
| B (%) | 3 (11.5%) | 6 (20.7%) |
| C (%) | 4 (15.4%) | 3 (10.3%) |
| D (%) | 10 (38.5%) | 10 (34.5%) |
| SAPSII score | 43 (32–52) | 32.5±27–47 |
| SOFA | 10 (8–11) | 9.5 (8.5–11) |
| Mechanical ventilation, n (%) | 19 (86%) | 19 (86%) |
| Body temperature, °C | 37.4 (36.7–38.3) | 37.5 (36.7–38.4) |
| White cell count, 103/mm3 | 13.7 (9.1–17.8) | 11.4 (7.5–15.2) |
| PCT, µg/l | 0.7 (0.4–2.4) | 1 (0.3–5) |
| Patients with procalcitonin <0.25 µg/l, n (%) | 2 (7%) | 6 (20%) |
| C reactive protein, mg/l | 141 (77–220) | 87 (52–142) |
*Three patients withdrew consent in the control arm and one in the experimental arm.†Levels of activity limitation are defined as follows: (A) prior good health, no functional limitations; (B) mild to moderate limitation of activity because of chronic medical problem; (C) chronic disease producing serious but not incapacitating restriction of activity and (D) severe restriction of activity due to disease, includes persons bedridden or institutionalised due to illness.
ICU, intensive care unit; PCT, procalcitonin; SAPSII, Simplified Acute Physiology Score, 2nd edition; SOFA, Sepsis-related Organ Failure Assessment.
Primary outcome: proportion of antibiotic-treated patients at day 5
Five days after randomisation, among survivors, 18/27 patients (67%) in the experimental arm were receiving antibiotics, compared with 21/26 in the control arm (81%) (p=0.24; relative risk=0.83, 95% CI 0.60 to 1.14; table 2).
Table 2.
Main outcome measures
| Variable | PCT-based algorithm (n=30) | Controls (n=28) | RR (95% CI) | p |
|---|---|---|---|---|
| Patients on antibiotics at day 5, n (%) | ||||
| Survivors only | 18/27 (67%) | 21/26 (81%) | 0.83 (0.60 to 1.14) | 0.24 |
| All patients with non-survivors considered as being antibiotic-free | 18/30 (60%) | 21/28 (75%) | 0.80 (0.56 to 1.15) | 0.22 |
| All patients with non-survivors considered as being treated with antibiotic | 21/30 (70%) | 23/28 (82%) | 0.85 (0.64 to 1.14) | 0.28 |
| All patients with last information carried over for non-survivors | 18/30 (60%) | 22/28 (79%) | 0.76 (0.54 to 1.08) | 0.13 |
| Days on antibiotic therapy | 5 (2–5) | 5 (3–5) | 0.52 | |
| Antibiotic therapy-free days | 0 (0–3) | 0 (0–2) | ||
| Days on mechanical ventilation | 11 (5–25) | 14 (8–25) | 0.56 | |
| SOFA score | ||||
| At day 3 | 8 (5–10) | 8 (7–11) | 0.85 | |
| At day 5 | 8 (5–9) | 8 (7–11) | 0.61 | |
| Mortality | ||||
| At day 5 | 3/31 (10%) | 3/31 (10%) | 1.00 (0.22 to 4.58) | 1.00 |
| At ICU discharge | 7/31 (23%) | 10/30 (33%) | 0.68 (0.30 to 1.55) | 0.40 |
| At hospital discharge | 7/31 (23%) | 10/30 (33%) | 0.68 (0.30 to 1.55) | 0.40 |
| Length of stay, days | ||||
| In ICU | 22 (8–42) | 23 (10–60) | 0.58 | |
| In hospital | 27 (9–49) | 33 (11–69) | 0.22 |
Continuous variables are expressed as medians and IQR.
Mortality data are reported for all randomised patients, regardless of consent withdrawal.
ICU, intensive care unit; PCT, procalcitonin; RR, relative risk; SOFA, Sepsis-related Organ Failure Assessment.
Secondary outcomes
The median (IQR) time on antibiotic therapy was 5 (4–5) days in the experimental arm and 5 (2–5) days in the control arm (p=0.52). At day 1 postrandomisation, among survivors, 4/27 patients (15%) in the experimental arm had not started antibiotics, compared with 4/26(15%) in the control arm (p=1.00).
At day 5 post-randomisation, 3/31 patients (10%) in the PCT-based algorithm arm and 3/31 (13%) in the control arm had died (p=1.00). Likewise, there were no differences between the two arms for ICU mortality, or in-hospital mortality, for the SOFA score at day 3 or day 5 or for ICU or hospital length-of-stay (table 2).
The proportion of patients who acquired an infection postrandomisation was 2/18 (11%) in the experimental arm and 3/19 (16%) in the control arm (p=1.00; table 3). The proportion of patients who were colonised with methicillin-resistant Staphylococcus aureus, extended spectrum β-lactamase enterobacteria or Klebsiella pneumonia was not different between the two groups (table 3).
Table 3 .
Postrandomisation acquisition of infection and colonisation
| Variable | Experimental group (n=30) | Controls (n=28) | RR (95% CI) | p |
|---|---|---|---|---|
| Acquired infections | ||||
| At day 3 | 1/18 (6%) | 1/19 (5%) | 1.06 (0.07 to 15.64) | 1.00 |
| At day 5 | 1/18 (6%) | 2/19 (11%) | 0.53 (0.05 to 5.33) | 1.00 |
| At any time post-randomisation | 2/18 (11%) | 3/19 (16%) | 0.70 (0.13 to 3.73) | 1.00 |
| Nasal swabs | ||||
| MRSA | 1/28 (4%) | 2/25 (8%) | – | 0.60 |
| Rectal swabs | ||||
| ESBL | 1/25 (4%) | 0/22 (0%) | – | 1.00 |
| Enterobacter Klebsiella | 0/24 (0%) | 0/24 (0%) | – | – |
ESBL, extended spectrum β-lactamase-resistant; MRSA, methicillin-resistant Staphylococcus aureus; RR, relative risk.
Compliance with the PCT-based algorithm
Table 4 presents PCT concentration categories and antibiotic use according to the category in both groups over time. In the experimental arm, physicians were non-compliant with the PCT-based algorithm in 19% of patients at 6 h, 17% on day 3 and 37% on day 5.
Table 4 .
Procalcitonin categories and antibiotic use
| Procalcitonin category | Antibiotic use category | 6 h |
Day 3 |
Day 5 |
|||
|---|---|---|---|---|---|---|---|
| Experimental arm (n=27) | Controls (n=26) | Experimental arm (n=24) | Controls (n=21) | Experimental arm (n=19) | Controls (n=21) | ||
| <0.25 µg/l | Yes | 4 | 2 | 2 | 7 | 5 | 9 |
| No | 2 | 0 | 3 | 2 | 3 | 2 | |
| Total | 6 | 2 | 5 | 9 | 8 | 11 | |
| 0.25–<1 µg/l | Yes | 0 | 5 | 4 | 4 | 1 | 5 |
| No | 1 | 0 | 2 | 2 | 2 | 1 | |
| Total | 1 | 5 | 6 | 6 | 3 | 1 | |
| 1–<5 µg/l | Yes | 12 | 12 | 8 | 4 | 5 | 2 |
| No | 1 | 4 | 2 | 1 | 1 | 1 | |
| Total | 13 | 16 | 10 | 5 | 6 | 3 | |
| >5 µg/l | Yes | 7 | 3 | 3 | 1 | 1 | 0 |
| No | 0 | 0 | 0 | 0 | 1 | 1 | |
| Total | 7 | 3 | 3 | 1 | 2 | 1 | |
Discussion
This study cannot not show evidence that a PCT-based algorithm could influence antibiotic exposure of ICU patients with non-microbiologically proven apparent severe sepsis.
This study specifically addressed the question of the usefulness of PCT levels to rule out infection in patients with the phenotype of severe sepsis or septic shock but doubtful bacterial infection. Very few patients had PCT levels <0.25 µg/l at 6 h, day 3 or even day 5 postrandomisation (table 4). The fact that PCT levels were persistently elevated in most patients in both arms may explain the lack of an intergroup difference in proportion and duration of antibiotic use. These findings suggest that PCT levels may not discriminate between infectious and non-infectious causes of organ dysfunction or shock.
We might have failed to identify true bacterial infections in this population. However, we very carefully excluded patients with previous antibiotic exposure, and comprehensively screened for any potential source of infection by sampling multiple matrices. We believe that the likelihood of misdiagnosing infections was thus very low. PCT levels have been shown to increase in the presence of various non-infectious causes of systemic inflammatory response syndrome or organ dysfunction such as trauma18 or surgery.19 In the current trial, we had almost no surgical patients. Nevertheless, PCT levels also have been shown to correlate with the intensity of organ dysfunction and the number of affected organs.20 The population investigated in the present study had a mean SOFA score of 10 which may per se result in PCT elevations of up to 10–15 µg/l.20 Moreover, there were some patients with procalcitonin levels of more than 1 µg/dl who had good outcome though not being treated with antibiotics.
Interestingly, in patients with PCT levels <0.25 µg/l, physicians were most often non-compliant with the PCT-based algorithm and used antibiotics. Similarly, poor compliance was also observed in the PRORATA trial.8 It has been shown that in sepsis, in the absence of shock or bacteraemia, PCT levels may be in the low range of 0.25–0.50 µg/l in up to 30% of cases.21 Thus, in the context of a phenotype of severe sepsis, the possibility of false-negative PCT findings and the prognostic value of early antibiotic intervention22 may explain physicians’ antibiotic use despite low PCT values.
Because this study investigated a different patient population, its findings do not contradict those of previous studies demonstrating that a PCT-guided strategy may reduce the time on antibiotics in patients with proven infection.7 8 Indeed, in patients with documented or highly suspected infections, the decrease in PCT levels correlated well with the resolution of infection and the duration of antibiotic therapy. However, in patients with the phenotype of severe sepsis but without overt source of infection or sterile cultures, the present study did not confirm the usefulness of low PCT levels to rule out sepsis.1 Together with a previous trial that showed increased duration of mechanical ventilation and ICU length of stay with PCT-guided strategy, our study did not favour the routine use of procalcitonin in such patients.
The trial was designed to demonstrate, at day 5, a 25% absolute reduction in the number of antibiotic-treated patients in the PCT arm, a magnitude of effect that was observed in a previous trial.7 This study was stopped prematurely owing to low numbers of eligible patients (65/1250, 5%). These findings suggest that, in practice, the proportion of patients with the phenotype of severe sepsis, no overt source of infection or identified pathogen, but no antibiotic treatment is very low. This observation may question the need for a sepsis biomarker. The observed rarity of this population also questioned the feasibility of a larger trial. We did not consider that altering the exclusion criteria would help fasting recruitment without deterioration of trial quality. In this trial, previous antibiotic exposure of 2 days or more was an exclusion criterion as this treatment may interfere with bacterial cultures and procalcitonin levels.
Supplementary Material
Acknowledgments
A native English-speaking medical editor, Robert J. Marlowe, Spencer-Fontayne Corporation, Jersey City, NJ, USA, edited the manuscript.
Footnotes
Contributors: DA is the guarantor for the study. He conceived and designed the study protocol, found funds, coordinated the group of investigators, recruited patients, interpreted the data, drafted the manuscript and was responsible for the communication with the sponsor, the study statistician and editors of peer-reviewed Journal. JPF, CC, JCN, VM, ON, MG, CM and HG contributed designing of the protocol, interpretating and reporting the data. MG was responsible for data monitoring. PM and PA performed the statistical analysis and contributed to interpreting and reporting the data. VM, JPF, CM, CC, HG, ON, MF, AR, YC, JCN, GC and DA contributed recruiting and following up patients. All authors have read and approved the manuscript.
Funding : This work was partly funded by Thermo Fisher B.R.A.H.M.S. France, a subsidiary of the maker of the PCT assay used in this study. The sponsor has no input in study design, conduct and reporting. It helped with logistic support when organising investigators meeting.
Competing interests: None.
Patient consent: Obtained.
Ethics approval: CPP of Saint Germain en Laye, France.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: Individual data are available by contacting DA at djillali.annane@rpc.aphp.fr.
References
- 1.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;365:63–78 [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323–9 [DOI] [PubMed] [Google Scholar]
- 3.Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomized trial. Lancet 2007;370:676–84 [DOI] [PubMed] [Google Scholar]
- 4.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008;358:111–24 [DOI] [PubMed] [Google Scholar]
- 5.Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008;358:877–87 [DOI] [PubMed] [Google Scholar]
- 6.Annane D, Cariou A, Maxime V, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 2010;303:341–8 [DOI] [PubMed] [Google Scholar]
- 7.Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302:1059–66 [DOI] [PubMed] [Google Scholar]
- 8.Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomized controlled trial. Lancet 2010;375:463–74 [DOI] [PubMed] [Google Scholar]
- 9.Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011;39:2048–58 [DOI] [PubMed] [Google Scholar]
- 10.Matthaiou D, Ntani G, Kontogeorgi M, et al. Systematic review and meta-analysis of procalcitonin-guided antibiotic therapy algorithms in adult critically-ill patients. An ESICM Systematic Review. Intens Care Med 2012;38:940–9 [DOI] [PubMed] [Google Scholar]
- 11.Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections. Lancet 2004;363:600–7 [DOI] [PubMed] [Google Scholar]
- 12.Clec'h C, Fosse JP, Karoubi P, et al. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med 2006;34:102–7 [DOI] [PubMed] [Google Scholar]
- 13.McCabe WA, Jackson GG. Gram negative bacteremia. I. Etiology and ecology. Arch Intern Med 1962;110:847–55 [Google Scholar]
- 14.Knaus WA, Zimmerman JE, Wagner DP, et al. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med 1981;9:591–7 [DOI] [PubMed] [Google Scholar]
- 15.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPSII) based on a European/North American multicenter study. JAMA 1993;270:2957–63 [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intens Care Med 1996;22:707–10 [DOI] [PubMed] [Google Scholar]
- 17.Gould AL. Interim analyses for monitoring clinical trials that do not materially affect the Type I error rate. Stat Med 1992;11:55–66 [DOI] [PubMed] [Google Scholar]
- 18.Mimoz O, Benoist JF, Edouard AR, et al. Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intens Care Med 1998;24:185–8 [DOI] [PubMed] [Google Scholar]
- 19.Meisner M, Tschaikowsky K, Hutzler A, et al. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intens Care Med 1998;24:680–4 [DOI] [PubMed] [Google Scholar]
- 20.Castelli GP, Pognani C, Cita M, et al. Procalcitonin as a prognostic and diagnostic tool for septic complications after major trauma. Crit Care Med 2009;37:1845–9 [DOI] [PubMed] [Google Scholar]
- 21.Chan YL, Tseng CP, Tsay PK, et al. Procalcitonin as a marker of bacterial infection in the emergency department: an observational study. Crit Care 2004;8:R12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension prior to initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589–96 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.