Abstract
Background Previous studies have shown disparities between Black and Hispanic patients compared with other populations in response to asthma medications.
Objective: The aim of this analysis was to assess the effect of budesonide/formoterol pressurized metered-dose inhaler (BUD/FM pMDI) and BUD on predefined criteria for asthma worsening, an asthma control metric generally aligned with definitions of moderate asthma exacerbations, across four different populations.
Methods: Data were from four 12-week, randomized, double-blind,US studies of BUD/FM pMDI treatment in patients aged 12 years or older with varying asthma severities and of varying races. Predefined asthma events and withdrawals due to predefined events were assessed as secondary study endpoints. Study I (NCT00651651) includes data from predominantly White patients with mild to moderate asthma who were randomized to BUD/FM pMDI 160/9 μg twice daily (bid; n = 123) or BUDpMDI 160 μg bid (n = 121). Study II (NCT00652002) includes data from predominantly White patients withmoderate to severe asthma who were randomized to BUD/FM pMDI 320/9 μg bid (n = 124) or BUD pMDI 320 μg bid (n = 109). Study III (NCT00702325) included self-reported Black patients with moderate to severe asthma who were randomized to BUD/FM pMDI 320/9 μg bid (n = 153) or BUD dry powder inhaler 360 μg bid (n = 148). Study IV (NCT00419757) included self-reported Hispanic patients with moderate to severe asthma who were randomized to BUD/FM pMDI 320/9 μg bid (n = 127) or BUD pMDI 320 μg bid (n = 123). Patients were to be withdrawn from the studies if they developed an asthma event, as determined by predefined criteria, except for night-time awakenings, where withdrawal was left to the study physician’s judgment.
Results: Overall, fewer patients in the studies (study I, II, III, and IV, respectively) experienced ≧1 asthma event in the BUD/FM group (18.7%, 29.8%, 37.3%, 25.2%) versus the BUD group (21.5%, 44.0%, 45.3%, 31.7%); only study II results showed a statistically significant difference between treatments. Fewer patients with moderate to severe asthma (studies II, III, and IV) were withdrawn due to ≧1 asthma event in the BUD/FM group (10.5%, 11.8%, 3.1%, respectively) than in the BUD group (20.2%, 18.9%, 6.5%, respectively); however, percentages were similar in the BUD/FM (7.3%) and BUD (6.6%) groups in patients with mild to moderate asthma (study I).
Conclusions: Predefined asthma event rates were numerically or significantly lower for patients with asthma receiving BUD/FMpMDI versusBUD, regardless of race or disease severity. Differences between the BUD/FM pMDI and BUD groupswere smaller in patients with mild to moderate asthma than in those with moderate to severe asthma, most likely because patients with milder disease had lower asthma event rates. Overall, these findings support the efficacy of BUD/FM pMDI in achieving asthma control in patients with moderate to severe asthma.
Introduction
Asthma disproportionately affects racial and ethnic populations. In the US in 2006, the age-adjusted, asthma-related mortality rates were approximately 3 times higher in non-Hispanic Blacks than in non-Hispanic Whites and Hispanics.[1] Although typical safety and efficacy studies are underpowered or too short in duration to make definitive conclusions regarding severe asthma exacerbations (i.e. those requiring systemic corticosteroids), important insight into the efficacy of medications can be gained from analyzing related moderate exacerbation events characterized by a sustained loss of asthma control (beyond normal day-to-day variation) that does not meet the definition of a severe exacerbation.[2] For the purpose of asthma research protocol development, moderate exacerbation events have been captured using various terminology, such as asthma deterioration,[3] asthma worsenings,[4] and asthma events.[5] Few US studies have evaluated the safety and efficacy of an inhaled corticosteroid (ICS)/long-acting β2-adrenergic agonist (LABA) combination therapy in Black or Hispanic patients with asthma. The efficacy of budesonide/formoterol (BUD/FM) pressurized metered-dose inhaler (pMDI) has been evaluated in randomized, double-blind studies in predominantly White patients with mild to moderate asthma[6] and predominantly White,[5] Black,[7] and Hispanic[8] patients with moderate to severe asthma. Results for a predefined asthma event definition, which encompass moderate to severe asthma deteriorations, are presented as these findings have not been presented previously in detail or compared across patient populations.
Methods
Table I includes a brief summary of the studies that were included in this exploratory analysis. Additional details of the individual studies, including study design and methods, have been previously described.[5–8] This analysis of predefined asthma events (table II) includes data from patients aged ≥12 years with asthma who were enrolled in randomized, double-blind, 12-week US studies that differed according to baseline asthma severity and/or race or ethnicity (table III). Only the BUD/FM and BUD treatment arms, which were common to all four studies, are presented; these studies were not originally powered for comparison of asthma events.
Table I.
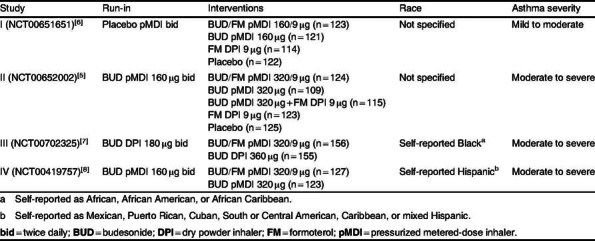
Table II.

Table III.
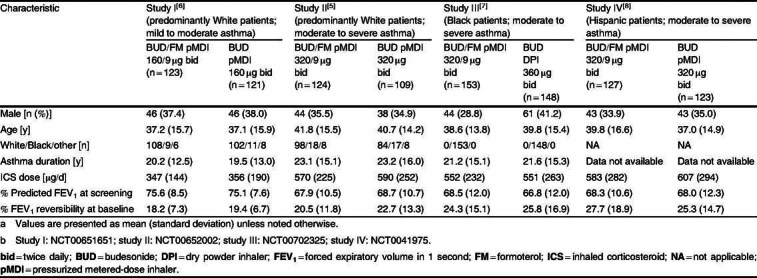
Patient demographic and baseline clinical characteristics[5–8] a,b
Statistical methods for this analysis are similar to those described previously.[5–8] Comparisons among treatment groups of percentages of patients who experienced ≥1 predefined asthma event and of percentages of patients who withdrew because of such an event were performed by χ2 test (study I) or Cochran-Mantel-Haenszel test with adjustment for treatment (studies III and IV) and ICS dose (medium or high; studies II, III, and IV) at study entry.
Results
Baseline demographics were similar across studies (table II). As expected, patients with mild to moderate asthma had better pulmonary function than those with moderate to severe asthma.
The percentage of patients with moderate to severe asthma who experienced ≥1 asthma event was lower in the BUD/FM groups versus the BUD group, with statistically significant differences observed in study II (p < 0.05) [figure 1]. In all studies, the most commonly met predefined criterion was night-time awakening. The predefined criterion of clinical exacerbation included the following subcategories that were not mutually exclusive:
study I (BUD/FM: one patient [one emergency department (ED) visit, one event of disallowed asthma medication use], BUD: three patients [one ED visit, three events of disallowed asthma medication use]);
study II (BUD/FM: seven patients [three ED visits, seven events of disallowed asthma medication use], BUD: five patients [one ED visit, four events of disallowed asthma medication use]);
study III (BUD/FM: three patients [two events of disallowed asthma medication use, one event of nebulized bronchodilator use, three events of oral corticosteroid use], BUD: three patients [one ED visit, three events of disallowed asthma medication use, one event of nebulized bronchodilator use, and three events of oral corticosteroid use]);
study IV (BUD/FM: seven patients [two ED visits, two hospitalizations — one due to multiple significant/active comorbidities and one due to viral infection, seven events of disallowed asthma medication use], BUD: two patients [two events of disallowed medication use]).
Fig. 1.
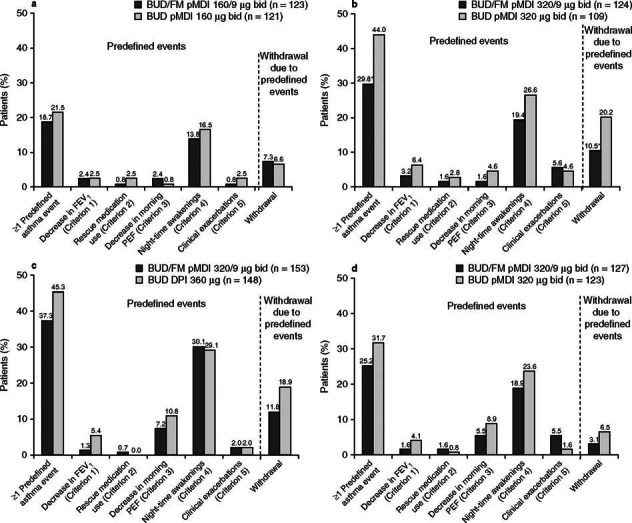
Percentages of patients with ≥1 predefined asthma event (overall and individual events) and withdrawals due to predefined asthma event in (a) study I (predominantly White patients with mild to moderate asthma), (b) study II (predominantly White patients with moderate to severe asthma), (c) study III (Black patients), and (d) study IV (Hispanic patients). bid = twice daily; BUD = budesonide; DPI = dry powder inhaler; FEV 1 = forced expiratory volume in 1 second; FM = formoterol; PEF = peak expiratory flow; pMDI = pressurized metered-dose inhaler; * p < 0.05 vs BUD.
Overall, withdrawal rates were lower in studies I and IV than in studies II and III (figure 1). The percentage of patients with mild to moderate asthma (study I) who withdrew due to ≥1 predefined asthma event was similar in the BUD/FM and BUD groups. Percentages of patients with moderate to severe asthma (studies II, III, and IV) who withdrew due to ≥1 asthma event were numerically lower in the BUD/FM versus BUD groups, regardless of race. Additional results from the individual studies have been previously described.[5–8]
Conclusions
Predefined asthma events are increasingly being utilized in clinical research studies as a sensitive composite control metric. An asthma event metric encompassing measures of pulmonary function, symptoms, rescue medication use, and the need for additional medications was investigated in the present analysis. While individual studies were not powered for statistical analyses, predefined asthma event rates in four 12-week, randomized studies consistently showed numerical or significant differences favoring BUD/FM pMDI over BUD across White, Black, and Hispanic patients, regardless of disease severity. Notably, the results of this analysis showing similar predefined asthma event rates among patients of differing racial backgrounds is consistent with the primary analyses showing the efficacy of BUD/FM pMDI in Blacks[7] and Hispanics,[8] as well as a study demonstrating the efficacy of ICS/LABA in Blacks.[9] Additional discussion of findings and limitations of the individual studies have been previously discussed.[5–8] Differences between the BUD/FM pMDI and BUD groups were smaller in patients with mild to moderate asthma than in patients with moderate to severe asthma, most likely because patients with milder disease had overall lower asthma event rates. These data further support the efficacy of BUD/FM pMDI in achieving asthma control in patients with moderate to severe asthma, regardless of race.
Acknowledgements
This study was supported by AstraZeneca LP, Wilmington, DE, USA. Medical writing services, provided by Lisa Feder, PhD (Scientific Connexions, Newtown, PA, USA), were funded by AstraZeneca LP.
K.R. Murphy, T. Uryniak, U.J. Martin, and J. Zangrilli made substantial contributions to the analysis and interpretation of data, drafted and revised the manuscript critically for important intellectual content, and provided final approval of the version to be published.
K.R. Murphy is a(n) consultant and advisor to and has received lecture fees and grants from AstraZeneca LP. T. Uryniak, U.J. Martin, and J. Zangrilli are shareholders and employees of AstraZeneca LP.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.2165/11632060-000000000-00000.
References
- 1.American Lung Association. Trends in asthma morbidity and mortality. 2011. [Google Scholar]
- 2.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 3.Nathan RA, Nolte H, Pearlman DS. P04334 Study Investigators. Twenty-six-week efficacy and safety study of mometasone furoate/formoterol 200/10 μg combination treatment in patients with persistent asthma previously receiving medium-dose inhaled corticosteroids. Allergy Asthma Proc. 2010;31(4):269–79. doi: 10.2500/aap.2010.31.3364. [DOI] [PubMed] [Google Scholar]
- 4.Kavuru M, Melamed J, Gross G, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, doubleblind, placebo-controlled trial. J Allergy Clin Immunol. 2000;105(6Pt1):1108–16. doi: 10.1067/mai.2000.105711. [DOI] [PubMed] [Google Scholar]
- 5.Noonan M, Rosenwasser LJ, Martin P, et al. Efficacy and safety of budesonide and formoterol in one pressurised metered-dose inhaler in adults and adolescents with moderate to severe asthma: a randomised clinical trial. Drugs. 2006;66(5):2235–54. doi: 10.2165/00003495-200666170-00006. [DOI] [PubMed] [Google Scholar]
- 6.Corren J, Korenblat PE, Miller CJ, et al. Twelve-week, randomized, placebo-controlled, multicenter study of the efficacy and tolerability of budesonide and formoterol in one metered-dose inhaler compared with budesonide alone and formoterol alone in adolescents and adults with asthma. Clin Ther. 2007;29(5):823–43. doi: 10.1016/j.clinthera.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Spector SL, Martin UJ, Uryniak T, et al. Budesonide/ formoterol pressurized metered dose inhaler versus budesonide: a randomized controlled trial in Black patients with asthma. J Asthma. 2012;49(1):70–7. doi: 10.3109/02770903.2011.633788. [DOI] [PubMed] [Google Scholar]
- 8.Zangrilli J, Mansfield LE, Uryniak T, et al. Efficacy of budesonide/formoterol pressurized metered-dose inhaler versus budesonide pressurized metered-dose inhaler alone in Hispanic adults and adolescents with asthma: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2011;107(3):258–65. doi: 10.1016/j.anai.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Bailey W, Castro M, Matz J, et al. Asthma exacerbations in African Americans treated for 1 year with combination fluticasone propionate and salmeterol or fluticasone propionate alone. Curr Med Res Opin. 2008;24(6):1669–82. doi: 10.1185/03007990802119111. [DOI] [PubMed] [Google Scholar]


