Abstract
Objectives
To describe the design and baseline population characteristics of an adapted lifestyle intervention trial aimed at reducing weight and increasing physical activity in people of Indian and Pakistani origin at high risk of developing type 2 diabetes.
Design
Cluster, randomised controlled trial.
Setting
Community-based in Edinburgh and Glasgow, Scotland, UK.
Participants
156 families, comprising 171 people with impaired glycaemia, and waist sizes ≥90 cm (men) and ≥80 cm (women), plus 124 family volunteers.
Interventions
Families were randomised into either an intensive intervention of 15 dietitian visits providing lifestyle advice, or a light (control) intervention of four visits, over a period of 3 years.
Outcome measures
The primary outcome is a change in mean weight between baseline and 3 years. Secondary outcomes are changes in waist, hip, body mass index, plasma blood glucose and physical activity. The cost of the intervention will be measured. Qualitative work will seek to understand factors that motivated participation and retention in the trial and families’ experience of adhering to the interventions.
Results
Between July 2007 and October 2009, 171 people with impaired glycaemia, along with 124 family volunteers, were randomised. In total, 95% (171/196) of eligible participants agreed to proceed to the 3-year trial. Only 13 of the 156 families contained more than one recruit with impaired glycaemia. We have recruited sufficient participants to undertake an adequately powered trial to detect a mean difference in weight of 2.5 kg between the intensive and light intervention groups at the 5% significance level. Over half the families include family volunteers. The main participants have a mean age of 52 years and 64% are women.
Conclusions
Prevention of Diabetes & Obesity in South Asians (PODOSA) is one of the first community-based, randomised lifestyle intervention trials in a UK South Asian population. The main trial results will be submitted for publication during 2013.
Trial registration
Current controlled trials ISRCTN25729565 (http://www.controlled-trials.com/isrctn/).
Keywords: randomised controlled trial; Prevention; diabetes mellitus, Type 2; ethnic groups
Article summary.
Article focus
Randomised controlled trial.
Diabetes prevention via weight loss and physical activity.
South Asians living in Scotland, UK.
Key messages
The worldwide prevalence of type 2 diabetes has doubled over the past 25 years and South Asians, including those in the UK, are at particularly high risk of developing the disease.
PODOSA is one of the first community-based, randomised lifestyle intervention trials focusing on the UK South Asian population, and it is taking place in Scotland, UK.
The dietitian-led intervention is family focused, based in the home and is culturally adapted from the Finnish Diabetes Prevention Study, for people of Indian and Pakistani origin.
The primary outcome is weight change over 3 years, the main driver for prevention or delay of onset of type 2 diabetes. The trial is on course to report in 2013.
Strengths and limitations of this study
The study is one of the few randomised trials specifically for an ethnic minority population in the UK.
The results should provide valuable evidence for tackling the high levels of diabetes in UK South Asians.
The study could not recruit sufficient people to examine progression to diabetes within the original time frame, but it is planned do this over the longer term via data linkage to national health records.
Background
Diabetes mellitus is a serious disease that reduces life expectancy by around 6 years from middle age,1 and increases the risk of blindness, heart disease, stroke and kidney failure. The age-standardised prevalence of diabetes worldwide was over 9% (9.8% for men, 9.2% for women) in 2008. This translates to around 347 million people with diabetes globally, more than double the number from 1980 (153 million).2 Estimates show that, in India, the number of adults with diabetes will increase from 50 million in 2010 to at least 87 million by 2030.3 In the UK, in 2010, there were an estimated 3.4 million people with type 2 diabetes mellitus (henceforth diabetes), resulting in direct costs to the National Health Service (NHS) of £8.8 billion.4 The adult age-standardised prevalence of diabetes in UK South Asians (defined here as UK residents with ancestral origins in the Indian Subcontinent) is about 2–6 times that of the general population with probably higher progression rates from impaired glycaemia to diabetes, although robust data are lacking.5–7
There is clear evidence from a number of diabetes prevention trials in adults at elevated risk for diabetes that lifestyle intervention focusing on modest weight loss (5–10% of body weight) and increased physical activity is effective at preventing or delaying diabetes.8–10 Such interventions have been found to be effective in a number of ethnic groups including ‘Asians’ living in the USA10 and Indians living in India.11 The evidence from randomised trials has been summarised and published by the Centres for Disease Control and Prevention Primary Prevention Working Group (2004).12 They conclude that maintenance of moderate weight loss through diet and physical activity reduces the incidence of diabetes by 40–60% over 3–4 years. A review of evidence and application in a UK setting by Davies et al13 showed that major benefits accrue from 5% to 10% body weight loss and 150 min/week of physical activity, similar in intensity to brisk walking. They suggest a need to develop and evaluate interventions that target communities and populations at risk in the UK.
In the UK, the first standard of the National Service Framework for Diabetes (NSFD) is to reduce the number of people who develop diabetes and to reduce inequalities in this disease. The NSFD14 and the Scottish Government Diabetes Action Plan 201015 place emphasis on the need to prevent and control diabetes in the UK's minority ethnic populations (highlighting the high rates in South Asians) through implementation of effective and culturally relevant interventions. The main risk factors for diabetes, principally weight gain and physical inactivity, need to be tackled in these populations, but there are no UK trial data to guide either practice or policy.
The current challenge is to adapt existing interventions to meet the cultural needs of South Asians, and to demonstrate efficacy in the UK context, as it has been suggested that strategies that work in some societies may not work in others, as different social, economic, political and cultural environments will affect diet and lifestyle.13 We based the Prevention of Diabetes & Obesity in South Asians (PODOSA) trial on the Finnish Diabetes Prevention study, which demonstrated the effectiveness of an individual-focused behavioural intervention that promoted weight loss and increased physical activity in preventing diabetes in a general population.9 PODOSA's key adaptations are to shift the emphasis from the individual to the family and from the clinic to the household. A cluster design was chosen first to maximise participation and help achieve behaviour change, by recognising the fact that most health-related behaviours take place within the family or home setting and that other family members may, for example, be involved in food preparation (supplementary information in the NSFD).14 Second, this design would limit potential ‘contamination’ where close members of a family were in different arms of the trial, but sharing information. For reasons already described,16 17 recruitment proved difficult and the primary aim was changed from a reduction in the incidence of diabetes to weight loss, as this needed a smaller sample size.
The PODOSA trial thus aims to test the effectiveness and cost-effectiveness of an intervention designed to reduce weight and increase physical activity in adults at high risk of diabetes, indicated by Impaired Glucose Tolerance (IGT) or Impaired Fasting Glycaemia (IFG), thereby preventing or delaying diabetes. The aim of this paper was to describe the trial design and methods and baseline characteristics of participants and family volunteers.
Methods/design
Study design and questions
PODOSA is a cluster, randomised controlled trial, the ‘cluster’ represented by a family. The original protocol written in 2007 was designed to answer the primary question, ‘Does a family-based three-year programme promoting weight loss and increased physical activity in South Asians, modelled on interventions of proven effectiveness internationally, reduce the incidence of type 2 diabetes in South Asians?’ However, owing to recruitment challenges,16 a substantial amendment was approved by the ethics committee in 2009 to alter the primary outcome as detailed above.
The principal research questions that we are pursuing are therefore now:
Does a family-based 3-year programme promoting weight loss and increased physical activity in South Asians with IGT and/or IFG, modelled on interventions of proven effectiveness internationally, result in a clinically meaningful weight loss in the intensive intervention (15-visit) group compared with the light intervention (four-visit) group?
What is the cost-effectiveness of the intervention?
What factors assist recruitment, adherence to advice given and retention in the trial?
In addition to participants with impaired glycaemia, we invited adult members of their families to take part, mainly to support the trial participants in the process of lifestyle change. We made limited measurements on the family volunteers both to motivate them in changing their own lifestyles and to help assess the potential benefits to family members outside the main intervention groups.
The secondary research questions which are designed to help interpret the main outcomes are:
- During the trial, what changes occur over time among participants with IGT and/or IFG, and volunteer members of their families (analysed separately), in
- Waist circumference?
- Hip circumference?
- Fasting and 2 h blood glucose (IGT/IFG recruits only)?
- Incidence of type 2 diabetes presently (and in the longer term, to be assessed via data linkage)?
The primary outcome is mean weight change between baseline and 3 years. Our goal is weight loss of 2.5 kg more (or 2.5 kg lower weight gain) in the intensive intervention than in the light intervention group and increase in physical activity to at least 30 min daily. Ideally, we would reduce the body mass index (BMI) to at least 25 or preferably 23 (the interim WHO recommendation for Asian populations).18
Secondary outcome measures of interest are:
Mean changes between baseline and 3 years in waist and hip circumference, BMI, fasting and 2 h post-Oral Glucose Tolerance Test (OGTT) glucose;
Cost-effectiveness of the intervention (focusing on health service costs of the intervention and the opportunity cost of time for trial participants);
Progression to type 2 diabetes in the longer term.
For the volunteer members of the family, these are:
Mean changes between baseline and 3 years in weight, BMI and waist and hip circumference.
Ethical approval
Ethical approval was obtained from the Scotland A Research Ethics Committee. All recruits gave written, informed consent to take part in the screening stage of the study and then further written consent for participation in the 3-year trial.
Setting and recruitment
Recruitment took place between July 2007 and October 2009. Men and women of Indian and Pakistani origin, aged 35 years and over, and living in the Lothian and Greater Glasgow & Clyde Health Board areas, were invited to be screened with the (OGTT).
Eligibility criteria
Eligible participants for the trial were those with
Waist sizes ≥90 cm for men and ≥80 cm for women;
IGT (ie, fasting plasma glucose of <7 mmol/l and, following a standard OGTT, a 2 h plasma glucose of 7.8–11.0 mmol/l);
IFG (ie, plasma fasting glucose of 6.1–6.9 mmol/l);
No previous diagnosis of diabetes;
‘Family cook’ agreed to cooperate (whether a family volunteer or another family member).
Participants on prescribed long-term oral corticosteroids, suffering from a health condition where adherence to the intervention was contraindicated or improbable, or unlikely to remain in the UK for 3 years, were excluded from trial entry.
The waist criteria correspond to the cut-off points recommended by the International Diabetes Federation Consensus Group in 2005 to identify South Asians at risk of diabetes.19
Eligible family volunteers were
≥18 years of age;
Close relative of the IGT/IFG participant either living within the same household, or living nearby and interacting with main recruit(s) on at least a weekly basis.
Definition of a family (cluster)
The composition of families was established in consultation with the family itself. Criteria were defined to identify each extended family unit prior to randomisation. To minimise contamination, first-degree relatives (parents, siblings, children) living in the same city could not be randomised separately. The cluster is ‘the core family’ consisting of the participant(s) with IGT/IFG, plus any family volunteer(s). In practice, given the relatively low prevalence of IGT or IFG (15%), compared with 30% expected, clustering was less common than predicted.
Sample size considerations
In the original study design, it was anticipated that there would often be at least two eligible individuals per family (ie, per cluster), so the fact that we were using a cluster randomised design was critical in the power calculation. However, when the primary endpoint was amended to weight change, it was clear that the vast majority of ‘clusters’ would comprise a single individual. Thus, in practice, the impact of clustering will be negligible, and the modified power calculation did not take this into account. The target sample size for the amended trial was calculated to be 175 recruits to allow for a 10% drop-out. This would result in at least 150 recruits having complete follow-up at 3 years. This sample size gives adequate (86%) power to detect a difference of 50% of the SD (ie, a mean difference in weight change of 2.5 kg between the two groups against a common background SD of 5 kg, derived using nQuery Advisor V.7.0) at the 5% significance level (two-sided).
Randomisation and allocation of interventions
Randomisation lists were produced by the trial statistician using a random number generator programme. Permuted blocks were used and block size varied randomly. Stratification was by location (Edinburgh or Glasgow), ethnic group (Indian or Pakistani) and number of IGT/IFG recruits in the family (one or more than one). All members of a ‘family’ gave written informed consent and completed the baseline visit prior to randomisation. Allocation of the intervention group was then performed centrally, by the trial statistician or a deputy, independently from the dietitians and trial office staff. This strategy was implemented to minimise selection bias during the recruitment process and meant that both the dietitians and the families did not know the allocated intervention until the enrolment and baseline measurements had been completed. As in other lifestyle prevention trials, blinding of the intervention was not feasible.
The study has two groups for comparison, one group having more frequent and tailored contact (intensive or 15-visit intervention) with the research dietitians than the ‘control’ group (light or 4-visit intervention), which largely receives information. The 15-visit group of 78 families received 15 contacts over 3 years, monthly for 3 months and quarterly thereafter. The four-visit group of 78 families had annual contact over 3 years with the dietitian. The dietitians visited the participating families at their home or community setting of their choice.
Measurements and data collection
Prior to the start of recruitment, pilot work was carried out for the main trial procedures, covering consent, measurements and OGTT, and the screening and baseline visits. Table 1 outlines the time-points for consent, randomisation and collection of data for the main trial outcomes for all trial participants. Anthropometric measurements, background and outcome data were collected by the dietitians in the case record forms at each visit. Standard operating procedures were written for all the main study procedures including anthropometric measurements and the oral glucose tolerance test. Two measurements for height, weight, waist and hip were performed and if the difference was more than a specified value (height, waist and hip >1 cm, weight >0.2 kg), a third measurement was carried out. Physical activity was assessed by the short form of the International Physical Activity Questionnaire (IPAQ).20 Time spent sitting, walking and undertaking moderate and vigorous activities was extracted from the IPAQ with time spent walking and in moderate and vigorous activities truncated at 180 min/day, in line with the published IPAQ data processing guidelines (http://www.ipaq.ki.se).
Table 1.
Time points of outcome measures and data collection
| Time point (months) | Name of visit | Informed consent | OGTT & blood sample for storage | Anthropometric measurements | Demographic, socioeconomic self-reported medical history | Costs and health resource use | Physical activity data | Delivery of intervention (intensive or light) |
|---|---|---|---|---|---|---|---|---|
| –1* | Screen | ✓ | ✓ | ✓ | ||||
| 0*† | Baseline | ✓† | ✓† | ✓ | ✓ | ✓ | General information on diabetes, diet and physical activity to all participants | |
| 0*† (plus 1 week) | Family (as the cluster) randomised to 15 or four-visit group | |||||||
| 1 | Interim | ✓ | ✓ | ✓ | ||||
| 2 | Interim | ✓ | ✓ | ✓ | ||||
| 3 | Interim | ✓ | ✓ | ✓ | ||||
| 6 | Interim | ✓ | ✓ | ✓ | ||||
| 9 | Interim | ✓ | ✓ | ✓ | ||||
| 12*† | Annual | ✓† | ✓ | ✓ | ✓ | Intensive or light | ||
| 15 | Interim | ✓ | ✓ | ✓ | ||||
| 18 | Interim | ✓ | ✓ | ✓ | ||||
| 21 | Interim | ✓ | ✓ | ✓ | ||||
| 24*† | Annual | ✓ † | ✓ | ✓ | ✓ | Intensive or light | ||
| ✓ | ✓ | ✓ | ||||||
| 27 | Interim | ✓ | ✓ | ✓ | ||||
| 30 | Interim | ✓ | ✓ | ✓ | ||||
| 33 | Interim | ✓ | ✓ | ✓ | ||||
| 36*† | Annual | ✓ (OGTT repeated if positive for diabetes) | ✓† | ✓ | ✓ | ✓ | Intensive or light | |
*Measurements and data collected similarly for participants in intervention and control groups—or prior to randomisation.
†Indicates time points and data collection for Family Volunteers.
OGTT, Oral Glucose Tolerance Test.
Data were entered by the study assistant into a Microsoft Access database which has inbuilt validity and consistency checks. Subsequent data cleaning was performed by further manual and statistical checking. Double data entry was carried out for the key variables relating to the main trial outcomes, including randomisation criteria, all anthropometric and biomedical measures and demographic and health economics data.
Three of four research dietitians were employed throughout the full study period and followed up the families for the full 3 years. The fourth dietitian left the research team in 2009 and her families were distributed among the remaining three dietitians. To counteract any potential observer bias when recording the key endpoint variables at the 3-year visits, an independent set of anthropometric measures (in addition to those recorded by the dietitians) was recorded by trained research nurses blinded to the study group.
Measurements for volunteer members of the family
As shown in table 1, weight and waist and hip circumferences were measured annually in adult family volunteers with and without diabetes (no blood tests were performed).
Intervention for 15-visit group
The research dietitians were trained in venepuncture, measurement, delivery of information, behaviour change and promotion of physical activity. The contacts with the families were, in effect, the intervention, and in general, each family was seen by the same dietitian for the duration of the trial. The content of the contacts was tailored, using a range of culturally adapted change management tools, to the needs of the individuals and families. The dietitians motivated the participating families to achieve weight loss through a calorie deficit diet in conjunction with physical activity. Verbal and written advice was provided including information on shopping, cooking (with demonstrations) and entertaining. Participants were invited to annual group sessions consisting of a food shopping tour, understanding food labels, exchange of recipes, food tasting and brisk walking. The dietitians’ toolkit (which will be published on the PODOSA website, http://www.podosa.org, by the end of March 2013), contained culturally adapted and translated existing resources on diet and physical activity such as Counterweight.21 A paper on the cultural adaptation process of the study materials has been accepted for publication (subject to minor revisions) by Health Promotion International. Pedometers were integral to the physical activity programme, providing motivation through self-monitoring and a tool for the dietitians to assess progress. Daily food diaries and pedometer logs, body weight and waist circumference data and the Chester Step Test22 were used as educational and motivational tools by the dietitians.
Intervention for four-visit group
This group had a baseline and then annual contact with the research dietitian. The dietitians gave both written and verbal advice on healthy eating, diabetes prevention, promotion of physical activity and on accessing available health services for weight control and physical activity. The research team agreed which resources should be given to families in the four-visit group at each visit, to ensure consistency. Although these actions were better than the routine service, it is not anticipated that they will reduce weight substantially and sustainably, though they may stabilise it and counteract the secular trend and age effects of increasing weight.23 In our opinion, this level of intervention was necessary on ethical grounds. It also offered something in return for participation and measurements.
Measurement and valuation of costs
The PODOSA trial design included an integrated cost analysis. Cost data were collected prospectively from randomisation (baseline) to the 3-year follow-up. We chose a societal perspective for the analysis which encompassed the health service costs of the intervention and the opportunity cost of time for trial participants. The programme costs included the number and length of home visits by research dietitians and self-reported health service use in primary and acute care settings. Initial screening and trial recruitment costs were excluded. We valued dietitians’ time (face-to-face contact, previsit and postvisit review and travel to participants’ households) using NHS salary scales inclusive of salary on-costs and overheads. Standard NHS unit costs were used to value general practitioner visits and hospital out-patient clinic attendances. Participant time included the number and length of dietitian visits and self-reported time spent doing moderate physical activities and on household allocation of time for food shopping and meal preparation. Median hourly wages by gender and ethnicity reported by the National Equality Panel/Labour Force Survey24 were used to value participant time. No estimate of diet costs was included. The present value of the 3-year cumulative costs was calculated using a 3.5% annual rate of discount following the UK Treasury and National Institute for Health and Clinical Excellence (NICE) guidance. All costs are reported in UK pounds using 2010 pay and price levels.
All analyses will be conducted on an intention-to-treat basis. The conditional mean cost comparison between 15-visit and 4-visit groups will be modelled using linear regression and generalised linear parametric methods. The mean cost difference between the groups will also be assessed using a non-parametric bootstrap. Quantile regression will be used to examine and compare the median cost differences. The relatively small sample size precludes assessment of heterogeneous treatment effects or subgroup differences in costs.
The robustness of results will be investigated by using a strategy of comparing different specifications within the generalised linear model and conducting a series of one-way sensitivity analyses, where we will alter key assumptions on programme intensity and frequency as measured by the number, length and duration of visits. The cost implications arising from moving away from a one-to-one programme towards a group-based intervention will also be considered.
Qualitative study
An embedded qualitative study was undertaken to:
Obtain a rich and multifaceted understanding of the main motivations for participation in an intervention study of Indian and Pakistani adults who are at high risk of developing diabetes.
Investigate participants’ perceptions of fidelity and faithfulness to the interventions offered both during and after participation.
Understand the factors that may help promote retention of participants once enrolled.
We utilised the storytelling concept to collect narratives describing the live experiences of participation in PODOSA from families at completion of the trial. The objective was to try to understand what factors motivate the ethnic minority people to engage with research and to understand more about the facilitators to participation through understanding the perspectives and experiences of those who chose to participate in the trial.
A detailed description of our methods will be reported in due course, but in summary, we undertook purposeful sampling on the basis of age, sex, ethnicity, faith group, geographical location and trial arm to ensure recruitment of a maximum diversity sample. We also sought to include family volunteers when possible. Biographical narrative interviews were undertaken usually in participants’ homes and in their preferred language, with the aid of a translator, if necessary. These interviews were digitally recorded, translated (if necessary) and then transcribed together with accompanying field notes. Analysis was undertaken in an iterative fashion, thus informing of further data collection. Thematic and performance analysis25 of the data utilised the constant comparison method26 concurrent to data generation, utilising NVivo9 software to code data during analysis.
Laboratory assessments
The 75 g OGTT followed standardised procedures, with venous blood samples being taken after an overnight fast of 10–16 h and then 2 h after ingestion of 75 g glucose. Samples were then transported to a central hospital laboratory (Western General Hospital, Edinburgh or Glasgow Royal Infirmary) where plasma glucose concentration was determined using the Ortho clinical diagnostics, Fusion dry ice method (Edinburgh), or the Abbott Architect, hexokinase/glucose-6-phosphate dehydrogenase method (Glasgow). Both laboratories participate in the UK National External Quality Assessment Service (UK NEQAS) scheme. In addition, an EDTA sample was obtained from all recruits at baseline and at 3 years, and with the participant's specific informed consent, plasma and DNA aliquots stored at –80°C, for future analyses out with the remit of the current trial.
Statistical analysis
Analyses will be performed on an intention-to-treat basis, that is, participants will be analysed in the group that they were assigned to regardless of how much of the intervention they received, unless specified otherwise.
Owing to the clustering inherent in the design, the primary outcome will be analysed using a random effects linear regression model (to accommodate the clustering of individuals within families) with maximum likelihood estimation. The model will be adjusted for the stratification variables (ethnicity and location). Change over time will be incorporated into the model using an extension to the analysis of covariance approach, adjusting for baseline value. Treatment group will be included in the model as a fixed effect. Results will be reported as an adjusted (for ethnicity and location) mean difference in weight between baseline and 3 years, with a 95% CI and corresponding p value. The intraclass correlation coefficient will be reported.
Analyses of secondary outcomes will mirror those for the primary outcome, where the distribution of the relevant outcome is continuous. Where the outcome is a proportion, the approach will be to fit a generalised linear mixed model with terms for stratification variables and treatment group as above and adjusting for baseline value where applicable. Results will be reported as an adjusted OR with a 95% CI and corresponding p value.
Results
Recruitment
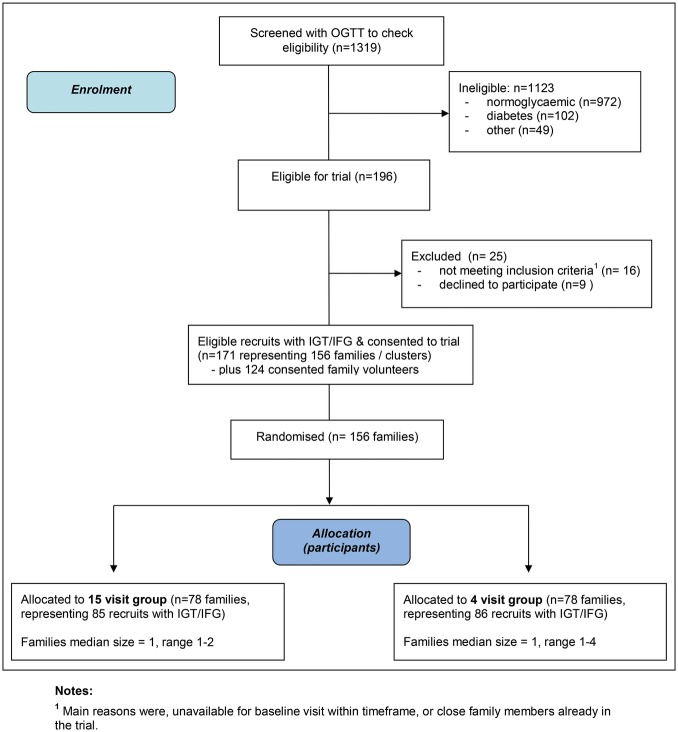
As shown in figure 1, 1319 participants were screened with an OGTT over a 27-month period between July 2007 and October 2009. In total, 102 recruits (8%) had OGTT results indicative of diabetes and 196 (15.4%) were found to have impaired glycaemia. Sixteen participants did not meet eligibility criteria to proceed into the full trial and nine declined to participate further. Thus, 95% (171/196) agreed to continue into the 3-year trial. In total, 156 family clusters comprising the 171 eligible participants with IGT and/or IFG, along with 124 family volunteers, were randomised into either the 15-visit or 4-visit intervention groups.
Figure 1.
PODOSA (Prevention of Diabetes & Obesity in South Asians) trail consort flowchart.
Baseline characteristics of the trial population
Baseline characteristics are shown in table 2. The families range from single participants to a family comprising four IGT/IFG recruits with five family volunteers. Only 13 of the 156 trial families have more than one recruit with IGT/IFG. Family volunteers were recruited to 85 families. The trial population is well established in the UK with a mean residency time of around 31 years. Approximately 33% of the participants have no formal educational qualifications.
Table 2.
Recruits with IFG and/or IGT, demographic, social, lifestyle, anthropometric, biochemical and other background characteristics of trial participants
| Variables | All participants number (column %) |
|---|---|
| Demographic | |
| Number of families with | |
| 1 IGT/IFG recruit | 143 (91.7) |
| 2 IGT/IFG recruits | 12 (7.7) |
| 4 IGT/IFG recruits | 1 (0.6) |
| Number of families with | |
| With family volunteer(s) | 85 (54.5) |
| Number of IGT/IFG individuals | 171 (100) |
| Number of family volunteers | 124 (100) |
| Individual IGT/IFG recruits | |
| Sex—male | 78 (45.6) |
| Age—mean (SD) | 52.3 (10.1) |
| Age—range | 35–80 |
| Location | |
| Glasgow | 132 (77.2) |
| Edinburgh | 39 (22.8) |
| Ethnic group | |
| Indian | 57 (33.3) |
| Pakistani | 114 (66.7) |
| Religion | |
| Muslim | 114 (66.7) |
| Hindu | 15 (8.8) |
| Sikh | 39 (22.8) |
| Other | 3 (1.8) |
| Social circumstances | |
| Cook was a participant | 85 (49.7) |
| Cook was a family volunteer | 59 (34.5) |
| Cook was simply cooperating | 27 (15.8) |
| Blood relative with diabetes | 118 (69.0) |
| Years lived in UK (mean, SD) | 31.4 (13.1) |
| Education | |
| No qualifications | 56 (32.7) |
| School level | 49 (28.7) |
| Further or higher education | 66 (38.6) |
| Lifestyle | |
| Current smoking/chewing tobacco | 11 (6.4) |
| Currently drinks alcohol | 19 (11.1) |
| Vegetarian | 26 (15.2) |
| Physical activity (mean minutes per day, SD) | |
| Total (moderate, vigorous, walking) | 51.0 (61.0) |
| Moderate and vigorous only | 23.3 (44.7) |
| Walking only | 27.7 (37.1) |
| Sitting time (mean hours per day, SD) | 6.5 (3.0) |
| Anthropometric (values are given as mean and SD) | |
| Height (cm) | 161.9 (9.3) |
| Weight (kg) | 80.2 (15.6) |
| BMI (kg/m2) | 30.5 (4.8) |
| Waist (cm) | 103.0 (11.1) |
| Hip (cm) | 107.1 (9.5) |
| Waist/hip ratio | 0.96 (0.07) |
| BMI <25 (n, %) | 20 (11.7) |
| BMI ≥25 and <30 (n, %) | 67 (39.2) |
| BMI ≥30 (n, %) | 84 (49.1) |
| Biomedical measures (values are given as mean and SD) | |
| Systolic BP (mm Hg) | 136.9 (20.6) |
| Diastolic BP (mm Hg) | 83.0 (11.5) |
| Fasting plasma glucose (mmol/l) | 5.8 (0.6) |
| 2 h post-OGTT plasma glucose (mmol/l) | 8.3 (1.6) |
| Current medications (n, %) | |
| Antihypertensives | 48 (28.1) |
| Cholesterol lowering | 39 (22.8) |
Figures are numbers and column percentages unless otherwise stated.
BMI, body mass index; IFG, Impaired Fasting Glycaemia; IGT, Impaired Glucose Tolerance; OGTT, Oral Glucose Tolerance Test.
Table 2 shows that approximately 84% of family cooks are either the IGT/IFG person or a family volunteer. The remainder all agreed to cooperate. Over a third of the participants had a close family history of diabetes.
Part (c) of table 2 describes the lifestyle characteristics of the participants. The average total activity time (comprising vigorous, moderate and brisk walking) for the trial population was 51 min/day. The mean sitting time was 6.5 h/day.
The mean BMI for all recruits was 30.5 kg/m2, and overall, 49% of participants had BMI >30 kg/m2. Table 3 shows demographic and anthropometric characteristics of the 124 family volunteers. Most volunteers were female (77%) and 64/124 (52%) were the spouse or partner of the index recruit. Over 90% of the family volunteers were recruited in Glasgow. The mean BMI of family volunteers was 27.4 kg/m2.
Table 3.
Family volunteers, Demographic, anthropometric and other background characteristics of family volunteers
| All family volunteers number (column %) | |
|---|---|
| Demographic | |
| Sex—male | 28 (22.6) |
| Age—mean (SD) | 41.9 (14.9) |
| Age—range | 18–75 |
| Location | |
| Glasgow | 114 (91.9) |
| Edinburgh | 10 (8.1) |
| Ethnic group | |
| Indian | 42 (33.9) |
| Pakistani | 79 (63.7) |
| Other | 3 (2.4) |
| Relationship to main recruit | |
| Spouse/partner | 64 (51.6) |
| Parent | 2 (1.6) |
| Son/daughter | 26 (21.0) |
| Brother/sister | 5 (4.0) |
| Other | 27 (21.7) |
| Anthropometric (values are given as mean and SD) | |
| Height (cm) | 161.7 (8.5) |
| Weight (kg) | 71.4 (13.9) |
| BMI (kg/m2) | 27.4 (5.3) |
| Waist (cm) | 92.7 (12.4) |
| Hip (cm) | 104.7 (8.6) |
| Waist/hip ratio | 0.89 (0.08) |
| Biomedical | |
| Number of with diabetes (self-reported) | 15 (12.1) |
Figures are numbers and column percentages unless otherwise stated.
Discussion
Principal achievements
The PODOSA trial's key achievements include: establishing the infrastructure for the trial; recruiting, training and forging a multiethnic team to implement the trial; and the involvement and support from within the wider South Asian community, particularly in the recruitment phase.17 It was encouraging that 95% of eligible recruits consented to participate in the 3-year trial (171/196). We emphasised the need for family involvement as a means of motivating behaviour change and set the complex intervention in the home setting. Our only entry criterion relating to the family was that the main cook agree to co-operate, and this was always achieved. We consider this a major success. It proved harder to recruit family volunteers in Edinburgh than in Glasgow. It was difficult to identify clear reasons for this, but the dietitians reported that, in many instances, the potential volunteers were either unavailable or did not interact or eat with the main recruits with sufficient frequency.
The proportion of Pakistani to Indian recruits (2:1) in PODOSA closely reflects the wider resident South Asian population as reported in the 2001 Scottish census where those of Pakistani origin represented 31% of the total minority ethnic population and 15% were of Indian origin.27
Strengths and weaknesses
PODOSA, to our knowledge, is one of the first culturally adapted, community-based, randomised intervention trials on lifestyle and health issues in South Asians in the UK. PODOSA will contribute evidence for weight control and diabetes specifically; however, its long-term legacy will be the experience, lessons and example of the evaluation of complex interventions in ethnic minority populations set in the community in the UK multiethnic society.
Although we were unable to recruit sufficient numbers to examine, with sufficient power, progression to diabetes within the life of the trial, we have participants’ consent to link trial data to the Scottish national morbidity records and Scottish diabetes register during a 10-year follow-up period. This may allow analysis of this outcome in the longer term. However, weight loss, our new primary outcome, is the main driver for diabetes prevention, and physical activity, a secondary outcome, is also important.
Putting the study in context
Based on the available evidence in 2005 at the design stage of the trial,5 19 we set eligibility criteria for waist circumference (≥90 cm for men and ≥80 cm for women) as those with central obesity are more likely to have impaired glycaemia. We estimated that we would identify IGT in around 30% of such volunteers screened for trial eligibility.5 Within PODOSA, the prevalence rate for IGT and/or IFG was approximately 15%, much lower than expected. The Leicester (UK) Addition study reported finding 19.8% IGT or IFG in South Asians aged 40–75 years, with no minimum waist size.28 A recent systematic review29 of cross-sectional studies in South Asians also suggests a stable or falling IGT prevalence, although the natural history of prediabetes and its progression to diabetes still remains unclear. Our lower prevalence rate of impaired glycaemia was one of the contributory factors to our difficulty in achieving the original intended sample size.
Within the UK, the case for national screening programmes for both diabetes and impaired glycaemia remains equivocal.30 Recent research has suggested that the case is stronger than it was, although evidence from good quality trials showing a subsequent reduction in morbidity and mortality is still required.31 Hanif et al32 argue that a stepwise screening strategy aimed at the South Asian population could be effective, although further work is needed to examine implementation within primary care. The Addition Leicester trial,6 a community screening programme and cardiovascular risk intervention, includes a significant South Asian population and is due to report in 2013. The results from PODOSA, also expected in 2013, will contribute to urgently needed evidence about the effectiveness of prevention interventions in a UK ethnic minority population at high risk of developing diabetes.
Implications
South Asians are at high risk of developing type 2 diabetes and effectiveness data for culturally tailored healthcare interventions are urgently required in order to help prevent this epidemic and to help inform healthcare services and policy in the UK. The trial results, including cost-effectiveness and qualitative findings, will be submitted for publication in 2013. In particular, this study has focused on the family rather than the individual and moved from the traditional clinic to a home setting. This kind of approach has been promoted in guidance from NSFD and NICE,14 33 so evidence from PODOSA will be pertinent to this line of argument. More generally, PODOSA will also contribute to the evidence base for conducting randomised lifestyle intervention trials in ethnic minority populations in the UK and contribute to future meta-analyses with ongoing diabetes prevention trials in other South Asian populations.
Supplementary Material
Acknowledgments
The authors would like to thank other PODOSA Investigators and Collaborators (Dr Naureen Ahmed, Dr Colin Fischbacher, Dr Rafik Gardee, Dr Sonja Hunt, Dr Lubna Kerr, Dr Fraser McLeod); the PODOSA Trial Steering Committee (Prof Nigel Unwin, Prof Graham Hitman, Dr Nita Forouhi, Dr Deepak Bhatnagar, Dr Marlie Ferenczi and Mr Iqbal Anwar); the PODOSA Trial Data Monitoring and Ethics Committee (Prof Iain Crombie, Dr Mike Small, Dr Mike Kelly, Prof Kamlesh Khunti); PODOSA Trial Staff (Alyson Hutchison, Alex Celini, Maninder Kaur, Arti Nair, Anne Houghton, Zoe Morrison, Rachael Samuel); the Trial Sponsor (University of Edinburgh); other supporting research networks—Scottish Primary Care Research Network, Scottish Diabetes Research Network, Welcome Trust Clinical Research Facility Edinburgh, BHF Glasgow Cardiovascular Research Centre (Dr Lynne Cherry, Pauline Watt); Glasgow & Edinburgh Diabetes Managed Clinical Networks; all Health Care Professionals in NHS Lothian and NHS Greater Glasgow & Clyde and South Asian Community & religious organisations and individuals who contributed to the recruitment for PODOSA, and finally to all the participants who gave their time to take part in this study. Prof Mike J E Lean and Prof Jaakko Tuomilehto contributed to the planning of the trial but are not authors on this paper.
Footnotes
Contributors: AD and RSB drafted the manuscript and are joint guarantors. RSB, SW,JFF, JMRG, JMK, GM, NS, SWild, ASheikh and AD contributed to the design of the trial, and RuB and AS contributed to the design of the intervention. SW, RuB and AS were the research dietitians responsible for the acquisition of data. AD, JMRG, GM and RSB contributed to the analysis. All authors contributed to the interpretation of data and a critical review of the manuscript during the writing process. All authors approved the final version to be published.
Funding: This work was supported by the National Prevention Research Initiative (grant number G0501310), a funding consortium comprising the British Heart Foundation; Cancer Research UK; Department of Health; Diabetes UK; Economic and Social Research Council; Medical Research Council; Health & Social Care Research & Development Office for Northern Ireland; Chief Scientist Office, Scottish Government Health Directorate; the Welsh Assembly Government and World Cancer Research Fund. Additional financial support was provided from NHS Lothian and NHS Greater Glasgow & Clyde R&D, Chief Scientist Office, NHS Health Scotland and NHS National Services Scotland.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics approval: Ethics approval was obtained from the Scotland A Research Ethics Committee (reference number 07-MRE10–2).
Data sharing statement: No additional data are available.
References
- 1.Seshasai SR, Kaptoge S, Thompson Aet al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40 [DOI] [PubMed] [Google Scholar]
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 4.Hex N, Bartlett C, Wright D, et al. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855–62 [DOI] [PubMed] [Google Scholar]
- 5.Bhopal R, Unwin N, White M, et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. BMJ 1999;319:215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webb DR, Gray LJ, Khunti K, et al. Screening for diabetes using an oral glucose tolerance test within a western multi-ethnic population identifies modifiable cardiovascular risk: the ADDITION-Leicester study. Diabetologia 2011;54:2237–46 [DOI] [PubMed] [Google Scholar]
- 7.Forouhi NG, Merrick D, Goyder E, et al. Diabetes prevalence in England, 2001—estimates from an epidemiological model. Diabet Med 2006;23:189–97 [DOI] [PubMed] [Google Scholar]
- 8.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–44 [DOI] [PubMed] [Google Scholar]
- 9.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50 [DOI] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006; 49:289–97 [DOI] [PubMed] [Google Scholar]
- 12.Williamson DF, Vinicor F, Bowman BA. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med 2004;140:951–7 [DOI] [PubMed] [Google Scholar]
- 13.Davies MJ, Tringham JR, Troughton J, et al. Prevention of Type 2 diabetes mellitus. A review of the evidence and its application in a UK setting. Diabet Med 2004;21:403–14 [DOI] [PubMed] [Google Scholar]
- 14.Department of Health, National Service Framework for Diabetes. London, 2001. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4032932.pdf (accessed 18 Jan 2013).
- 15.2010. Diabetes Action Plan 2010 Quality Care for Diabetes in Scotland. Scottish Government. http://www.scotland.gov.uk/Resource/Doc/321699–0103402.pdf (accessed 18 Jan 2013).
- 16.Douglas A, Bhopal RS, Bhopal R, et al. Recruiting South Asians to a lifestyle intervention trial: experiences and lessons from PODOSA (Prevention of Diabetes & Obesity in South Asians). Trials 2011;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samsudeen BS, Douglas A, Bhopal RS. Challenges in recruiting South Asians into prevention trials: health professional and community recruiters’ perceptions on the PODOSA trial. Public Health 2011;125:201–9 [DOI] [PubMed] [Google Scholar]
- 18.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63 [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet 2005;366:1059–62 [DOI] [PubMed] [Google Scholar]
- 20.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95 [DOI] [PubMed] [Google Scholar]
- 21.Gibbs HD, Broom J, Brown J, et al. Current approaches to obesity management in UK Primary Care: the Counterwieght Programme. J Hum Nutr Diet 2004;17:183–90 [DOI] [PubMed] [Google Scholar]
- 22.Sykes K. Chester Step Test; resource pack(Version 3). Chester College, UK, 1998 [Google Scholar]
- 23.Lindstrom J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–6 [DOI] [PubMed] [Google Scholar]
- 24.Government Equalities Office, National Equality Panel 2010 http://sta.geo.useconnect.co.uk/national_equality_panel/publications.aspx (accessed 18 Jan 2013).
- 25.Reissman CK. Narrative methods for the human sciences. Sage, 2007 [Google Scholar]
- 26.Barbour R. Introducing qualitative research. Sage, 2007 [Google Scholar]
- 27.2001. Scottish Executive, Analysis of Ethnicity in the 2001 Census. http://www.scotland.gov.uk/Resource/Doc/47210–0025543.pdf (accessed 18 Jan 2013).
- 28.Gray LJ, Yates T, Davies MJ, et al. Defining obesity cut-off points for migrant South Asians. PLoS ONE 2011;6:e26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katikireddi SV, Morling JR, Bhopal R. Is there a divergence in time trends in the prevalence of impaired glucose tolerance and diabetes? A systematic review in South Asian populations. Int J Epidemiol 2011;40:1542–53 [DOI] [PubMed] [Google Scholar]
- 30.Simmons RK, Echouffo-Tcheugui JB, Sharp SJ, et al. Screening for type 2 diabetes and population mortality over 10 years (ADDITION-Cambridge): a cluster-randomised controlled trial. Lancet 2012;380:1741–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waugh N, Scotland G, McNamee P, et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11:iii–xi1 [DOI] [PubMed] [Google Scholar]
- 32.Hanif MW, Valsamakis G, Dixon A, et al. Detection of impaired glucose tolerance and undiagnosed type 2 diabetes in UK South Asians: an effective screening strategy. Diabetes Obes Metab 2008;10:755–62 [DOI] [PubMed] [Google Scholar]
- 33.2011. Preventing type 2 diabetes: population and community-level interventions in high-risk groups and the general population. Nice.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.