Abstract
Osteoarthritis (OA), also called degenerative joint disease, is the most frequently occurring chronic musculoskeletal disease, particularly affecting the aging population. The use of viscosupplementation, i.e. intra-articular (IA) hyaluronic acid (HA) drug therapy, to treat OA, is growing worldwide, due to important results obtained from several clinical trials, which reported IA HA-related improvements in functional activity and pain management. This review is an update of the IA use of this compound in the treatment of OA, with clinical evidence from the last few years being discussed and used to delineate new trends for the future.
1. Introduction
The principal forms of chronic arthritis can be grouped as follows: (i) atrophic arthritis with synovial inflammation and erosion or atrophy of the cartilage and bone (e.g rheumatoid arthritis [RA]); and (ii) hypertrophic arthritis, characterized by focal loss of cartilage with little evidence of the typical form of inflammation (it is not a systemic disease and the ‘inflammatory component’ seems to be restricted to the cartilage and bone), and by growth (hypertrophy) of the adjacent bone and soft tissue (i.e osteoarthritis [OA]).[1] The term ‘osteoarthritis’ is derived from the Greek word osteon, meaning ‘of the bone’, arthron meaning ‘joint’, and itis meaning ‘inflammation’ OA is the most common form of arthritis affecting the aging population, causing significant pain and functional disability worldwide.[2] It has been observed that about one-third of adults show radiologic signs of OA,[3] although an epidemiologic study found clinically significant OA of the knee, hand, or hip in only 8.9% of adults; knee OA was the most common type of OA, occurring in 6% of adults.[4]
Endogenous risk factors for OA of the knee include age, sex, family history, ethnic origin (OA is more common in people of European descent), and post-menopausal changes Exogenous risk factors include macrotrauma, repetitive microtrauma, being overweight, resective joint surgery, and lifestyle factors (e.g alcohol and tobacco use).[5] The relationship between running and OA has been studied for a long time in both humans and animals A review in 2006 concluded that a moderate level of running does not increase the risk of knee or hip OA in healthy people, and that this activity might even have a protective effect; however, a history of injury from overuse or acute trauma, running or excessive running, intrinsic anatomical instability in the joints, or a high body mass index, can accelerate the onset of OA and cause disability.[6]
OA is a degenerative joint disease characterized by an accumulation of mechanical stresses to joints, leading to the destruction of articular cartilage It is caused by a combination of (i) increased degradation of extracellular matrix (ECM; some members of the matrix metalloproteinase [MMP] and a disintegrin and metalloproteinase with thrombospondin motifs [ADAMTS] gene families have been correlated with the process of cartilage ECM degradation); (ii) decreased production of ECM; and (iii) chondrocyte death.[7]
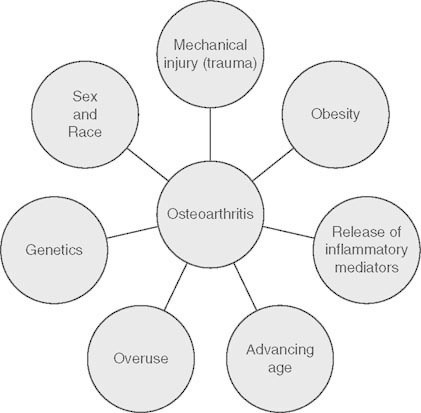
OA has historically been classified as ‘primary’ if no discernible cause is evident (e.g related to age and genetics) and ‘secondary’ if a triggering factor is apparent (e.g associated with a history of joint injury, such as those caused by trauma, infection, surgery, mineral deposition, or autoimmune disorders).[6,8] Aggrecan and type II collagen are the most abundant proteins found in the ECM, and are both produced by chondrocytes Their destruction is crucial in the alteration of cartilage homeostasis and ECM destruction in OA Mechanical stress, aging, genetic background, inflammation, and phenotypic changes of chondrocytes are thought to influence the pathogenesis of OA[9] (figure 1).
Fig. 1.
During the osteoarthritic process, it has been observed that articular chondrocytes exhibit an age-related increase in cytokine and MMP levels, a decline in the synthetic capacity of growth factors, and an increase in the features of senescence (e.g increased activity of senescence-associated β-galactosidase, p53, p21, and p16, and telomere shortening) Moreover, because stress-induced senescence is caused by the reactive oxygen species (ROS), and ROS are induced by the mechanical stress loaded onto the chondrocyte, these molecules may play a key role in the pathogenesis of OA.[14]
Drugs, such as glucocorticoids and NSAIDs, have been widely used for the treatment of OA; however, none of these agents provide a complete treatment and they have all been associated with adverse effects Recently, new strategies, such as anti-cytokine therapy, gene therapy, delivery of growth factors, stem-cell therapy, and new lubricant agents, such as lubricin, have been proposed.[15] The use of viscosupplementation (i.e intra-articular [IA] hyaluronic acid [HA] drug therapy) to treat OA is growing worldwide due to important results obtained from several clinical trials reporting improvements in functional activity and pain management HA is a constitutive component of matrix cartilage, which plays a key role in the maintenance of joint homeostasis HA is also a biologically active component, secreted by chondrocytes, that protects the cartilage from degradation by interacting with MMPs and pain mediators This review provides an up-to-date analysis of the IA HA for the treatment of OA.
1.1 Search Criteria
Data from clinical trials published between January 2007 and April 2010 that involved the use of IA HA for the treatment of OA were derived from a literature search in MEDLINE and PubMed using the keyword “hyaluronic acid” combined with the keywords “intra-articular”, “injection”, and “osteoarthritis”.
2. Hyaluronic Acid
2.1 Background
In 1934, Karl Meyer isolated an unknown glycosaminoglycan from the vitreous humor of the bovine eye and proposed, for convenience, to name it ‘hyaluronic acid’ (derived from hyaloid [vitreous] and uronic acid).[16] Originally, this compound was known as hyaluronan when referring to its synthesis in vivo, and HA when referring to research performed in vitro.[16] However, the terms are now used synonymously; the compound is also sometimes referred to as hyaluronate.
2.2 Pharmacologic Properties
HA is a non-sulfated, naturally occurring glycosaminoglycan with distinct physicochemical properties, consisting of alternately repeating D-glucuronic acid and N-acetylglucosamine units (figure 2).[17] HA exists naturally in various animal tissues, including rooster combs (the largest content of HA), shark skin, bovine eyeballs, bovine nasal cartilage, rabbit brain, and rabbit heart, and in various human tissues, including the umbilical cord, synovial fluid (SF), vitreous body, dermis, epidermis, thoracic lymph, urine, and serum.[18] However, the highest amounts of HA in the human body are found in the ECM of soft connective tissues.[19]
Fig. 2.

The chemical structure of hyaluronic acid.
The two types of HA currently available are (i) low molecular weight HA (LMW HA) – hyaluronans, sodium hyaluronans, or hyaluronates (molecular weight 0.5–3.6 million Da); and (ii) high molecular weight HA (HMW HA) - chemically crosslinked hyaluronan (molecular weight 6.0 million Da).[20] HA physicochemical characteristics (i.e hydrophilic, rheologic, signaling, and viscoelastic properties) and biologic functions (e.g involvement in embryogenesis, inflammation, metastasis, tumor progression, tissue turnover, and wound healing) depend on its molecular weight and interactions with specific binding proteins called ‘hyaladherins’ These interactions modify the conformation of HA The best characterized hyaladherins are structural hyaluronan-binding proteins of the ECM (i.e link protein and the aggregating proteoglycans aggrecan, versican, brevican, and neurocan), and the cell surface hyaluronan receptor CD44 These molecules all have structurally similar hyaluronan-binding domains, which are termed link modules The receptor for hyaluronic acid-mediated motility (RHAMM) is another hyaladherin, but this molecule does not have link modules.[21]
HA is involved in various biologic processes because it stimulates cell migration, differentiation, and proliferation, and regulates ECM organization and metabolism.[22] In the cartilage, HA plays an important structural role in the matrix, forming an aggregation center for aggrecan, a large chondroitin sulfate proteoglycan that retains its macromolecular assembly in the matrix due to specific HA-protein interactions.[23] Cartilage homeostasis and modulation of cartilage metabolism is maintained by the interaction of HA with the CD44 receptor on chondrocytes (CD44 isoform), suggesting that disruption of these interactions may promote matrix remodeling.[24,25] This receptor is also present in the osteocyte plasma, basolateral membrane, and the cytoplasmic processes of active osteoblasts The interaction of the CD44 receptor with HA restricts osteoblast-mediated osteoclastogenesis.[26–28]
HA interacts with other biomacromolecules, such as collagen, to promote ECM assembly HA can also interact with cells through receptors on the plasmatic membrane, such as the CD44 receptor, which activates several biologic effects, such as modulation of angiogenic processes, induction of pro-inflammatory MMP expression, enhancement of cell motility and invasion, and amplification of cell proliferation.[29] Moreover, there is evidence that the interaction between HA and CD44 plays a key role at several stages of embryogenesis,[30] as well as in the development of adult tissues.[31–33] HA also plays an important role in signal transduction, and the molecule is associated with cancer invasiveness and metastasis.[34]
In addition, hyaluronan possesses chondroprotective effects, which make it useful for the treatment of OA and RA (sections 2.2.1 and 2.3) The cell surface receptors of hyaluronan are involved in (i) the inhibition of the catabolic actions exerted by pro-inflammatory cytokines and matrikines (matrix degradation products), which are known to be increased in OA and RA joints; and (ii) the induction of catabolic enzymes (e.g collagenase and aggrecanase) that cause cartilage degradation.[35]
The use of HA and HA-based scaffolds in the repair of ligament, cartilage, adipose tissue, bone, and osteochondral defects has been investigated in various tissue engineering studies.[36] In addition, clinical data on the efficacy of HA scaffolds in the tissue engineering of cartilage[37,38] and artery regeneration[39] have been reported.
2.2.1 In Osteoarthritis or Rheumatoid Arthritis
From a clinical perspective, patients with OA have been shown to have a variable degree of synovitis, and it is thought that several cytokines and other mediators, particularly tumor necrosis factor (TNF)-α and interleukin (IL)-1, may play a key role in both synovial inflammation, and in the activation of chondrocytes and synovial fibroblasts.[40] These cytokines can stimulate their own production, and induce synovial cells and chondrocytes to produce IL-6, IL-8, and leukocyte inhibitory factor, along with proteases and prostaglandins In fact, it has been hypothesized that TNFα and IL-1 are key mediators of inflammation and articular cartilage destruction, supporting a future possibility of anticytokine therapy in OA or the design of specific disease-modifying anti-OA drugs.[41]
A recent study performed in 82 patients, aged 90 years, showed that low innate production of the pro-inflammatory cytokines IL-1β and IL-6 was associated with the absence of OA in old age In particular, the absence of hand OA was associated with low innate production of IL-6 and IL-1 receptor antagonist, and the absence of hip OA was associated with low IL-1β production These findings suggest the presence of protective factors against OA development, and underline the importance of cytokine pathways in the pathophysiology of this degenerative condition.[42]
Joint destruction in RA is caused by the pro-inflammatory cytokine-stimulated (e.g IL-1β) production of MMPs by rheumatoid synovial fibroblasts (RSFs).[43] In contrast, TNF-α or IL-1β-stimulated production of hyaluronan inhibits MMP-1 production by RSFs, suggesting a therapeutic role of HA in the treatment of rheumatoid joints.[44] In addition, the binding of HA to CD44 is directly involved in the suppression of MMP-1 production.[45]
In normal SF, the high concentration of HMW HA is responsible for joint lubrication and shock absorption The HA viscoelastic behavior differs depending on the force applied If a shear stress is applied, HA acts as a lubricant, becoming less viscous and allowing free and easy movements However, if a compressive force is applied, HA acts as a shock absorber, preventing injury to the joint HMW HA in the SF also provides a renewed source of HA to joint tissues, thus restoring the normal levels.[46] Under inflammatory conditions of arthritic diseases, such as OA or RA, HMW HA is degraded by ROS, which reduces its viscosity and impairs its lubricant and shock absorbing properties, leading to deteriorated joint movement and pain.[47] It has been speculated that the removal of pathologic osteoarthritic SF and its replacement with HA-based products (viscosupplementation) may have beneficial therapeutic effects (section 2.3).[48]
HMW HA in the SF is also essential for the structural integrity of the joint In fact, it protects synovial cells and controls the movement of large molecules in the joint, preventing the release of free radicals and inflammatory factors Moreover, IA HA-related decreases in pain have been associated with reduced production of bradykinin, prostaglandin E2, and substance P, and the direct inhibition of nociceptive afferents.[49,50]
Injection of exogenous HA into a joint may restore the articular viscoelastic properties.[51] A possible mechanism that describes HA therapeutic action in OA has been proposed Macroscopically, HA restores the synovium and the organization of healthy cartilage so that the joint can react viscoelastically to force application.[52,53] HA exerts a beneficial effect on the cartilage integrity and response to OA damage, which may be related to a primary effect of HA on the cartilage surface HA may also act on the synovial membrane by limiting the synovial reaction HA exerts a chondroprotective action, which can be explained at different levels It seems to have a trophic effect on chondrocytes, enhancing their metabolism Moreover, HA inhibits the development of the fibroblast-like cells in damaged joints.[54] HA protects chondrocytes from oxidative stress through preservation of mitochondrial function,[55] and it inhibits apoptotic and dedifferentiative effects of nitic oxide (NO) on chondrocytes, reverting the block of protein kinase C-α.[56,57]
In addition, HA therapeutic effects can also be explained at the molecular level In fact HA treatment decreases the expression of vascular endothelial growth factor (VEGF), VEGF receptor, and connective tissue growth factor (CTGF), which are known to be significantly upregulated in OA causing detrimental effects on cartilage.[58,59] VEGF induces capillary formation and is involved in the inflammatory process, while CTGF stimulates MMP-3 and cellular matrix degradation.[60–63] HA also inhibits IL-1β preventing MMP-1 and MMP-9 release, and it increases the cartilage catabolism of the joint.[43–45,62]
These effects may depend on the HA molecular weight and the duration of time that HA is present within the joint space.[52,64–66] There is evidence that injected HA remains within the joint space for hours or even days, and that it exerts its clinical effects for months, possibly due to its anti-inflammatory and anti-nociceptive properties.[67,68]
The ability of HA to bind to its cellular receptors is strictly related to its molecular size, so that LMW HA elicits a cellular response opposite to that of HMW HA Experimental animal models of OA showed that HA with a molecular weight within the range 0.5–1.0 million Da was responsible for more effective reductions in indices of synovial inflammation, and greater restoration of SF rheologic properties (visco-induction) than HA with a molecular weight >2.3 million Da.[52] It was also observed that, although HA with a molecular weight >40 kDa produced an analgesic effect, HA with a molecular weight of 860 or 2300 kDa produced high and long-lasting analgesia These effects of HA appear to be caused by the interaction between HA and its receptors.[69]
The effects of HA on human OA chondrocytes are also related to its molecular weight, so that the 500–730; kDa HA at 200 μg/mL reduces the synthesis of both IL-1-induced NO and prostaglandin E2 by 70% and 45%, respectively, while the 6000 kDa HA does not.[70]
2.3 Clinical Use
Pure high molecular weight hyaluronan was first developed by Balazs[71] in the 1960s It was known as non-inflammatory fraction sodium hyaluronan (NIF-NaHA) and was marketed for use in ocular surgery Hyaluronan was injected into the joints of racehorses for traumatic OA with effective results,[72] leading to the first use of viscosupplementation for human knee OA in the early 1970s.[73]
The clinical use of viscosupplementation to treat OA in humans is growing worldwide The procedure involves the introduction of HA into the joint, and aims to provide initial lubrication and shock absorption, and to change the long-term disease process, thereby restoring the rheologic properties of the SF.[74]
During the last 20 years, several clinical trials using different HA formulations (LMW HA has been commonly used; and less often, HMW HA has been injected) have shown that HA is more active than placebo in reducing arthritic pain.[17,75,76]
In a literature review conducted by Brzusek and Petron[77] that used data from all MEDLINE- or Embase-identified randomized, placebo-controlled trials involving the use of hyaluronans, HA and sodium hyaluronate plus hylan G-F 20 (a cross-linked HA derivative, approved by the US FDA since 1997 for the treatment of patients with symptomatic knee OA) were both associated with significant improvements in pain and physical function in patients (mostly adults aged over 40 years) with knee OA Both regimens were most effective between 5 and 13 weeks after injection, but improvements were also observed at 14–26 weeks and sometimes even longer; both were well tolerated with a low incidence of adverse events Moreover, the same authors also reported beneficial treatment effects when HA was coadministered with other therapies, and concluded that the drug was not only effective, but also safe and tolerable for the treatment of symptomatic knee OA.
A recent systematic review of randomized, controlled, prospective clinical studies (eight trials; n = 1674 patients), showed that hylan G–F 20 significantly improved Western Ontario and McMaster Universities (WOMAC) OA index physical function versus appropriate/;conventional care or corticosteroid treatment Moreover, hylan G–F 20 was associated with a significant improvement in loss of activity compared with saline, and with similar functional improvements compared with progressive knee exercises or NSAIDs.[78]
In a 2008 literature review of all trials involving the use of hylan G–F 20, the authors concluded that the clinical use of this drug was safe and effective for decreasing pain and improving function in patients experiencing knee OA.[79]
A systematic review and meta-analysis comparing the efficacy of IA HA with that of corticosteroids for knee OA (seven trials; n = 606 patients), showed that corticosteroids were more effective than HA in the short term (up to 4 weeks), but that HA was more effective in the long term (4–26 weeks) This highlights the importance of determining whether coadministration of the two agents may lead to a synergistic effect useful in clinical practice.[80]
In addition, it has been pointed out that HA may relieve pain and improve function in patients with hip OA Moderate improvements in pain and function were reported for 3–6 months after HA injection, with no serious adverse events observed.[81]
All the clinical trials involving the use of HA in knee, carpometacarpal joint, temporomandibular joint, and ankle OA are summarized in tables sI, sII, sIII, and sIV (see Supplemental Digital Content 1, http://links.adisonline.com/DRZ/A2) Table sV (Supplemental Digital Content) summarizes all the other applications of HA in clinical studies.
3. Intra-Articular Injections: Other Drugs
3.1 Corticosteroids
The efficacy and safety of IA corticosteroids in the treatment of knee OA was evaluated by Bellamy et al.[82] in 2006 This Cochrane systematic review analyzed results from 28 trials (n = 1973), which compared IA corticosteroids with placebo, IA HA/;hyaluronan/;hylan, joint lavage, or other IA corticosteroids This review showed that IA corticosteroids were more effective than IA placebo for pain reduction and functional improvement (patient global assessment) at 1 week post-injection There was evidence of a between-group difference in pain reduction at 2 weeks post-injection that was in favor of corticosteroids, but not of a significant between-group difference in functional improvement At 4–24 weeks post-injection, there was no evidence of a between-group difference in the effect on pain or function.
Comparing corticosteroids with HA products, no statistically significant differences were detected at 1–4 weeks post-injection, according to the Cochrane systematic review.[83] However, between 5 and 13 weeks post-injection, HA products were more effective than corticosteroids for one or more of the following variables: WOMAC osteoarthritis index, Lequesne Index, pain, range of motion (flexion), and number of responders In one study,[83] there was also a between-group difference in the range of motion (flexion) at 14 and 26 weeks that was in favor of HA, but no differences in efficacy were detected at 45–52 weeks In general, HA products and IA corticosteroids had a similar onset of effect, but HA products had a more durable response.
In the Cochrane systematic review,[82] comparisons between various IA corticosteroids showed that triamcinolone hexacetonide was superior to betamethasone with regard to the number of patients reporting pain reduction up to 4 weeks post-injection Comparisons between IA corticosteroid and joint lavage showed no differences in any of the efficacy or safety outcome measures.
In another systematic literature review conducted in 2009, the efficacy (including duration of action) of IA corticosteroid injections in reducing pain caused by knee OA was assessed This review analyzed data from six trials (reported in five articles) that compared IA corticosteroids with placebo, and four articles that compared different IA corticosteroids The review observed that IA corticosteroids were associated with reductions in knee pain that lasted for at least 1 week It concluded that IA corticosteroids can only be considered as a short-term treatment for a chronic problem Two of four trials showed triamcinolone to be more effective in pain reduction than the other corticosteroids assessed.[84]
In a review of the literature that included results from eight trials (four randomized controlled trials) examining the efficacy of IA corticosteroid injection for hip OA, there was strong evidence to support the use of corticosteroid injections for short-term reductions in pain, and in patients refractory to non-pharmacologic therapy or pharmacologic therapy with analgesic or NSAID therapy The authors reported that, when an IA hip injection is performed, the use of radiological guidance is recommended The same review reports a comprehensive list of contraindications for IA hip corticosteroid injection, which include suspected or known joint infection, presence of joint fracture, coagulopathy, overlying cellulitis or infection, hypersensitivity to corticosteroids, and the presence of a prosthetic joint Relative contraindications include anticoagulation therapy, joint instability, poorly controlled diabetes mellitus, and adjacent skin abrasions Adverse effects related to IA injections of the hip are septic arthritis, osteonecrosis, and the risk of joint infection after total hip replacement following pre-operative IA corticosteroid injection.[85]
A postoperative, IA methylprednisolone and lidocaine injection in 58 patients with chondromalacia undergoing meniscectomy was tested in a randomized controlled trial The patients were randomized to receive active treatment (n = 29) or saline plus lidocaine (n = 30) Results showed that the addition of a postoperative corticosteroid injection improved pain relief and function at an early timepoint, but did not provide a lasting effect compared with a local anesthetic injection.[86]
A dual-center, single blind, randomized, parallel-group trial was performed to compare the benefits of IA corticosteroid (40 mg triamcinolone acetonide and 1% lidocaine) injections and tidal irrigation (TI) in patients with OA of the knee.[87] TI is the repeated distention and irrigation of a joint with saline through a 14 gauge Vere’s needle.[88] In this study, the Vere’s needle was introduced using a 3.2 mm arthroscope under local anesthesia.[87] Patients were followed up for 6 months Both procedures provided significant short-term pain relief of at least 4 weeks duration in >80% of patients, and TI displayed a significantly greater duration of benefit than IA corticosteroid therapy (statistical analyses not available) Patients with effusions and milder radiographic change obtained the best response to treatment Both treatments were well tolerated with few adverse effects The benefits of corticosteroid injection were seen most greatly in patients with milder radiographic OA, and in those with a clinically detectable effusion.[87]
3.2 Analgesics/Anti-Inflammatory Drugs
The efficacy and safety of an IA cocktail analgesic injection was assessed in a randomized, double-blind, placebo-controlled, parallel-group study in 80 patients with OA undergoing unilateral total knee arthroplasty (TKA) Patients in this study received an IA intraoperative injection containing 5 mg morphine, 30 mg bupivacaine (mg/;1.5 mL), and 1 mL betamethasone (mixed with sterile normal saline solution to make up a combined volume of 60 mL) or normal saline as control In this study, the IA cocktail analgesic injection significantly (p < 0.05) reduced the morphine consumption during the 0–36 hours postoperative period and the total morphine consumption Visual analog scale (VAS) was significantly (p < 0.05) lower in the trial group than in the control group at rest (6, 10, 24, and 36 hours postoperatively) and during exercise (24 and 36 hours postoperatively) In addition, active treatment was better than placebo with regard to other study endpoints, including time to perform an active straight leg raise, time to actively reach 90° knee flexion, and the range of motion of the knee on postoperative day 15 The occurrence of nausea and vomiting in the trial group was lower than in the control group Overall, this study demonstrated that use of an IA injection containing a cocktail of analgesics following TKA reduces the need for morphine, and offers better pain control than placebo without any apparent risks.[89]
Another randomized, double-blind study (n = 39) assessed the efficacy of IA morphine (1 mg [1 mL] of morphine diluted in 9 mL of saline [group 1]) and that of bupivacaine (25 mg [10 mL] of 0.25% bupivacaine without epinephrine [group 2]) on the joint flexion and extension angles (among other endpoints) of patients with knee OA Mean angle of flexion or extension of the knee significantly increased from baseline to endpoint in both treatment groups (p ≤ 0.05) Furthermore, the analgesic effects of both drugs were similar, with both significantly reducing pain at rest and during movement (p ≤ 0.05).[90] However, no significant between-group differences were observed for any endpoint.
Thirty patients with an acute effusion of the knee joint that was related to grade II–III OA (according to the Kellgren-Lawrence system) were randomly assigned to receive either an IA injection of tenoxicam 20 mg following aspiration or oral tenoxicam 20 mg/day for 10 days (n = 15 in each group) The study showed that IA injection of tenoxicam provided rapid pain relief in patients with an acute flare-up of knee OA It also showed that IA tenoxicam helped to prevent effusion, with the number of knee effusions being significantly (p < 0.01) lower in the IA treatment group than in the oral treatment group 1 year after treatment.[91]
3.3 Polymerized Collagen
The efficacy of polymerized type I collagen (polymerized collagen; a compound that has anti-inflammatory and tissue regeneration properties) was investigated in a study involving 53 patients with knee OA Patients in this trial were treated with 12 IA injections of 2 mL of polymerized collagen (n = 27) or 2 mL of placebo (n = 26) Polymerized collagen was safe and well tolerated, and patients in the polymerized collagen group had statistically (p < 0.05) greater improvements in primary outcomes (i.e Lequesne index, WOMAC OA index, and VAS) from baseline to follow-up (at 3–6 months, depending on endpoint) than those in the placebo group.[92]
3.4 Stem-Cell Therapy
The development of techniques that cause multipotent adult mesenchymal stem cells (MSCs) to differentiate into cells of the chondrogenic lineage have led to new insights in the attempt to restore the damaged cartilage in OA patients It has been proposed that MSCs may be used as progenitor cells to engineer cartilage implants that can be used to repair chondral and osteochondral lesions, and as trophic producers of bioactive factors to initiate endogenous regenerative activities in the OA joint.[93] Nöth et al.[83] proposed that MSCs may be delivered to the point of action by direct IA injection or by graft of engineered constructs derived from cell-seeded scaffolds.
3.5 Anti-Cytokine Drugs
Anakinra, a recombinant IL-1 receptor antagonist, at a dose of 50 or 150 mg was well tolerated as a single IA injection in a multicenter, double-blind, placebo-controlled study involving patients with OA of the knee However, over a 12-week follow-up period, the drug was not associated with improvements in OA symptoms compared with placebo.[94]
Orthokin®, a product that is produced by incubating whole human venous blood with CrSO4-treated glass beads, has previously demonstrated the ability to increase IL-1 receptor antagonist production in vitro.[95] In a randomized, multicenter, double-blind, placebo-controlled trial involving 167 patients with symptomatic knee OA, patients received six IA injections of Orthokin® or physiologic saline Study results showed significantly greater improvements with Orthokin® than with placebo in the Knee injury and Osteoarthritis Outcome Score (KOOS)-assessed symptom (p = 0.002) and sport parameters (p = 0.042) Furthermore, the biologic response induced by Orthokin® in this trial was different to that induced by placebo.[96]
In a pilot study involving ten women (12 months’ follow-up), the effect of IA infliximab on erosive OA of the hands was evaluated Patients with bilateral hand OA received monthly IA injections of infliximab (a monoclonal antibody against TNF-α) in the most involved hand, and of physiologic saline in the other hand (control) VAS for spontaneous pain at baseline was significantly (p < 0.01) higher for the hand treated with IA infliximab than for the control hand IA infliximab was associated with reductions in VAS for spontaneous pain at 6 months, with the difference from baseline becoming significant (p < 0.002) at 12 months Similar results were obtained in the evaluation of pain on lateral pressure Furthermore, on radiologic evaluation, there was a difference between infliximab- and saline-treated joints in the progression of the anatomical lesion progression score that was in favor of infliximab, and a tendency towards stability or slight bone remodeling in the infliximab group compared with a tendency towards a worsening in the saline group The treatment showed no side effects or local adverse reactions, and the hematochemical parameters showed no significant modifications and remained within the normal range.[97]
3.6 Neurotransmitter Antagonists
Recently, the efficacy and safety of botulinum toxin type A (BoNT-A) injected IA have been evaluated in 60 patients with moderate pain and functional impairment secondary to knee OA In this double-blind, randomized, single tertiary care academic medical center trial with 6-month follow-up, patients were randomized to receive a single injection of corticosteroid, low-dose BoNT-A (100 units), or high-dose BoNT-A (200 units) It was observed that VAS score decreased within each group, reaching statistical significance (p = 0.01) only in the low-dose BoNT-A group at 8 weeks In addition, all groups showed statistically significant improvements in WOMAC OA index scores (pain, stiffness, function) at 8 weeks (p-value not available), and no serious adverse events were noted in any group.[98]
3.7 Bisphosphonates
In a phase II, randomized, partially-blind clinical trial, 150 patients (aged 50–75 years) with primary knee OA were randomized to one of the following five IA therapies: (i) clodronate 0.5 mg, one IA injection/;week for 4 weeks; (ii) clodronate 1 mg, one IA injection/;week for 4 weeks; (iii) clodronate 2 mg, one IA injection/;week for 4 weeks; (iv) clodronate 1 mg, two IA injections/;week for 2 weeks (clodronate 1 + 1 mg); or (v) HA 20 mg, one IA injection/;week for 4 weeks In all treatment groups, significant (p < 0.001) improvements in VAS for different types of pain and the Lequesne index were reported after the first injection and continued to improve for up to 2–4 weeks after the last injection; however, no significant between-group differences were noted for the latter results A significant (p = 0.03) linear trend for a dose-response (0.5–2 mg clodronate) relationship was found for active movement VAS pain In addition, both joint extension and mobility scores improved significantly from baseline at all timepoints in all treatment groups, although no between-group differences were seen (p-values ranging from 0.06 to 0.34) These results indicate that both IA clodronate and HA provide symptomatic and functional improvements in knee OA.[99]
4. Discussion
The best treatment for OA is not yet clear, and an early diagnosis still plays a key role in the management of this condition Current OARSI (Osteoarthritis Research Society International) recommendations for the optimal management of patients with hip or knee OA were summarized by Zhang et al.,[100] and include a combination of both non-pharmacologic and pharmacologic modalities of therapy that are based on a critical appraisal of existing guidelines, a systematic review of research evidence, and the consensus opinions of an international, multidisciplinary group of experts These recommendations create evidence-based and consensus guidelines for the treatment of knee and/;or hip OA, providing assistance to physicians and allied healthcare professionals who deal with patients with these conditions in both primary and secondary care settings.
The recommendations are as follows:[100] (i) 11 non-pharmacologic modalities, including education and self-management; regular telephone contact; referral to a physical therapist; aerobic, muscle strengthening, and water-based exercises; weight reduction; walking aids; knee braces; footwear and insoles; thermal modalities; transcutaneous electrical nerve stimulation; and acupuncture; (ii) eight pharmacologic modalities, including acetaminophen (paracetamol); cyclooxygenase-2 (COX-2) non-selective and selective oral NSAIDs; topical NSAIDs and capsaicin; IA injections of corticosteroids, IA injections of hyaluronates; glucosamine and/;or chondroitin sulfate for symptom relief; glucosamine sulfate, chondroitin sulfate, and diacerein for possible structure-modifying effects; and opioid analgesics for the treatment of refractory pain; and (iii) five surgical modalities, including total joint replacements; unicompartmental knee replacement; osteotomy and joint preserving surgical procedures; joint lavage and arthroscopic debridement (in knee OA); and joint fusion as a salvage procedure when joint replacement has failed.
In the past, OA was not considered an inflammatory disorder, but nowadays it is associated with several inflammatory mediators Finding a way to modulate these inflammatory mediators could lead to new insights into the treatment of this pathologic condition (see figure s1 in the Supplemental Digital Content).
Recently, a systematic review examined the influence of the ‘placebo effect’ (defined as the change in various efficacy endpoint parameters from baseline to study end in the placebo group) on the management of OA in 198 randomized, placebo-controlled trials (including a total of 193 placebo groups [n = 16 364] and 14 untreated control groups [n = 1167]) investigating the use of a broad range of OA therapies (non-pharmacologic, pharmacologic, and surgical treatments) The authors pointed out that it is difficult to distinguish this change from natural disease remission and chance regression to the mean According to this review, placebo effectively relieved pain, and improved patient function and stiffness In particular, the review showed that the pain-relieving effect of placebo increases when the active treatment effect, baseline pain level, or sample size increases, and when placebo has been given as an injection.[101]
Although some active compounds are rapidly cleared from the OA joint, meaning that multiple injections are required with the subsequent potential for an increased incidence of adverse effects (e.g infection and joint disability), IA injections offer several benefits for the treatment of OA These benefits include the ability to reach high drug concentrations at the site of delivery, use drugs with low bioavailability, and limit the occurrence of adverse effects related to systemic administration However, although rare, complications of IA injections, such as infection, post-injection flare, crystal-induced synovitis, cutaneous atrophy, and steroid arthropathy may occur The incidence of septic joints related to local corticosteroid injection is about 1 in 10 000 injections, while for post-injection flare, the frequency is around 200 in 10 000 Due to the crystalline nature of corticosteroids, crystals present in the joint produce transient synovitis in about 10% of patients; however, this condition generally disappears after a few days.
Because IA injection is an invasive procedure, it has the disadvantage of being painful and putting the patient at risk of infection.[102] As for any IA joint injection, various adverse effects may occur with IA joint injections, such as injection-related pain, post-injection flare, skin pigment changes, fat atrophy, and joint infection Systemic effects can also occur, including disruption of diabetes and hypertension control, facial flushing, inhibition of the hypothalamo-pituitary-adrenal axis, sepsis, and death.[103–106] Prefilled syringes, rigorous aseptic maneuvers, proper needle choice, and isotonicity (a pH of 7.4 or sometimes less can stabilize the drug without activating inflammatory enzymes) are all techniques that may help to reduce the risk of adverse effects.
There is also evidence to suggest that sonographic needle guidance of IA injections improves performance and clinical outcomes In a randomized controlled study, patients with painful joints (n = 148) received IA injections under sonographic guidance, or by the conventional palpation-guided anatomic technique Relative to the conventional technique, sonographic guidance resulted in a 43.0% reduction in procedural pain (p < 0.001), a 58.5% reduction in absolute pain scores at 2 weeks (p < 0.001), a 75.0% reduction in VAS-assessed significant pain (p < 0.001), a 25.6% increase in the responder rate (defined as a reduction in VAS score from baseline of ≥50%; p < 0.01), and a 62.0% reduction in the nonresponder rate (defined as a reduction in VAS score from baseline of <50%; p < 0.01) The sonographic-guided technique also increased the number of effusions detected by 200% and the volume of aspirated fluid by 337%.[107]
When choosing the optimal drug or drug combination for IA injection, the experience of each doctor, and individual factors (e.g bioavailability and mechanism of action) must be considered In our opinion, the short- and long-term perspective must be taken into account when selecting IA treatment When the short-term approach is required (e.g for the treatment of painful acute conditions), SF aspiration under local anesthetic is mandatory If long-term IA therapy is required, the SF will decrease after the first set of injections, allowing the subsequent ones to be directly performed within the joint.
Among various treatment choices (oral, parenteral, or IA drugs), viscosupplementation with hyaluronans seems to be a promising option for the treatment of OA (sections 2.2.1 and 2.3) This technique aims to replace HA, which is reduced in the OA process, and restore the elasticity and viscosity of the SF back to normal Although a review described earlier underlines the importance of taking into account the ‘placebo effect’ when speaking of IA injections,[101] literature supports the use of HA in the treatment of OA because of its immediate bioavailability and the absence of systemic effects, and its use should be encouraged.
HA plus corticosteroid therapy should form part of the short-term treatment plan for OA in order to take control of the acute inflammatory process as soon as possible Fixing a bandage to the joint and rest should complete the procedure In the follow-up, and depending on results from the SF examination and the local and general symptoms, the doctor should select the best procedure to repair or regenerate the synovial membrane and cartilage, preventing or delaying further relapse episodes At this time, HA alone or in combination with other compounds containing methotrexate, biphosphonates, somatostatin, NSAID, or biotechnologic products, should be selected, with administration frequency and dosages being determined according to the physiopathology of the disease and instrumental imaging of the process at follow-up.
Our efforts are now targeted at improving the drug delivery systems, such as drains, double-chamber syringes, dedicated needles or catheters, and kits for the joint medication, in order to achieve the patient’s best compliance and the doctor’s optimal standardized operative strategies Joint care is a well defined area of the rheumatologic, orthopedic, and physiatric specialties and requires proper tools, medications, and homogenous treatment planning to standardize the evidence-based results.
In summary, IA HA has numerous restorative effects in patients with acute or chronic joint disease In our opinion, to achieve the most effective outcome, an individually tailored approach should be taken for each case For this reason, further investigation into different HA formulations (including crosslinked and non-crosslinked formulations), and their synchronous or metachronous association with other compounds, is of importance.
Acknowledgements
The authors contributed equally to this work This review was not supported by grants The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the review The authors hereby certify that all work contained in this review is the original work of Tommaso Iannitti, Daniele Lodi, and Beniamino Palmieri All the data from other articles, including tables and figures, have been referenced The authors claim full responsibility for the contents of the article.
References
- 1.Attur MG, Dave M, Akamatsu M, et al. Osteoarthritis or osteoarthrosis: the definition of inflammation becomes a semantic issue in the genomic era of molecular medicine. Osteoarthritis Cartilage. 2002;10(1):1–4. doi: 10.1053/joca.2001.0488. [DOI] [PubMed] [Google Scholar]
- 2.Fox BA, Stephens MM. Glucosamine/chondroitin/primorine combination therapy for osteoarthritis. Drugs Today (Barc) 2009;45(1):21–31. doi: 10.1358/dot.2009.45.1.1314053. [DOI] [PubMed] [Google Scholar]
- 3.Felson DT, Couropmitree NN, Chaisson CE, et al. Evidence for a Mendelian gene in a segregation analysis of generalized radiographic osteoarthritis: the Framingham Study. Arthr Rheum. 1998;41:1064–71. doi: 10.1002/1529-0131(199806)41:6<1064::AID-ART13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Andrianakos AA, Kontelis LK, Karamitsos DG, et al. Prevalence of symptomatic knee, hand and hip osteoarthritis in Greece: the ESORDIG study. J Rheumatology. 2006;33:2507–13. [PubMed] [Google Scholar]
- 5.Michael JW, Schlü ter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–62. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cymet TC, Sinkov V. Does long-distance running cause osteoarthritis? J Am Osteopath Assoc. 2006;106(6):342–5. [PubMed] [Google Scholar]
- 7.Okada A, Okada Y. Progress of research in osteoarthritis: metalloproteinases in osteoarthritis. Clin Calcium. 2009;19(11):1593–601. [PubMed] [Google Scholar]
- 8.McGonagle D, Tan AL, Carey J, et al. The anatomical basis for a novel classification of osteoarthritis and allied disorders. J Anat. 2010;216(3):279–91. doi: 10.1111/j.1469-7580.2009.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okazaki K, Iwamoto Y. Progress of research in osteoarthritis: gene expression and its regulatory mechanisms in the degenerative cartilage in osteoarthritis. Clin Calcium. 2009;19(11):1578–85. [PubMed] [Google Scholar]
- 10.Sofat N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int J Exp Pathol. 2009;90(5):463–79. doi: 10.1111/j.1365-2613.2009.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q. Mouse models and new therapeutic targets for OA. J Musculoskelet Neuronal Interact. 2008;8(4):311–2. [PubMed] [Google Scholar]
- 12.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang K, Wu LD. YKL-40: a potential biomarker for osteoarthritis. J Int Med Res. 2009;37(1):18–24. doi: 10.1177/147323000903700102. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda K. Progress of research in osteoarthritis: involvement of reactive oxygen species in the pathogenesis of osteoarthritis. Clin Calcium. 2009;19(11):1602–6. [PubMed] [Google Scholar]
- 15.Chevalier X. Intraarticular treatments for osteoarthritis: newperspectives. Curr Drug Targets. 2010;11(5):546–60. doi: 10.2174/138945010791011866. [DOI] [PubMed] [Google Scholar]
- 16.Meyer K, Palmer J. The polysaccharide of the vitreous humor. J Biol Chem. 1934;107:629–34. [Google Scholar]
- 17.Goa KL, Benfield P. Hyaluronic acid: a review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing. Drugs. 1994;47(3):536–66. doi: 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- 18.Kogan G, Solté s L, Stern R, et al. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29(1):17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 19.Laurent TC. The chemistry, biology and medical applications of hyaluronan and its derivatives. London: Portland Press; 1998. [Google Scholar]
- 20.Arnold W, Fullerton DS, Holder S, et al. Viscosupplementation: managed care issue for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4):S3–19. doi: 10.18553/jmcp.2007.13.s4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12(2):79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 22.Olczyk P, Komosiń ska-Vassev K, Winsz-Szczotka K, et al. Hyaluronan: structure, metabolism, functions, and role in wound healing. Postepy Hig Med Dosw (Online) 2008;62:651–9. [PubMed] [Google Scholar]
- 23.Prehm P. Hyaluronan. In: Vandamme EJ, DeBaets S, Steinbü chel A, editors. Biopolymers: biology, chemistry, biotechnology, applications. Polysaccharides I: polysaccharides from prokaryotes. Vol. 5. Weinheim: Wiley-VCH; 2000. pp. 379–404. [Google Scholar]
- 24.Salter DM, Godolphin JL, Gourlay MS, et al. Analysis of human articular chondrocyte CD44 isoform expression and function in health and disease. J Pathol. 1996;179:396–402. doi: 10.1002/(SICI)1096-9896(199608)179:4<396::AID-PATH606>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Knudson CB, Knudson W. Clin Orthop Relat Res. 2004. Hyaluronan and CD44: modulators of chondrocyte metabolism; pp. S152–62. [PubMed] [Google Scholar]
- 26.Nakamura H, Kenmotsu S, Sakai H, et al. Localization of CD44, the hyaluronate receptor, on the plasma membrane of osteocytes and osteoclasts in rat tibiae. Cell Tissue Res. 1995;280:225–33. doi: 10.1007/BF00307793. [DOI] [PubMed] [Google Scholar]
- 27.Fujii Y, Fujii K, Nakano K, et al. Crosslinking of CD44 on human osteoblastic cells upregulates ICAM-1 and VCAM-1. FEBS Lett. 2003;539:45–50. doi: 10.1016/S0014-5793(03)00182-0. [DOI] [PubMed] [Google Scholar]
- 28.Yamazaki M, Nakajima F, Ogasawara A, et al. Spatial and temporal distribution of CD44 and osteopontin in fracture callus. J Bone Joint Surg Br. 1999;81:508–15. doi: 10.1302/0301-620X.81B3.9398. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Fuentes M, Meinel AJ, Hilbe M, et al. Merkle HP Silk fibroin/hyaluronan scaffolds for human mesenchymal stem cell culture in tissue engineering. Biomaterials. 2009;30(28):5068–76. doi: 10.1016/j.biomaterials.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Toole BP. Hyaluronan in morphogenesis. J Intern Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 31.Pavasant P, Shizari T, Underhill CB. Hyaluronan contributes to the enlargement of hypertrophic lacunae in the growth plate. J Cell Sci. 1996;109(Pt2):327–34. doi: 10.1242/jcs.109.2.327. [DOI] [PubMed] [Google Scholar]
- 32.Fedarko NS, Termine JD, Young MF, et al. Temporal regulation of hyaluronan and proteoglycan metabolism by human bone cells in vitro. J Biol Chem. 1990;265:12200–9. [PubMed] [Google Scholar]
- 33.Fedarko NS, Vetter UK, Weinstein S, et al. Age-related changes in hyaluronan, proteoglycan, collagen, and osteonectin synthesis by human bone cells. J Cell Physiol. 1992;151:215–27. doi: 10.1002/jcp.1041510202. [DOI] [PubMed] [Google Scholar]
- 34.Kogan G, Solté s L, Stern R, et al. Hyaluronic acid: its function and degradation in in vivo systems. In: Attaur- Rahman, et al., editors. Studies in natural products chemistry: bioactive natural products (part N). Vol. 34. Amsterdam: Elsevier; 2008. pp. 789–882. [Google Scholar]
- 35.Yasuda T. Progress of research in osteoarthritis: pharmacological effects of hyaluronan. Clin Calcium. 2009;19(11):1644–52. [PubMed] [Google Scholar]
- 36.Grigolo B, Roseti L, Fiorini M, et al. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001;22:2417–24. doi: 10.1016/S0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 37.Park SH, Park SR, Chung SI, et al. Tissue-engineered cartilage using fibrin/hyaluronan composite gel and its in vivo implantation. Artif Organs. 2005;29:838–45. doi: 10.1111/j.1525-1594.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 38.Nehrer S, Domayer S, Dorotka R, et al. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3–8. doi: 10.1016/j.ejrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Lepidi S, Grego F, Vindigni V, et al. Hyaluronan biodegradable scaffold for small-caliber artery grafting: preliminary results in an animal model. Eur J Vasc Endovasc Surg. 2006;32:411–7. doi: 10.1016/j.ejvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Goldring MB, Goldring SB. Osteoarthritis. J Cell Physiol. 2007;213:626–34. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 41.Bondeson J, Blom AB, Wainwright S, et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62(3):647–57. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 42.Goekoop RJ, Kloppenburg M, Kroon HM, et al. Low innate production of interleukin-1beta and interleukin-6 is associated with the absence of osteoarthritis in old age. Osteoarthritis Cartilage. 2010;18(7):942–7. doi: 10.1016/j.joca.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Hiramitsu T, Yasuda T, Ito H, et al. Intercellular adhesion molecule-1 mediates the inhibitory effects of hyaluronan on interleukin-1beta-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts via downregulation of NF-kappaB and p38. Rheumatology (Oxford) 2006;45(7):824–32. doi: 10.1093/rheumatology/kel026. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu M, Yasuda T, Nakagawa T, et al. Hyaluronan inhibits matrix metalloproteinase-1 production by rheumatoid synovial fibroblasts stimulated by proinflammatory cytokines. J Rheumatol. 2003;30(6):1164–72. [PubMed] [Google Scholar]
- 45.Waddell DD, Kolomytkin OV, Dunn S, et al. Hyaluronan suppresses IL-1beta-induced metalloproteinase activity from synovial tissue. Clin Orthop Relat Res. 2007;465:241–8. doi: 10.1097/BLO.0b013e31815873f9. [DOI] [PubMed] [Google Scholar]
- 46.Volpi N, Schiller J, Stern R, et al. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009;16(14):1718–45. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- 47.Solté s L, Mendichi R. Molecular characterization of two host-guest associating hyaluronan derivatives. Biomed Chromatogr. 2003;17:376–84. doi: 10.1002/bmc.252. [DOI] [PubMed] [Google Scholar]
- 48.Marshall KW. Intra-articular hyaluronan therapy. Curr Opin Rheumatol. 2000;12:468–74. doi: 10.1097/00002281-200009000-00022. [DOI] [PubMed] [Google Scholar]
- 49.Notoya K, Jovanovic DV, Reboul P, et al. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. J Immunol. 2000;165:3402–10. doi: 10.4049/jimmunol.165.6.3402. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita I, Gomis A, Pawlak M, et al. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004;50:314–26. doi: 10.1002/art.11421. [DOI] [PubMed] [Google Scholar]
- 51.Kreger ST, Voytik-Harbin SL. Hyaluronan concentration within a 3D collagen matrix modulates matrix viscoelasticity, but not fibroblast response. Matrix Biol. 2009;28(6):336–46. doi: 10.1016/j.matbio.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10–37. doi: 10.1053/sarh.2002.33720. [DOI] [PubMed] [Google Scholar]
- 53.Pelletier JP, Martel-Pelletier J. The pathophysiology of osteoarthritis and the implication of the use of hyaluronan and hylan as therapeutic agents in viscosupplementation. J Rheumatol Suppl. 1993;39:19–24. [PubMed] [Google Scholar]
- 54.Schiavinato A, Lini E, Guidolin D, et al. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. II: Morphological findings. Clin Orthop Relat Res. 1989;241:286–99. [PubMed] [Google Scholar]
- 55.Grishko V, Xu M, Ho R, et al. Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J Biol Chem. 2009;284(14):9132–9. doi: 10.1074/jbc.M804178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi K, Hashimoto S, Kubo T, et al. Effect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritis. J Rheumatol. 2000;27(7):1713–20. [PubMed] [Google Scholar]
- 57.Peng H, Zhou JL, Liu SQ, et al. Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res. 2010;59:519–30. doi: 10.1007/s00011-010-0156-x. [DOI] [PubMed] [Google Scholar]
- 58.Lee YT, Shao HJ, Wang JH, et al. Hyaluronic acid modulates gene expression of connective tissue growth factor (CTGF), transforming growth factor-beta1 (TGF-beta1), and vascular endothelial growth factor (VEGF) in human fibroblast-like synovial cells from advanced-stage osteoarthritis in vitro. J Orthop Res. 2010;28(4):492–6. doi: 10.1002/jor.21029. [DOI] [PubMed] [Google Scholar]
- 59.Zhou JL, Liu SQ, Qiu B, et al. Effects of hyaluronan on vascular endothelial growth factor and receptor-2 expression in a rabbit osteoarthritis model. J Orthop Sci. 2009;14(3):313–9. doi: 10.1007/s00776-009-1329-8. [DOI] [PubMed] [Google Scholar]
- 60.Davidson ENB, Vitters EL, Mooren FM, et al. Connective tissue growth factor/ccn2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 2006;54:1653–61. doi: 10.1002/art.21795. [DOI] [PubMed] [Google Scholar]
- 61.Murata M, Yudoh K, Masuko K. The potential role of vascular endothelial growth factor (vegf) in cartilage: how the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthritis Cartilage. 2008;16:279–86. doi: 10.1016/j.joca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka E, Aoyama J, Miyauchi M, et al. Vascular endothelial growth factor plays an important autocrine/paracrine role in the progression of osteoarthritis. Histochem Cell Biol. 2005;123:275–81. doi: 10.1007/s00418-005-0773-6. [DOI] [PubMed] [Google Scholar]
- 63.Enomoto H, Inoki I, Komiya K, et al. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am J Pathol. 2003;162:171–81. doi: 10.1016/S0002-9440(10)63808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9. [PubMed] [Google Scholar]
- 65.Kelly MA, Moskowitz RW, Lieberman JR. Hyaluronan therapy: looking toward the future. Am J Orthop. 2004;33(2Suppl.):23–8. [PubMed] [Google Scholar]
- 66.Vitanzo PC, Sennett BJ. Hyaluronans: is clinical effectiveness dependent on molecular weight? Am J Orthop. 2006;35:421–8. [PubMed] [Google Scholar]
- 67.Wen DY. Intra-articular hyaluronic acid injections for knee osteoarthritis. Am Fam Physician. 2000;62:565–70. [PubMed] [Google Scholar]
- 68.Fraser JR, Kimpton WG, Pierscionek BK, et al. The kinetics of hyaluronan in normal and acutely inflamed synovial joints: observations with experimental arthritis in sheep. Semin Arthritis Rheum. 1993;22(Suppl.1):9–17. doi: 10.1016/S0049-0172(10)80015-0. [DOI] [PubMed] [Google Scholar]
- 69.Gotoh S, Onaya J, Abe M, et al. Effects of the molecular weight of hyaluronic acid and its action mechanism on experimental joint pain in rats. Ann Rheum Dis. 1993;52(11):817–22. doi: 10.1136/ard.52.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maneiro E, de Andres MC, Ferná ndez-Sueiro JL, et al. The biologicalaction of hyaluronan on human osteoarthritic articular chondrocytes: the importance of molecular weight. Clin Exp Rheumatol. 2004;22(3):307–12. [PubMed] [Google Scholar]
- 71.Balazs EA. Hyaluronic acid and matrix implantation. Arlington (MA): Biotrix Inc.; 1971. [Google Scholar]
- 72.Balazs EA, Denlinger JL. Sodium hyaluronate acid and joint function. J Equine Vet Sci. 1985;5:217–8. doi: 10.1016/S0737-0806(85)80102-7. [DOI] [Google Scholar]
- 73.Weiss C, Balazs EA, St Onge R, et al. Clinical studies of the intra-articular injections of Healon (sodium hyaluronate) in the treatment of osteoarthritis of human knees. Semin Arthritis Rheum. 1981;11:143–4. doi: 10.1016/0049-0172(81)90081-0. [DOI] [Google Scholar]
- 74.Grogan KA, Chang TJ, Salk RS. Update on viscosupplementation in the treatment of osteoarthritis of the foot and ankle. Clin Podiatr Med Surg. 2009;26(2):199–204. doi: 10.1016/j.cpm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Lee PB, Kim YC, Lim YJ, et al. Comparison between high and low molecular weight hyaluronates in knee osteoarthritis patients: open-label, randomized, multicentre clinical trial. J Int Med Res. 2006;34(1):77–87. doi: 10.1177/147323000603400110. [DOI] [PubMed] [Google Scholar]
- 76.Bongkotphet K, Tassanawipas W, Krittiyanunt S, et al. Comparative efficacy of low- and high-molecular weight intra-articular hyaluronic acids in patients with knee osteoarthritis. J Health Res. 2009;23(2):87–92. [Google Scholar]
- 77.Brzusek D, Petron D. Treating knee osteoarthritis with intra-articular hyaluronans. Curr Med Res Opin. 2008;24(12):3307–22. doi: 10.1185/03007990802490124. [DOI] [PubMed] [Google Scholar]
- 78.Brander VA, Stadler TS. Functional improvement with hylan G-F 20 in patients with knee osteoarthritis. Phys Sportsmed. 2009;37(3):38–48. doi: 10.3810/psm.2009.10.1728. [DOI] [PubMed] [Google Scholar]
- 79.Conrozier T, Chevalier X. Long-term experience with hylan GF-20 in the treatment of knee osteoarthritis. Expert Opin Pharmacother. 2008;9(10):1797–804. doi: 10.1517/14656566.9.10.1797. [DOI] [PubMed] [Google Scholar]
- 80.Bannuru RR, Natov NS, Obadan IE, et al. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–11. doi: 10.1002/art.24925. [DOI] [PubMed] [Google Scholar]
- 81.Dagenais S. Intra-articular hyaluronic acid (viscosupplementation) for hip osteoarthritis. Issues Emerg Health Technol. 2007;98:1–4. [PubMed] [Google Scholar]
- 82.Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:CD005328. doi: 10.1002/14651858.CD005328.pub2. [DOI] [PubMed] [Google Scholar]
- 83.Nö th U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4(7):371–80. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- 84.Hepper CT, Halvorson JJ, Duncan ST, et al. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. J Am Acad Orthop Surg. 2009;17(10):638–46. doi: 10.5435/00124635-200910000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Kruse DW. Intraarticular cortisone injection for osteoarthritis of the hip. Is it effective? Is it safe? Curr Rev Musculoskelet Med. 2008;1(3–4):227–33. doi: 10.1007/s12178-008-9029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koyonos L, Yanke AB, McNickle AG, et al. A randomized, prospective, double-blind study to investigate the effectiveness of adding DepoMedrol to a local anesthetic injection in postmeniscectomy patients with osteoarthritis of the knee. Am J Sports Med. 2009;37(6):1077–82. doi: 10.1177/0363546508331204. [DOI] [PubMed] [Google Scholar]
- 87.Arden NK, Reading IC, Jordan KM, et al. A randomised controlled trial of tidal irrigation vs corticosteroid injection in knee osteoarthritis: the KIVIS Study. Osteoarthritis Cartilage. 2008;16(6):733–9. doi: 10.1016/j.joca.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 88.Ike RW. Tidal irrigation in septic arthritis of the knee: a potential alternative to surgical drainage. J Rheumatol. 1993;20(12):2104–11. [PubMed] [Google Scholar]
- 89.Fu P, Wu Y, Wu H, et al. Efficacy of intra-articular cocktail analgesic injection in total knee arthroplasty: a randomized controlled trial. Knee. 2009;16(4):280–4. doi: 10.1016/j.knee.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 90.Gazi MB, Sakata RK, Issy AM. Intra-articular morphine versus bupivacaine for knee motion among patients with osteoarthritis: randomized double-blind clinical trial. Sao Paulo Med J. 2008;126(6):309–13. doi: 10.1590/S1516-31802008000600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oztuna V, Eskandari M, Bugdayci R, et al. Intra-articular injection of tenoxicam in osteoarthritic knee joints with effusion. Orthopedics. 2007;30(12):1039–42. doi: 10.3928/01477447-20071201-07. [DOI] [PubMed] [Google Scholar]
- 92.Furuzawa-Carballeda J, Muñ oz-Chablé OA, Mací as- Herná ndez SI, et al. Effect of polymerized-type I collagen in knee osteoarthritis: II. In vivo study. Eur J Clin Invest. 2009;39(7):598–606. doi: 10.1111/j.1365-2362.2009.02144.x. [DOI] [PubMed] [Google Scholar]
- 93.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26(6):1387–94. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61(3):344–52. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 95.Meijer H, Reinecke J, Tholen G, et al. The production of anti-inflammatory cytokines in whole blood by physicochemical induction. Inflamm Res. 2003;52(10):404–7. doi: 10.1007/s00011-003-1197-1. [DOI] [PubMed] [Google Scholar]
- 96.Yang KG, Raijmakers NJ, van Arkel ER, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage. 2008;16(4):498–505. doi: 10.1016/j.joca.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 97.Fioravanti A, Fabbroni M, Cerase A, et al. Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: a pilot study. Rheumatol Int. 2009;29(8):961–5. doi: 10.1007/s00296-009-0872-0. [DOI] [PubMed] [Google Scholar]
- 98.Boon AJ, Smith J, Dahm DL, et al. Efficacy of intraarticular botulinum toxin type A in painful knee osteoarthritis: a pilot study. PM R. 2010;2(4):268–76. doi: 10.1016/j.pmrj.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 99.Rossini M, Viapiana O, Ramonda R, et al. Intra-articular clodronate for the treatment of knee osteoarthritis: dose ranging study vs hyaluronic acid. Rheumatology (Oxford) 2009;48(7):773–8. doi: 10.1093/rheumatology/kep084. [DOI] [PubMed] [Google Scholar]
- 100.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 101.Zhang W, Robertson J, Jones AC, et al. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67(12):1716–23. doi: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 102.Needle pathology [Editorial]. C.M.A. Journal 1972; 106: 957 [PMC free article] [PubMed]
- 103.Ayral X. Injections in the treatment of osteoarthritis. Best Pract Res Clin Rheumatol. 2001;15:609–26. doi: 10.1053/berh.2001.0177. [DOI] [PubMed] [Google Scholar]
- 104.Rozental TD, Sculco TP. Intra-articular corticosteroids: an updated overview. Am J Orthop. 2000;29:18–23. [PubMed] [Google Scholar]
- 105.Cole BJ, Schumacher HR. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13:37–46. doi: 10.5435/00124635-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 106.Duclos M, Guinot M, Colsy M, et al. High risk of adrenal insufficiency after a single articular steroid injection in athletes. Med Sci Sports Exerc. 2007;39(7):1036–43. doi: 10.1249/mss.0b013e31805468d6. [DOI] [PubMed] [Google Scholar]
- 107.Sibbitt WL, Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36(9):1892–902. doi: 10.3899/jrheum.090013. [DOI] [PubMed] [Google Scholar]


