Abstract
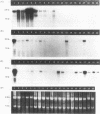
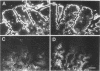
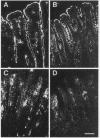
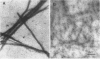
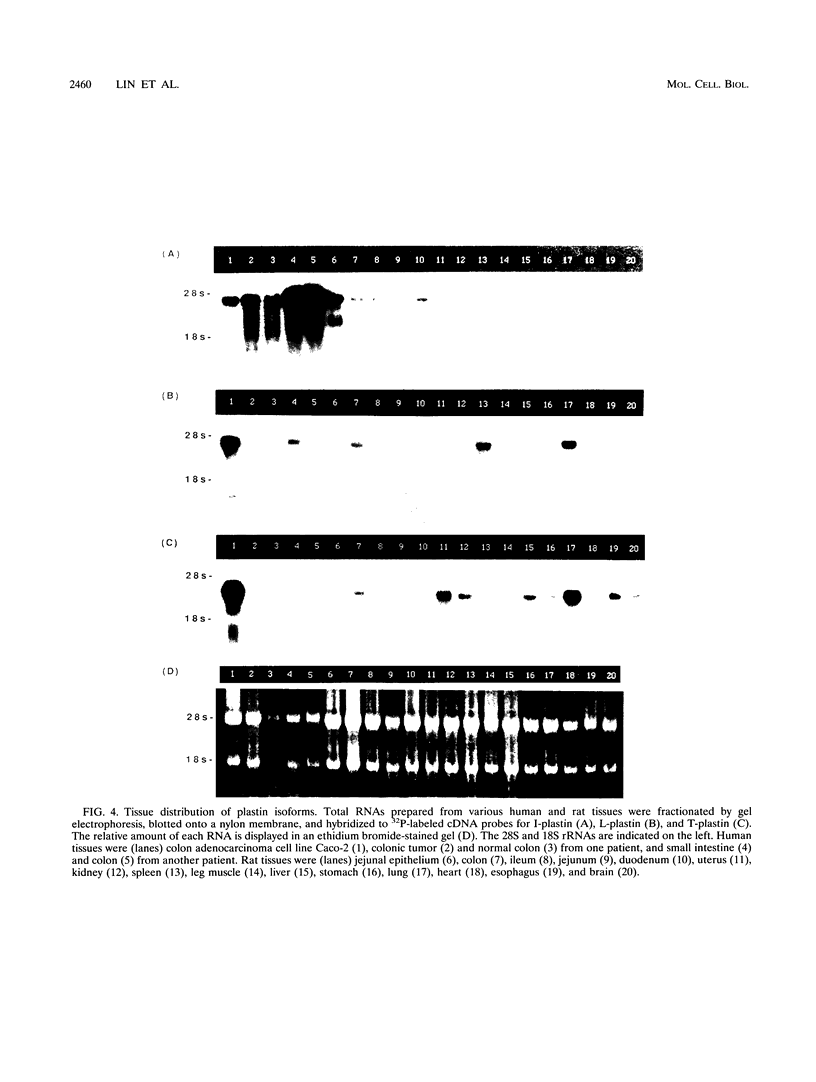
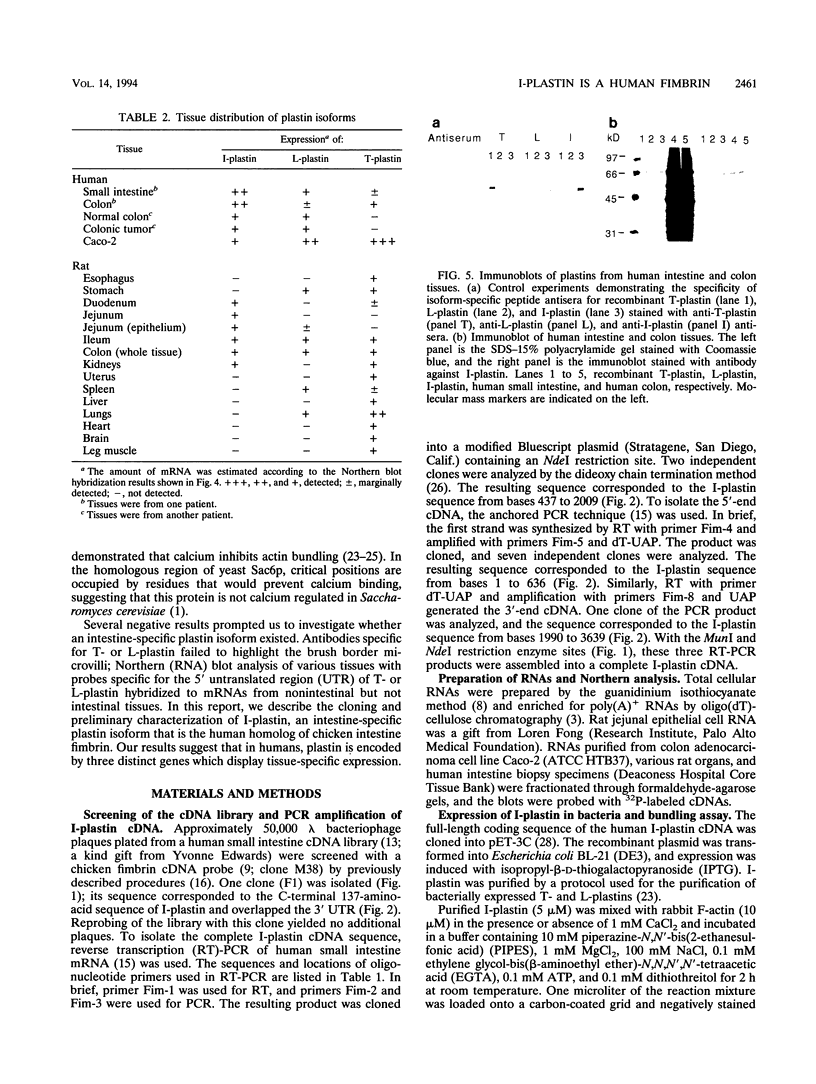
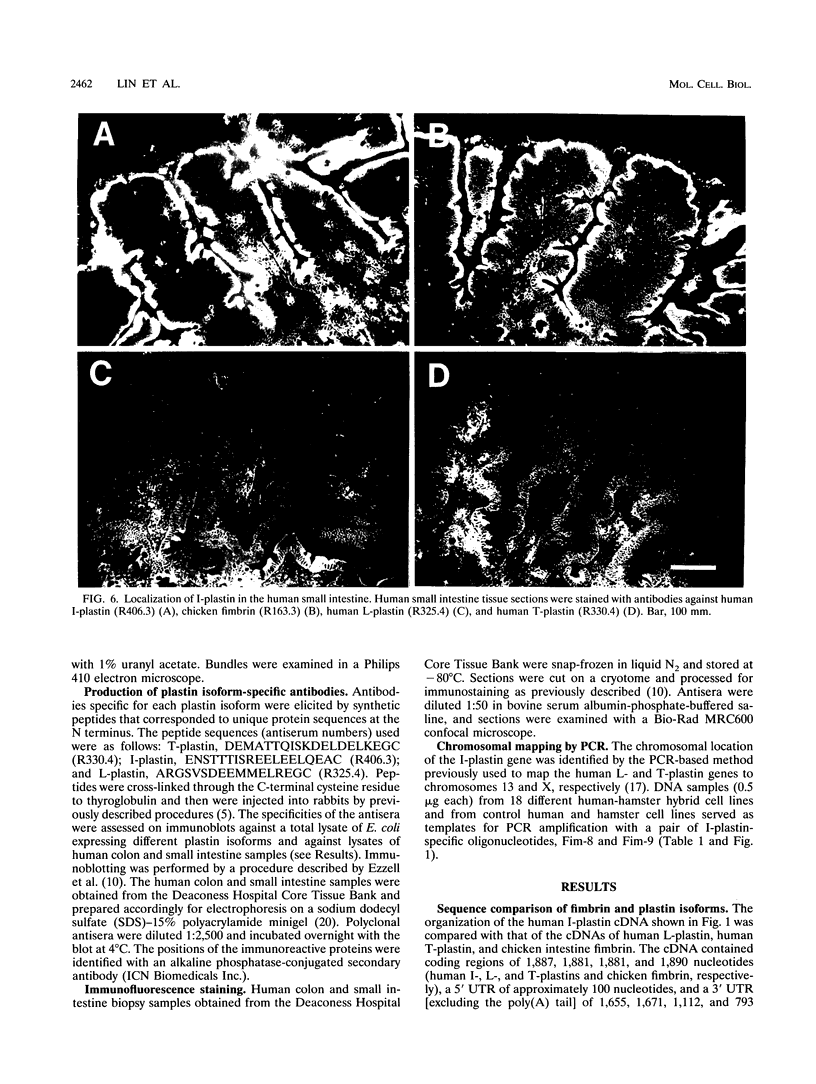
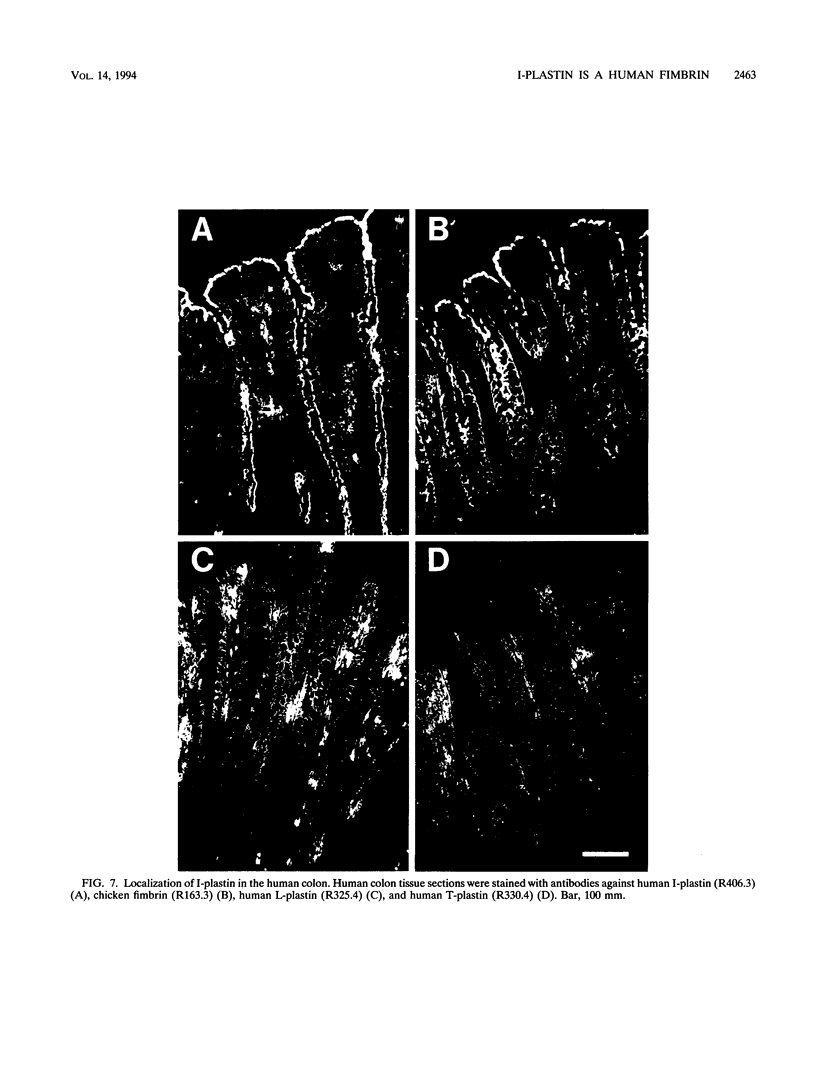
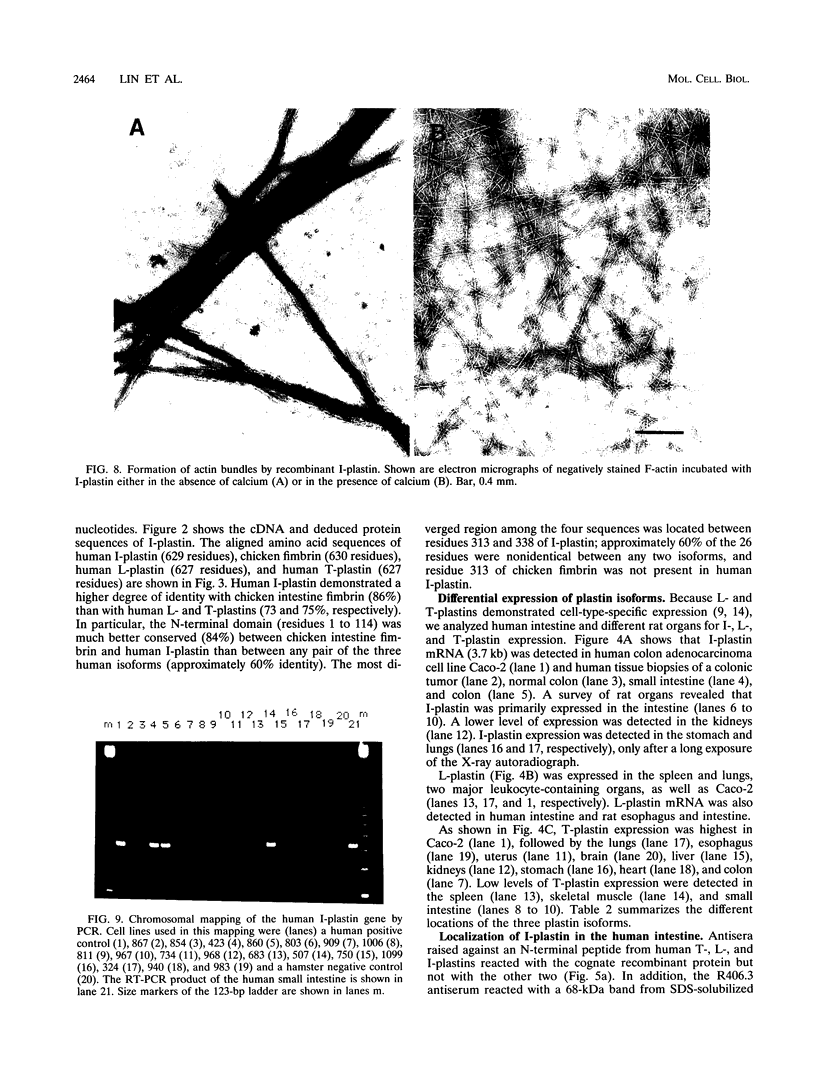
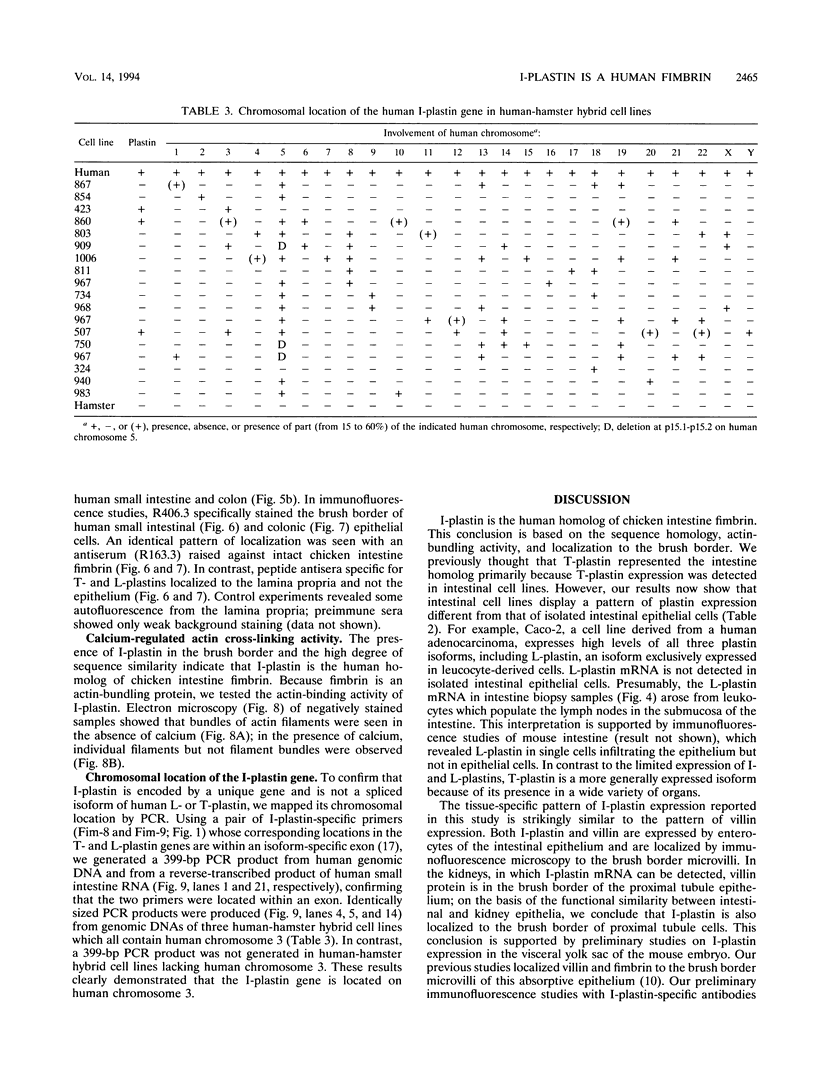
The complete cDNA sequence of human intestine-specific plastin (I-plastin) was determined from a clone derived by PCR. It consists of a 97-bp 5' untranslated region, a 1,887-bp coding region, and a 1,655-bp 3' untranslated region. The coding region predicts a 629-residue polypeptide whose sequence displays 86, 75, and 73% identities with chicken intestine fimbrin, human T-plastin, and human L-plastin, respectively. Recombinant I-plastin cross-linked actin filaments into bundles in the absence but not in the presence of calcium. The I-plastin gene was mapped by PCR to human chromosome 3; the L- and T-plastin genes were previously mapped to chromosomes 13 and X, respectively. I-plastin mRNA was detected in the small intestine, colon, and kidneys; relatively lower levels of expression were detected in the lungs and stomach. In contrast, L-plastin expression was restricted to the spleen and other lymph node-containing organs, while T-plastin was expressed in a variety of organs, including muscle, brain, uterus, and esophagus. In contrast to the situation for the intestine, high levels of L- and T-plastin mRNAs were detected in Caco-2, a human colon-derived cell line. Immunofluorescence microscopy detected I-plastin in the brush border of the small intestine and colon. These results identify I-plastin as the human homolog of chicken intestine fimbrin and as a third plastin isoform in humans.
Full text
PDF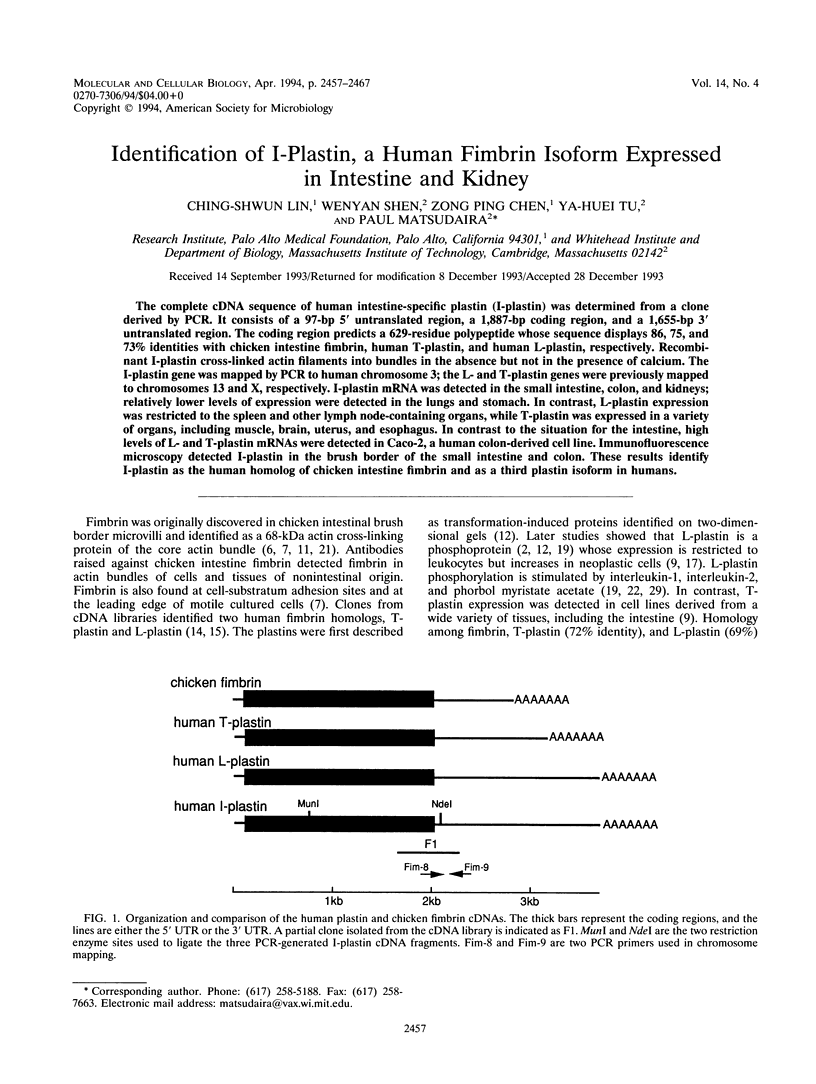




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Botstein D., Drubin D. G. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature. 1991 Dec 5;354(6352):404–408. doi: 10.1038/354404a0. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Gemmell M. A., Coussens P. M., Murao S., Huberman E. Specific protein phosphorylation in human promyelocytic HL-60 leukemia cells susceptible or resistant to induction of cell differentiation by phorbol-12-myristate-13-acetate. Cancer Res. 1985 Oct;45(10):4955–4962. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu A., Sonnenblick E., Gulati J. Molecular basis for the influence of muscle length on myocardial performance. Science. 1988 Apr 1;240(4848):74–76. doi: 10.1126/science.3353709. [DOI] [PubMed] [Google Scholar]
- Bazari W. L., Matsudaira P., Wallek M., Smeal T., Jakes R., Ahmed Y. Villin sequence and peptide map identify six homologous domains. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4986–4990. doi: 10.1073/pnas.85.14.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Fimbrin is a cytoskeletal protein that crosslinks F-actin in vitro. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6849–6853. doi: 10.1073/pnas.78.11.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J Cell Biol. 1980 Jul;86(1):335–340. doi: 10.1083/jcb.86.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Ezzell R. M., Chafel M. M., Matsudaira P. T. Differential localization of villin and fimbrin during development of the mouse visceral endoderm and intestinal epithelium. Development. 1989 Jun;106(2):407–419. doi: 10.1242/dev.106.2.407. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Kaulfus P., Matsudaira P., Weber K. F-actin binding and bundling properties of fimbrin, a major cytoskeletal protein of microvillus core filaments. J Biol Chem. 1981 Sep 10;256(17):9283–9288. [PubMed] [Google Scholar]
- Goldstein D., Djeu J., Latter G., Burbeck S., Leavitt J. Abundant synthesis of the transformation-induced protein of neoplastic human fibroblasts, plastin, in normal lymphocytes. Cancer Res. 1985 Nov;45(11 Pt 2):5643–5647. [PubMed] [Google Scholar]
- Green F., Edwards Y., Hauri H. P., Povey S., Ho M. W., Pinto M., Swallow D. Isolation of a cDNA probe for a human jejunal brush-border hydrolase, sucrase-isomaltase, and assignment of the gene locus to chromosome 3. Gene. 1987;57(1):101–110. doi: 10.1016/0378-1119(87)90181-8. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Aebersold R. H., Kent S. B., Varma M., Leavitt J. Molecular cloning and characterization of plastin, a human leukocyte protein expressed in transformed human fibroblasts. Mol Cell Biol. 1988 Nov;8(11):4659–4668. doi: 10.1128/mcb.8.11.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Aebersold R. H., Leavitt J. Correction of the N-terminal sequences of the human plastin isoforms by using anchored polymerase chain reaction: identification of a potential calcium-binding domain. Mol Cell Biol. 1990 Apr;10(4):1818–1821. doi: 10.1128/mcb.10.4.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Leavitt J. Cloning and characterization of a cDNA encoding transformation-sensitive tropomyosin isoform 3 from tumorigenic human fibroblasts. Mol Cell Biol. 1988 Jan;8(1):160–168. doi: 10.1128/mcb.8.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Park T., Chen Z. P., Leavitt J. Human plastin genes. Comparative gene structure, chromosome location, and differential expression in normal and neoplastic cells. J Biol Chem. 1993 Feb 5;268(4):2781–2792. [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp Cell Res. 1987 Mar;169(1):202–214. doi: 10.1016/0014-4827(87)90238-2. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. Identification and organization of the components in the isolated microvillus cytoskeleton. J Cell Biol. 1979 Dec;83(3):667–673. doi: 10.1083/jcb.83.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Shiroo M., Kung H. F., Copeland T. D. Purification and characterization of a cytosolic 65-kilodalton phosphoprotein in human leukocytes whose phosphorylation is augmented by stimulation with interleukin 1. Biochemistry. 1988 May 17;27(10):3765–3770. doi: 10.1021/bi00410a037. [DOI] [PubMed] [Google Scholar]
- Messier J. M., Shaw L. M., Chafel M., Matsudaira P., Mercurio A. M. Fimbrin localized to an insoluble cytoskeletal fraction is constitutively phosphorylated on its headpiece domain in adherent macrophages. Cell Motil Cytoskeleton. 1993;25(3):223–233. doi: 10.1002/cm.970250303. [DOI] [PubMed] [Google Scholar]
- Namba Y., Ito M., Zu Y., Shigesada K., Maruyama K. Human T cell L-plastin bundles actin filaments in a calcium-dependent manner. J Biochem. 1992 Oct;112(4):503–507. doi: 10.1093/oxfordjournals.jbchem.a123929. [DOI] [PubMed] [Google Scholar]
- Pacaud M., Derancourt J. Purification and further characterization of macrophage 70-kDa protein, a calcium-regulated, actin-binding protein identical to L-plastin. Biochemistry. 1993 Apr 6;32(13):3448–3455. doi: 10.1021/bi00064a031. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell N. M., Hartman A. L., Lessard J. L. Unique isoactins in the brush border of rat intestinal epithelial cells. Cell Motil Cytoskeleton. 1988;11(4):318–325. doi: 10.1002/cm.970110409. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Zu Y., Kohno M., Kubota I., Nishida E., Hanaoka M., Namba Y. Characterization of interleukin 2 stimulated 65-kilodalton phosphoprotein in human T cells. Biochemistry. 1990 Jan 30;29(4):1055–1062. doi: 10.1021/bi00456a030. [DOI] [PubMed] [Google Scholar]
- de Arruda M. V., Watson S., Lin C. S., Leavitt J., Matsudaira P. Fimbrin is a homologue of the cytoplasmic phosphoprotein plastin and has domains homologous with calmodulin and actin gelation proteins. J Cell Biol. 1990 Sep;111(3):1069–1079. doi: 10.1083/jcb.111.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]