Abstract
Oral fixed-dose niacin extended release/simvastatin is associatedwith clinically relevant improvements in plasma lipid profiles, including lowering of nonhigh- density lipoprotein cholesterol levels, relative to simvastatinmonotherapy in patients with mixed dyslipidemias who had not responded fully to simvastatin monotherapy, and is generally well tolerated.
1. What is the Rationale for Developing the Fixed-Dose Combination?
HMG-CoA reductase inhibitors (statins) and niacin (nicotinic acid) are well established lipid-modifying agents that are used, along with therapeutic life-style changes, in the primary and secondary prevention of coronary heart disease, carotid artery disease and other atherosclerotic vascular diseases.[1]
In US guidelines,[1] the lowering of low-density lipoprotein cholesterol (LDL-C) is the primary goal of lipid-modifying therapy in patients with atherosclerotic disease and those at risk for atherosclerotic disease due to dyslipidemia. However, in patients with atherogenic dyslipidemia (i.e. those with high triglyceride levels, low high-density lipoprotein cholesterol [HDL-C] levels and small dense LDL particles), LDL-C levels may underestimate the cardiovascular risk.[2] Therefore, the US guidelines[1] recommend lowering both LDL-C and non-HDL-C in patients with hypertriglyceridemia.
Of the available lipid-modifying drugs, statins are the most effective for lowering plasma LDL-C and are considered the cornerstone of treatment for dyslipidemia.[1,2] At pharmacologic doses, niacin displays wide-ranging lipid-modifying activity, reducing levels of all atherogenic lipid and lipoprotein subclasses, including total cholesterol, LDL-C, non-HDL-C, triglycerides, apolipoprotein B, and lipoprotein(a), and also significantly increasing levels of HDL-C and apolipoprotein A.[3,4]
As niacin extended release (ER) and simvastatin have complementary mechanisms of action, the combination of these agents in a fixed-dose formulation (Simcor ®) may lead to the attainment of lipid regulation goals when monotherapy with simvastatin or niacin ER is considered inadequate (table I).[3,4] Furthermore, the combination of two lipid-lowering agents in one formulation may potentially improve patient compliance.
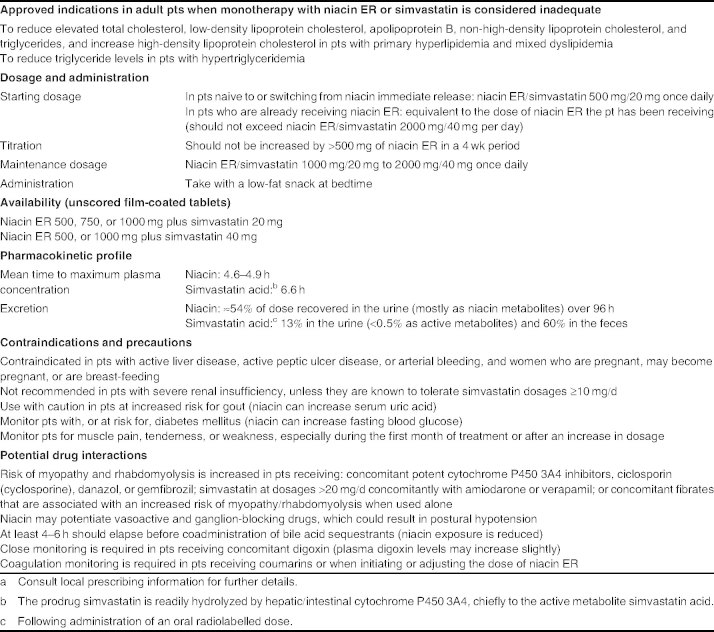
Table I.
Prescribing summary of oral niacin extended release (ER)/simvastatin (Simcor®) in patients (pts) in the US[4] a
2. Who Should Receive the Fixed-DoseCombination?
Niacin ER/simvastatin is approved in the US as an adjunct to diet in the treatment of patients with primary hypercholesterolemia and mixed dyslipidemia, or those with hypertriglyceridemia when monotherapy with simvastatin or niacin ER is considered inadequate for these purposes (table II).[4] Of note, two new dosage strengths of niacin ER/simvastatin containing 40 mg of simvastatin (i.e. niacin ER/simvastatin 500 mg/40 mg and 1000 mg/40 mg) were approved in the US in July 2010.
Table II.
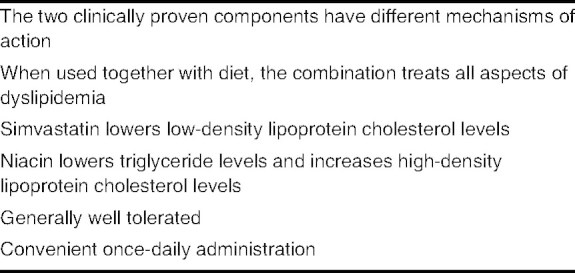
Key clinical benefits of the fixed-dose formulation of niacin extended release plus simvastatin (Simcor®)
3. What is the Efficacy of Niacin ExtendedRelease Plus a Statin on LipidLevels?
Treatment with niacin ER plus a statin administered as separate tablets has been shown to have beneficial effects on lipids in a number of randomized, controlled, double-blind,[5–8] or open-label[3,9–11] clinical trials in patients with dyslipidemia. In a 24-week trial,[3] patients receiving atorvastatin or rosuvastatin plus niacin ER had significantly greater improvements in several lipid parameters compared with those receiving simvastatin plus ezetimibe or rosuvastatin alone. These included significantly greater increases in HDL-C (+18% and +20% vs +8% and +7%) and large HDL (+69% and +85% vs +32% and +29%) levels, and significantly greater decreases in triglyceride (−41% and −33% vs −23% and −19%) and lipoprotein(a) [−7% and −5% vs +8% and +11%] levels (all p ≤ 0.05). In a 12-week trial, once-daily niacin ER (1000 mg for weeks 1-4, then 2000 mg for weeks 5–12) plus simvastatin 40 mg, relative to atorvastatin 40 mg/day, was associated with superior improvements in HDL-C (+30.1% vs +9.4%; p < 0.001), triglyceride (−44.0% vs −37.0%; p = 0.02) and lipoprotein(a) [−15.8% vs +16.0%; p < 0.001] levels and comparable improvements in non-HDL-C (−43.4% vs −43.3%) and LDL-C (−43.8% vs −46.0%) levels.[10] In other trials, the lipid-modifying effects of ER were often additive when used in combination with lovastatin,[5,6] rosuvastatin,[9] or atorvastatin.[11]
3.1 What is its Effect on Atherosclerosis?
Niacin ER in combination with a statin slows or regresses the progression of atherosclerosis.[12–15] In a double-blind trial[12] and its open-label extension[13] in patients with or at risk of coronary heart disease already receiving a statin (predominantly simvastatin), niacin ER plus a statin was associated with significant regression in atherosclerosis as measured by carotid intima-media thickness (CIMT) at 12 months[12] and over 12–24 months.[13] In a further trial in patients with coronary heart disease or equivalent risk,[14] a statin (usually simvastatin or atorvastatin) plus niacin ER produced significant reductions in mean and maximal CIMT relative to both baseline and ezetimibe, whereas ezetimibe was not associated with significant net changes from baseline in CIMT (last-observation-carried-forward analysis in all patients who completed a final CIMT measurement after ≤14 months of treatment). In another trial in patients receiving statin therapy, the addition of niacin ER significantly reduced carotid atherosclerosis (assessed by the change in carotid wall area using MRI), within 1 year relative to the addition of placebo.[15]
Niacin has also been associated with improvements in levels of high-sensitivity C-reactive protein levels relative to placebo, which were correlated with angiographic changes in patients with coronary artery stenosis.[16]
4. Is the Fixed-Dose Combination Effective?
The efficacy of niacin ER/simvastatin as a treatment for type II hyperlipidemia or mixed dyslipidemia was compared with that of simvastatin monotherapy in a 24-week, randomized, double-blind, multicenter, phase III SEACOAST (Safety and Efficacy of a Combination of Niacin-Extended Release and Simvastatin in Patients with Dyslipidemia) study.[7,8] This study consisted of the following two components.
SEACOAST I (low-dose trial).[7] Assessed whether once-daily niacin ER/simvastatin (1000 mg/20 mg or 2000 mg/20 mg) was superior to low-dose simvastatin 20 mg/day in reducing non-HDL-C in patients with elevated non-HDL-C, but with LDL-C at or below US guideline[1] goals after a ≥2-week run-in period/lipid qualification phase with simvastatin 20 mg/day.
SEACOAST II (high-dose trial).[8] Assessed whether once-daily niacin ER/simvastatin (1000 mg/40 mg or 2000 mg/40 mg) was noninferior to high-dose simvastatin 80 mg/day in reducing non-HDL-C in patients with elevated non-HDL-C levels after a ≥2-week run-in period/lipid qualification phase with simvastatin 40 mg/day.[8] Patients were included regardless of whether or not US National Cholesterol Education Adult Treatment Panel III (NCEP ATP III) LDL-C goals had been met during the run-in phase.[8]
In both trials, simvastatin monotherapy recipients also received niacin immediate release 50 mg/day to maintain study blinding.[7,8] Aspirin (acetylsalicylic acid) 325 mg, ibuprofen 200 mg, or another NSAID could be taken ≈30 minutes before the study medication to reduce flushing effects.[7,8] Patients were included in the modified intent-to-treat (mITT) analyses if they had one baseline non-HDL-C evaluation and a plasma lipid evaluation at 24 weeks.
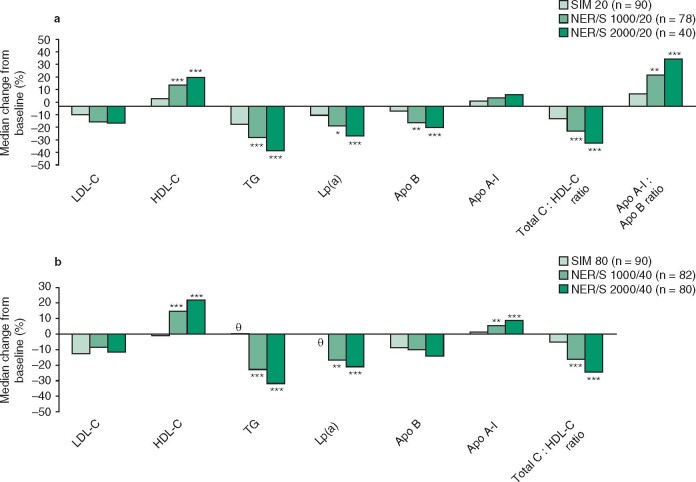
Niacin ER/simvastatin effectively modified lipid levels in patients with mixed dyslipidemias or type II hyperlipidemia.[7,8] In SEACOAST I, patients receiving niacin ER/simvastatin 1000 mg/20 mg or 2000 mg/20 mg per day for 24 weeks experienced ≈2- and ≈3-fold greater median percentage reductions in plasma non-HDL-C levels from statin-treated baseline (primary endpoint) than simvastatin 20 mg/day recipients (figure 1).[7] Both niacin ER/simvastatin dosage groups also experienced significantly greater reductions in plasma triglycerides, lipoprotein(a) and apolipoprotein B, total cholesterol : HDL-C ratio, and significantly greater increases in plasma HDL-C and apolipoprotein A-I : apolipoprotein B ratio than the simvastatin 20 mg/day group (figure 2).[7]
Fig. 1.
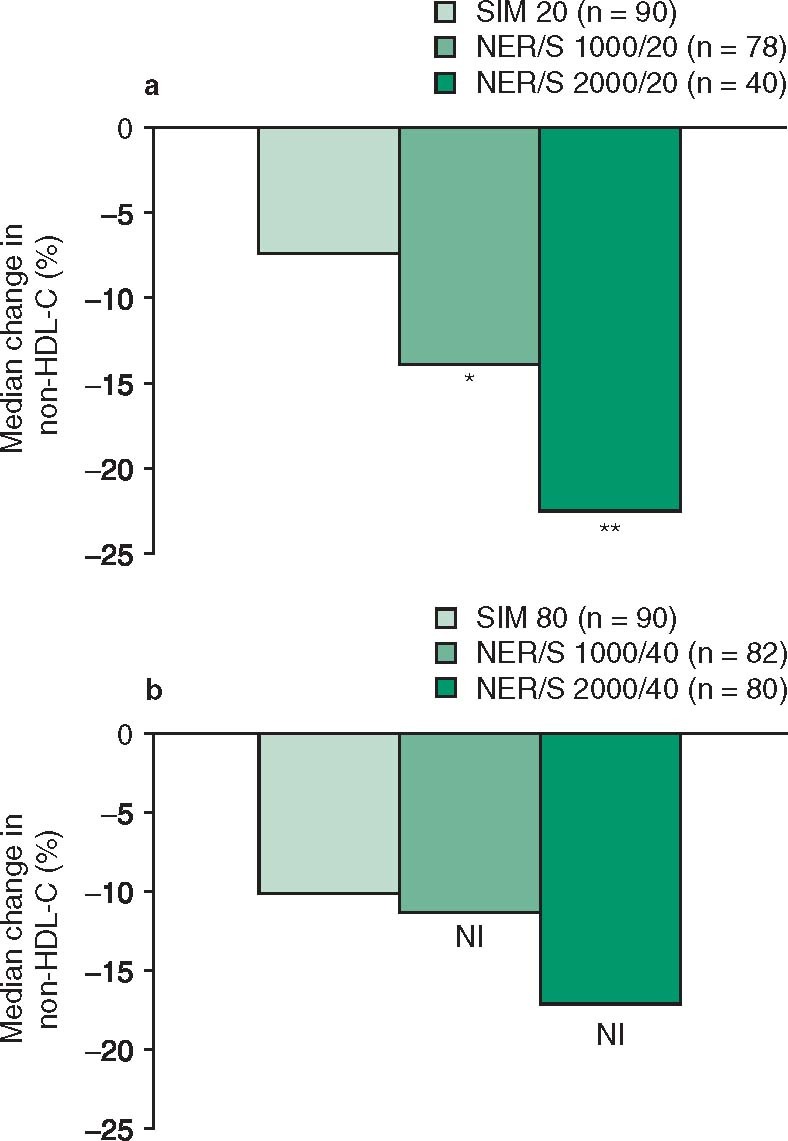
Efficacy of oral niacin extended release/simvastatin (NER/S) in the treatment of adult patients (pts) with dyslipidemia. Median change from statin-treated baseline to wk 24 in plasma non-high-density lipoprotein cholesterol (non-HDL-C) level in the SEACOAST trials of () NER/S 1000 mg/20 mg or 2000 mg/20 mg per day vs simvastatin (SIM) 20 mg/day (SEACOAST I)[7] and (b) NER/S 1000 mg/40 mg or 2000 mg/40 mg per day vs SIM 80 mg/day (SEACOAST II).[8] NI = meets noninferiority criterion. * p < 0.01, ** p < 0.001 vs SIM 20 mg/d.
Fig. 2.
Efficacy of niacin extended release/simvastatin (NER/S) in the treatment of dyslipidemia according to secondary endpoints. Median percentage change from baseline to wk 24 in plasma lipids in the SEACOAST trials comparing () NER/S 1000 mg/20 mg or 2000 mg/20 mg per day vs simvastatin (SIM) 20 mg/day (SEACOAST I)[7] and (b) NER/S 1000 mg/40 mg or 2000 mg/40 mg per day vs SIM 80 mg/day (SEACOAST II).[8] Apo A-I = apolipoprotein A-I; Apo B = apolipoprotein B; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; Lp(a) = lipoprotein(a); TG = triglycerides; Total C = total cholesterol; θ indicates percentage change <1%; * p < 0.05, ** p < 0.01, *** p < 0.001 vs SIM 20 or 80 mg/day.
In SEACOAST II, once-daily niacin ER/simvastatin 1000 mg/40 mg or 2000 mg/40 mg were considered noninferior to high-dose simvastatin 80 mg/day, since the upper limit of the 95% confidence interval for the between-group difference in percentage change from statin-treated baseline in median plasma non-HDL-C (primary endpoint) at 24 weeks was less than the predefined margin of 6% (figure 1).[8] Compared with high-dose simvastatin 80 mg/day, both dosages of niacin ER/simvastatin produced significantly greater decreases in triglycerides, lipoprotein(a) and total cholesterol : HDL-C ratio and significantly greater increases in HDL-C and apolipoprotein A-I (figure 2).[8]
Compared with simvastatin monotherapy, there was no significant difference in reduction in plasma LDL-C levels with niacin ER/simvastatin in either trial (figure 2).[7,8]
In post hoc analyses of the SEACOAST trials, a significantly higher percentage of niacin ER/simvastatin recipients than simvastatin monotherapy recipients reached the trial combined lipid goals (risk-adjusted goals for non-HDL-C or LDL-C, HDL-C ≥40 mg/dL and triglycerides <150 mg/dL).[7,8] In SEACOAST I, lipid goals were achieved in 16%, 25%, and 42% of patients receiving simvastatin monotherapy, niacin ER/simvastatin 1000 mg/20 mg and 2000 mg/20 mg per day, respectively (p < 0.001 for niacin ER/simvastatin 2000 mg/20 mg vs simvastatin monotherapy) [data estimated from a graph].[7] The respective values in SEACOAST II for patients receiving simvastatin 80 mg/day, niacin ER/simvastatin 1000 mg/40 mg, and 2000 mg/40 mg per day were 20%, 30%, and 50% (p < 0.05 and p < 0.001 for niacin ER/simvastatin 1000 mg/20 mg and 2000 mg/20 mg vs simvastatin monotherapy) [data estimated from a graph].[8]
4.1 Is Longer-Term Treatment Effective?
The randomized, 52-week OCEANS (Open-Label Evaluation of the Safety and Efficacy of a Combination of Niacin ER and Simvastatin in Patients with Dyslipidaemia) study[17] randomized patients to 8- or 12-week niacin ER/simvastatin titration schedules (maximum daily dosage 2000 mg/40 mg). Patients were withdrawn from the study if they did not reach LDL-C NCEP ATP III goals at 24 weeks.[17]
Treatment with once-daily niacin ER/simvastatin 2000 mg/40 mg significantly reduced plasma non-HDL-C.[17] In the mITT population (n = 463) at 24 weeks, the median reduction from baseline in non-HDL-C in the niacin ER/simvastatin 8- and 12-week titration groups was 23% and 21% (p < 0.0001).[17]
In the 24-week completer population, a similar reduction in non-HDL-C was observed (27% in both titration groups; both p < 0.0001 vs baseline). In a subgroup analysis in 85 patients in either treatment group who had been lipid-treatment naive prior to enrolment, the median decrease in non-HDL-C was 53.0%.[17] In the 52-week completer population, median percentage reductions in non-HDL-C were 25% and 28% in the 8- and 12-week titration groups.[17]
5. What is its Tolerability Profile?
Fixed-dose niacin ER/simvastatin (500 mg/20 mg to 2000 mg/40 mg per day) for 6–12 months was generally well tolerated in the SEACOAST[7,8] and OCEANS[17] studies. Only minimal differences in tolerability were seen between the 8- and 12-week titration groups in the OCEANS study.[17]
Flushing was the most common adverse reaction experienced by niacin ER/simvastatin recipients.[4] At least one episode of flushing was reported in up to 67% (SEACOAST)[7,8] and 71% (OCEANS)[17] of niacin ER/simvastatin recipients. Flushing was mostly mild to moderate in intensity and tended to occur early in treatment, with the incidence waning over time.[7,8,17] Flushing led to study discontinuation in 6% of 403 patients receiving niacin ER/simvastatin in a controlled study over a period of 6 months.[4]
Other adverse reactions occurring at a frequency ≥3% in overall recipients of once-daily niacin ER/simvastatin (500 mg/20 mg to 2000 mg/40 mg) or simvastatin (20 or 80 mg) in the pooled SEACOAST trial data were headache (4.5% vs 4.6% of patients), pruritus (3.2% vs 0%), nausea (3.2% vs 4.2%), back pain (3.2% vs 2.1%), and diarrhea (3.0% vs 2.9%).[4]
Discontinuations due to adverse events were reported in 11–16% of niacin ER/simvastatin recipients and 4–5% of simvastatin recipients in the SEACOAST trials,[7,8] and 23% of niacin ER/simvastatin recipients during the entire 52-week period of the OCEANS trial.[17]
Serious treatment-related adverse events occurred in <1% of niacin ER/simvastatin or simvastatin monotherapy recipients in the clinical trials.[7,8,17] Simvastatin (in common with other statins) is occasionally associated with myopathy, including rhabdomyolysis. However, no patients in the clinical trials developed myopathy or rhabdomyolysis.[7,8,17]
Niacin can lead to reversible increases in plasma uric acid and increases in hepatic transaminases. In the clinical trials,[7,8,17] there was only one report of a new case of gout[8] and niacin ER/simvastatin was not associated with clinically important changes in hepatic enzymes. When used at recommended doses, clinically significant elevations in hepatic transaminases are uncommon and hepatotoxicity is rare.[18]
Niacin can increase glucose levels and may worsen glucose tolerance in patients with diabetes mellitus.[18] This can usually be managed with alterations in diabetes drug treatment.[18] Fourteen niacin ER/simvastatin recipients developed new-onset diabetes during treatment in the pivotal clinical trials, all of whom had signs of pre-existing glucose intolerance at baseline.[7,8,17]
5.1 How can Flushing be Managed?
Flushing, a common and transient non-allergenic response to niacin, causes patient discomfort and may reduce medication compliance.[4,19] The following methods may be used to ameliorate flushing or the effects of flushing on patient compliance with niacin ER/simvastatin therapy.[4,19]
Inhibiting prostaglandin production. Taking NSAIDs, such as aspirin, 30 minutes prior to administration of niacin can reduce flushing.[4,19,20] In a double-blind, 5-week trial,[20] aspirin 325 mg 30 minutes before niacin ER (titrated from 500 or 1000 mg to 2000 mg once daily) reduced the severity and incidence of flushing (assessed using the validated Flushing ASsessment Tool [FAST©][21] ), but did not affect overall tolerability. At week 1, the proportion of patients with flushing episodes of moderate or greater intensity was 15% in aspirin recipients versus 29% in placebo recipients (p = 0.01; primary endpoint); the between-group difference was also significant (p < 0.001) at weeks 2, 3, and 4, and overall.[20] The aspirin group also had a lower rate of discontinuation due to flushing than the placebo group (1.8% vs 9.4%; p = 0.007).[20] Lower doses of aspirin (e.g. 81 mg) may not be sufficient to inhibit flushing.[22] As flushing improves over time due to tolerance, aspirin treatment may be discontinued after several weeks of niacin ER/simvastatin therapy, which reduces the risk of adverse events associated with the long-term use of aspirin.[20]
Patient education. The willingness of patients to tolerate the effects of transient flushing and comply with therapy may be improved by ensuring that patients understand the long-term benefits of niacin ER/simvastatin on cardiovascular outcomes and are advised on how to manage flushing (e.g. follow the niacin ER/simvastatin titration schedule, take an NSAID prior to niacin ER, and avoid ingestion of alcohol, hot beverages, and spicy foods near the time of taking niacin ER).[4,19]
6. What is its Current Status?
In the US, niacin ER/simvastatin is indicated for the treatment of primary hypercholesterolemia and mixed dyslipidemia or triglyceridemia when treatment with simvastatin or niacin ER monotherapy is considered inadequate.[4] In patients with mixed dyslipidemias who have not responded fully to a simvastatin monotherapy, niacin ER/simvastatin improves plasma lipid profiles, including lowering of non-HDL-C levels, and is generally well tolerated.[7,8]
Niacin ER/simvastatin has not been shown to reduce the incidence of cardiovascular events. However, the ongoing AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) is designed to determine the impact of the addition of niacin ER to simvastatin as secondary prevention of long-term clinical events in patients with vascular disease and atherogenic dyslipidemia, the majority of whom will have metabolic syndrome.[23] Results from this study are expected to be presented in 2012.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was also offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
References
- 1.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP. Combination therapy in the management of mixed dyslipidemia. J Intern Med. 2008;263(4):353–65. doi: 10.1111/j.1365-2796.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 3.McKenney JM, Jones PH, Bays HE, et al. Comparative effects on lipid levels of combination therapy with a statin and extended-release niacin or ezetimibe versus a statin alone (the COMPELL study) Atherosclerosis. 2007;192(2):432–7. doi: 10.1016/j.atherosclerosis.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 4.US prescribing information. North Chicago (IL): Abbott Laboratories; 2010. [Google Scholar]
- 5.Insull W, Jr, McGovern ME, Schrott H, et al. Efficacy of extended-release niacin with lovastatin for hypercholesterolemia: assessing all reasonable doses with innovative surface graph analysis. Arch Intern Med. 2004;164(10):1121–7. doi: 10.1001/archinte.164.10.1121. [DOI] [PubMed] [Google Scholar]
- 6.Hunninghake DB, McGovern ME, Koren M, et al. A doseranging study of a new, once-daily, dual-component drug product containing niacin extended-release and lovastatin. Clin Cardiol. 2003;26:112–8. doi: 10.1002/clc.4960260304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballantyne CM, Davidson MH, McKenney J, et al. Comparison of the safety and efficacy of a combination tablet of niacin extended release and simvastatin vs simvastatin monotherapy in patients with increased non-HDL cholesterol (from the SEACOAST I Study). Am J Cardiol 2008 May 15; 101 (10): 1428–36 [DOI] [PubMed]
- 8.Ballantyne CM, Davidson MH, McKenney JM, et al. Comparison of the efficacy and safety of a combination tablet of niacin extended-release and simvastatin with simvastatin 80mg monotherapy: the SEACOAST II (high-dose) study. J Clin Lipidol. 2008;2(2):79–90. doi: 10.1016/j.jacl.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Capuzzi DM, Morgan JM, Weiss RJ, et al. Beneficial effects of rosuvastatin alone and in combination with extended-release niacin in patients with a combined hyperlipidemia and low high-density lipoprotein cholesterol levels. Am J Cardiol. 2003;91(11):1304–10. doi: 10.1016/S0002-9149(03)00318-7. [DOI] [PubMed] [Google Scholar]
- 10.Insull W, Jr, Basile JN, Vo AN, et al. Efficacy and safety of combination therapy with niacin extended-release and simvastatin versus atorvastatin in patients with dyslipidemia: the SUPREME study. J Clin Lipid. 2009;3(2):109–18. doi: 10.1016/j.jacl.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Sang ZC, Wang F, Zhou Q, et al. Combined use of extendedrelease niacin and atorvastatin: safety and effects on lipid modification. Chin Med J (Engl) 2009;122(14):1615–20. [PubMed] [Google Scholar]
- 12.Taylor AJ, Sullenberger LE, Lee HJ, et al. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110(23):3512–7. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 13.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22(11):2243–50. doi: 10.1185/030079906X148508. [DOI] [PubMed] [Google Scholar]
- 14.Villines TC, Stanek EJ, Devine PJ, et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J Am Coll Cardiol. 2010;55(24):2721–6. doi: 10.1016/j.jacc.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Lee JM, Robson MD, Yu LM, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54(19):1787–94. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Frutkin AD, Baas A, O’Brien KD, et al. Reduction in high sensitivity C-reactive protein (hsCRP) during simvastatin and niacin therapy correlates with angiographic change in coronary artery stenosis: results from the HDL atherosclerosis treatment study (HATS) [abstract no. 2689] Circulation. 2003;108(17Suppl.IV):590–1. [Google Scholar]
- 17.Karas RH, Kashyap ML, Knopp RH, et al. Long-term safety and efficacy of a combination of niacin extended release and simvastatin in patients with dyslipidemia. Am J Cardiovasc Drug. 2008;8(2):69–81. doi: 10.2165/00129784-200808020-00001. [DOI] [PubMed] [Google Scholar]
- 18.Bays H. Safety of niacin and simvastatin combination therapy. Am J Cardiol. 2008;101(8A):3–8B. doi: 10.1016/j.amjcard.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Kamanna VS, Ganji SH, Kashyap ML. The mechanism and mitigation of niacin-induced flushing. Int J Clin Pract. 2009;63(9):1369–77. doi: 10.1111/j.1742-1241.2009.02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakkar RB, Kashyap ML, Lewin AJ, et al. Acetylsalicylic acid reduces niacin extended-release-induced flushing in patients with dyslipidemia. Am J Cardiovasc Drugs. 2009;9(2):69–79. doi: 10.1007/BF03256578. [DOI] [PubMed] [Google Scholar]
- 21.Kawata AK, Revicki DA, Thakkar R, et al. Flushing ASsessment Tool (FAST®): psychometric properties of a new measure assessing flushing symptoms and clinical impact of niacin therapy. Clin Drug Invest. 2009;29(4):215–29. doi: 10.2165/00044011-200929040-00001. [DOI] [PubMed] [Google Scholar]
- 22.Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007;99(6Suppl.):S22–31. doi: 10.1016/j.amjcard.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 23.National Heart, Lung, and Blood Institute (NHLBI). Niacin plus statin to prevent vascular events [ClinicalTrials.gov identifier NCT00120289]. ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2010 Oct 29]