Abstract
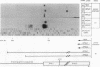
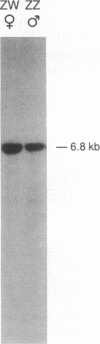
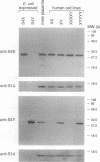
The human sex-linked genes RPS4X and RPS4Y encode distinct isoforms of ribosomal protein S4. Insufficient expression of S4 may play a role in the development of Turner syndrome, the complex human phenotype associated with monosomy X. In mice, the S4 protein is encoded by an X-linked gene, Rps4, and is identical to human S4X; there is no mouse Y homolog. We report here the organization of the human RPS4X and RPS4Y and mouse Rps4 genes. Each gene comprises seven exons; the positions of introns are conserved. The 5' flanking sequences of human RPS4X and mouse Rps4 are very similar, while RPS4Y diverges shortly upstream of the transcription start site. In chickens, S4 is encoded by a single gene that is not sex linked. The chicken protein differs from human S4X by four amino acid substitutions, all within a region encoded by a single exon. Three of the four substitutions are also present in human S4Y, suggesting that the chicken S4 gene may have arisen by recombination between S4X- and S4Y-like sequences. Using isoform-specific antisera, we determined that human S4X and S4Y are both present in translationally active ribosomes. S4Y is about 10 to 15% as abundant as S4X in ribosomes from normal male placental tissue and 46,XY cultured cells. In 49,XYYYY cells, S4Y is about half as abundant as S4X. In 49,XXXXY cells, S4Y is barely detectable. These results bear on the hypothesized role of S4 deficiency in Turner syndrome.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine M., Fried M. The organization of the intron-containing human S6 ribosomal protein (rpS6) gene and determination of its location at chromosome 9p21. Hum Mol Genet. 1992 Nov;1(8):565–570. doi: 10.1093/hmg/1.8.565. [DOI] [PubMed] [Google Scholar]
- Ashworth A., Rastan S., Lovell-Badge R., Kay G. X-chromosome inactivation may explain the difference in viability of XO humans and mice. Nature. 1991 May 30;351(6325):406–408. doi: 10.1038/351406a0. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Wool I. G. The structure of a gene containing introns and encoding rat ribosomal protein P2. Nucleic Acids Res. 1991 Sep 25;19(18):4895–4900. doi: 10.1093/nar/19.18.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. T., Roufa D. J. The transcriptionally active human ribosomal protein S17 gene. Gene. 1988 Oct 15;70(1):107–116. doi: 10.1016/0378-1119(88)90109-6. [DOI] [PubMed] [Google Scholar]
- Colombo P., Yon J., Fried M. The organization and expression of the human L7a ribosomal protein gene. Biochim Biophys Acta. 1991 Dec 2;1129(1):93–95. doi: 10.1016/0167-4781(91)90218-b. [DOI] [PubMed] [Google Scholar]
- Davies B., Feo S., Heard E., Fried M. A strategy to detect and isolate an intron-containing gene in the presence of multiple processed pseudogenes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6691–6695. doi: 10.1073/pnas.86.17.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Fried M. The structure of the human intron-containing S8 ribosomal protein gene and determination of its chromosomal location at 1p32-p34.1. Genomics. 1993 Jan;15(1):68–75. doi: 10.1006/geno.1993.1011. [DOI] [PubMed] [Google Scholar]
- De Falco S., Russo G., Angiolillo A., Pietropaolo C. Human L7a ribosomal protein: sequence, structural organization, and expression of a functional gene. Gene. 1993 Apr 30;126(2):227–235. doi: 10.1016/0378-1119(93)90371-9. [DOI] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- FERGUSON-SMITH M. A. KARYOTYPE-PHENOTYPE CORRELATIONS IN GONADAL DYSGENESIS AND THEIR BEARING ON THE PATHOGENESIS OF MALFORMATIONS. J Med Genet. 1965 Jun;2(2):142–155. doi: 10.1136/jmg.2.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch M. P., Thompson M. A., Holicky E. L., Schultz P. J., Hallett J. B., Wieben E. D. Conservation of coding and transcriptional control sequences within the snRNP E protein gene. Genomics. 1992 Dec;14(4):883–890. doi: 10.1016/s0888-7543(05)80109-0. [DOI] [PubMed] [Google Scholar]
- Feo S., Davies B., Fried M. The mapping of seven intron-containing ribosomal protein genes shows they are unlinked in the human genome. Genomics. 1992 May;13(1):201–207. doi: 10.1016/0888-7543(92)90221-d. [DOI] [PubMed] [Google Scholar]
- Fisher E. M., Beer-Romero P., Brown L. G., Ridley A., McNeil J. A., Lawrence J. B., Willard H. F., Bieber F. R., Page D. C. Homologous ribosomal protein genes on the human X and Y chromosomes: escape from X inactivation and possible implications for Turner syndrome. Cell. 1990 Dec 21;63(6):1205–1218. doi: 10.1016/0092-8674(90)90416-c. [DOI] [PubMed] [Google Scholar]
- Hammond M. L., Merrick W., Bowman L. H. Sequences mediating the translation of mouse S16 ribosomal protein mRNA during myoblast differentiation and in vitro and possible control points for the in vitro translation. Genes Dev. 1991 Sep;5(9):1723–1736. doi: 10.1101/gad.5.9.1723. [DOI] [PubMed] [Google Scholar]
- Hamvas R. M., Zinn A., Keer J. T., Fisher E. M., Beer-Romero P., Brown S. D., Page D. C. Rps4 maps near the inactivation center on the mouse X chromosome. Genomics. 1992 Feb;12(2):363–367. doi: 10.1016/0888-7543(92)90386-7. [DOI] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Equipotent mouse ribosomal protein promoters have a similar architecture that includes internal sequence elements. Genes Dev. 1989 Nov;3(11):1789–1800. doi: 10.1101/gad.3.11.1789. [DOI] [PubMed] [Google Scholar]
- Huxley C., Fried M. The mouse rpL7a gene is typical of other ribosomal protein genes in it's 5' region but differs in being located in a tight cluster of CpG-rich islands. Nucleic Acids Res. 1990 Sep 25;18(18):5353–5357. doi: 10.1093/nar/18.18.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley C., Williams T., Fried M. One of the tightly clustered genes of the mouse surfeit locus is a highly expressed member of a multigene family whose other members are predominantly processed pseudogenes. Mol Cell Biol. 1988 Sep;8(9):3898–3905. doi: 10.1128/mcb.8.9.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay G. F., Ashworth A., Penny G. D., Dunlop M., Swift S., Brockdorff N., Rastan S. A candidate spermatogenesis gene on the mouse Y chromosome is homologous to ubiquitin-activating enzyme E1. Nature. 1991 Dec 12;354(6353):486–489. doi: 10.1038/354486a0. [DOI] [PubMed] [Google Scholar]
- Levy S., Avni D., Hariharan N., Perry R. P., Meyuhas O. Oligopyrimidine tract at the 5' end of mammalian ribosomal protein mRNAs is required for their translational control. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3319–3323. doi: 10.1073/pnas.88.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O., Klein A. The mouse ribosomal protein L7 gene. Its primary structure and functional analysis of the promoter region. J Biol Chem. 1990 Jul 15;265(20):11465–11473. [PubMed] [Google Scholar]
- Mitchell M. J., Woods D. R., Tucker P. K., Opp J. S., Bishop C. E. Homology of a candidate spermatogenic gene from the mouse Y chromosome to the ubiquitin-activating enzyme E1. Nature. 1991 Dec 12;354(6353):483–486. doi: 10.1038/354483a0. [DOI] [PubMed] [Google Scholar]
- Monk R. J., Meyuhas O., Perry R. P. Mammals have multiple genes for individual ribosomal proteins. Cell. 1981 May;24(2):301–306. doi: 10.1016/0092-8674(81)90319-6. [DOI] [PubMed] [Google Scholar]
- Noël B., Bénézech M., Bouzon M. T., Coudrot D. Un garçon de sept ans 49,XYYYY. Ann Genet. 1988;31(2):111–116. [PubMed] [Google Scholar]
- Page D. C., Fisher E. M., McGillivray B., Brown L. G. Additional deletion in sex-determining region of human Y chromosome resolves paradox of X,t(Y;22) female. Nature. 1990 Jul 19;346(6281):279–281. doi: 10.1038/346279a0. [DOI] [PubMed] [Google Scholar]
- Pata I., Hoth S., Kruppa J., Metspalu A. The human ribosomal protein S6 gene: isolation, primary structure and location in chromosome 9. Gene. 1992 Nov 16;121(2):387–392. doi: 10.1016/0378-1119(92)90149-j. [DOI] [PubMed] [Google Scholar]
- Plauchu H., Charrin C., Kossmann J. C. Le syndrome 49,XYYYY: à propos d'un cas dépisté à la naissance et suivi depuis deux ans et demi. J Genet Hum. 1984 Sep;32(4):299–306. [PubMed] [Google Scholar]
- Rhoads D. D., Dixit A., Roufa D. J. Primary structure of human ribosomal protein S14 and the gene that encodes it. Mol Cell Biol. 1986 Aug;6(8):2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salido E. C., Yen P. H., Koprivnikar K., Yu L. C., Shapiro L. J. The human enamel protein gene amelogenin is expressed from both the X and the Y chromosomes. Am J Hum Genet. 1992 Feb;50(2):303–316. [PMC free article] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Beer-Romero P., Brown L. G., Nussbaum R., Page D. C. ZFX has a gene structure similar to ZFY, the putative human sex determinant, and escapes X inactivation. Cell. 1989 Jun 30;57(7):1247–1258. doi: 10.1016/0092-8674(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Sherton C. C., Wool I. G. Determination of the number of proteins in liver ribosomes and ribosomal subunits by two-dimensional polyacrylamide gel electrophoresis. J Biol Chem. 1972 Jul 25;247(14):4460–4467. [PubMed] [Google Scholar]
- Sirota L., Zlotogora Y., Shabtai F., Halbrecht I., Elian E. 49,XYYYY. A case report. Clin Genet. 1981 Feb;19(2):87–93. doi: 10.1111/j.1399-0004.1981.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Perry R. P. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol Cell Biol. 1985 Dec;5(12):3560–3576. doi: 10.1128/mcb.5.12.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Zinn A. R., Page D. C., Nishimoto T. Functional equivalence of human X- and Y-encoded isoforms of ribosomal protein S4 consistent with a role in Turner syndrome. Nat Genet. 1993 Jul;4(3):268–271. doi: 10.1038/ng0793-268. [DOI] [PubMed] [Google Scholar]
- Wiedemann L. M., Perry R. P. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol Cell Biol. 1984 Nov;4(11):2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool I. G., Chan Y. L., Paz V., Olvera J. The primary structure of rat ribosomal proteins: the amino acid sequences of L27a and L28 and corrections in the sequences of S4 and S12. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):69–73. doi: 10.1016/0167-4781(90)90143-p. [DOI] [PubMed] [Google Scholar]
- Wool I. G. The structure and function of eukaryotic ribosomes. Annu Rev Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]
- Yoganathan T., Bhat N. K., Sells B. H. A positive regulator of the ribosomal protein gene, beta factor, belongs to the ETS oncoprotein family. Biochem J. 1992 Oct 15;287(Pt 2):349–353. doi: 10.1042/bj2870349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn A. R., Bressler S. L., Beer-Romero P., Adler D. A., Chapman V. M., Page D. C., Disteche C. M. Inactivation of the Rps4 gene on the mouse X chromosome. Genomics. 1991 Dec;11(4):1097–1101. doi: 10.1016/0888-7543(91)90037-f. [DOI] [PubMed] [Google Scholar]