Abstract
Objective
Largely, watchful waiting is the initial policy for patients with small-sized or medium-sized vestibular schwannoma, because of slow growth and relatively minor complaints, that do not improve by an intervention. If intervention (microsurgery, radiosurgery or fractionated radiotherapy) becomes necessary, the choice of intervention appears to be driven by the patient's or clinician's preference rather than by evidence based. This study addresses the existing evidence based on controlled studies of these interventions.
Design
A systematic Boolean search was performed focused on controlled intervention studies. The quality of the retrieved studies was assessed based on the Sign-50 criteria on cohort studies.
Data sources
Pubmed/Medline, Embase, Cochrane Central Register of Controlled Trials and reference lists.
Study selection
Six eligibility criteria included a controlled intervention study on a newly diagnosed solitary, vestibular schwannoma reporting on clinical outcomes. Two prospective and four retrospective observational, controlled studies published before November 2011 were selected.
Data analysis
Two reviewers independently assessed the methodological quality of the studies and extracted the outcome data using predefined formats.
Results
Neither randomised studies, nor controlled studies on fractionated radiotherapy were retrieved. Six studies compared radiosurgery and microsurgery in a controlled way. All but one were confined to solitary tumours less than 30 mm in diameter and had no earlier interventions. Four studies qualified for trustworthy conclusions. Among all four, radiosurgery showed the best outcomes: there were no direct mortality, no surgical or anaesthesiological complications, but better facial nerve outcome, better preservation of useful hearing and better quality of life.
Conclusions
The available evidence indicates radiosurgery to be the best practice for solitary vestibular schwannomas up to 30 mm in cisternal diameter.
Keywords: Vestibular Schwannoma, Excision, Radiosurgery, RADIOTHERAPY, NEUROSURGERY
Article summary.
Article focus
Search for the best practice if an intervention for solitary vestibular schwannoma is considered necessary.
Systematic review of evidence from controlled intervention studies on the effectiveness of interventions for solitary vestibular schwannomas.
Key messages
The literature search yielded cohort studies comparing microsurgery and radiosurgery.
Quality assessment showed four studies likely to give unbiased results.
Radiosurgery consistently emerges as the best practice for tumours smaller than 30 mm in cisternal diameter.
Strengths and limitations of this study
All eligible studies compared the same interventions: microsurgical excision and radiosurgery.
All four trustworthy controlled studies pointed to the same intervention as the best practice.
Patients’ outcomes in the assessed comparative studies are in accordance with long-term outcomes in sizeable contemporary case-series.
The conclusion is limited to solitary vestibular schwannomas smaller than 30 mm.
Introduction
Vestibular schwannoma, also called acoustic neuroma, is not an uncommon benign brain tumour. It accounts for about 6% of all intracranial tumours.1 A reliable register is available in Denmark, since almost all patients with a vestibular schwannoma are referred to one specialist clinic. The incidence approaches 20 per million per year.2 Owing to its benign nature the prevalence accumulates to 200 per million.3 The tumour originates from the Schwann cells of the vestibular section of the vestibulocochlear nerve at the borders of the central and peripheral myelin, usually slightly lateral to the rim of the internal auditory meatus. A vestibular schwannoma visualises characteristically at MRI (figure 1). In combination with symptoms like asymmetric hearing loss, tinnitus, vertigo or imbalance, the diagnosis is accepted without histological verification. The majority grows slowly or not at all; the average growth is 1–2 mm/year.4 5 However, if the tumour grows, the rate in the first year is on an average 5–10 mm.6 There are no parameters known that predict which tumour will grow and to what extent.7 8
Figure 1.
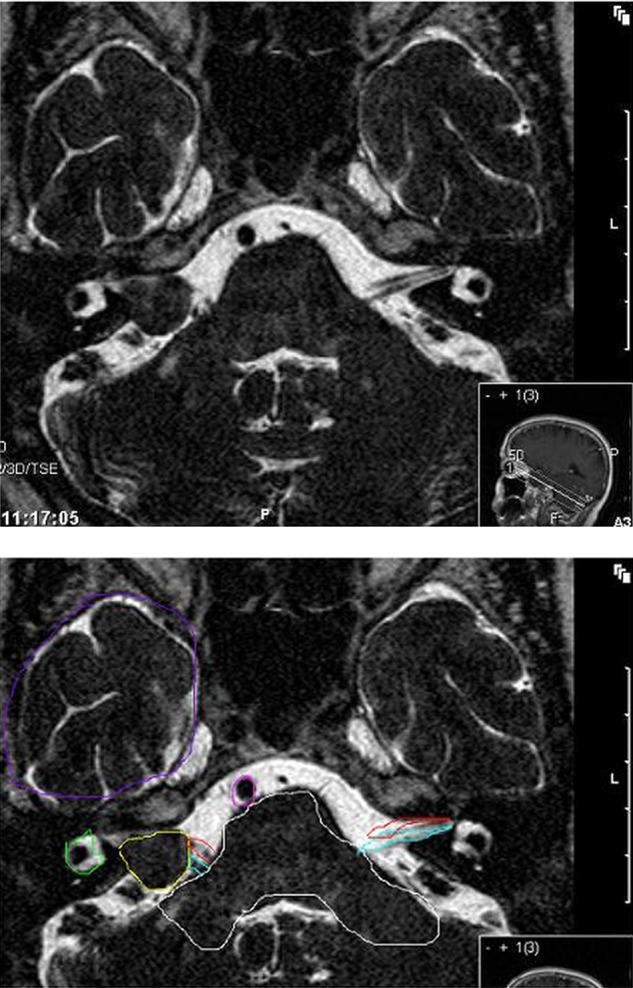
Axial T2-weighted MRI with a still visible CSF-interface between tumour and brain. The largest diameter of the tumour in the CPA cistem is 14 mm. Yellow: vestibular schwannoma; green: labyrinth; red: ipsilateral and contralateral facial nerve; blue: ipsilateral and contralateral vestibulocochlear nerve; white: brainstem and cerebellar peduncle; purple: caudal temporal lobe; pink: basilar artery.
The mild natural course and relatively minor symptoms—that do not improve by any intervention—justifies for small-sized and medium-sized tumours an initial policy of watchful waiting by sequential MRI follow-up. However, if the tumour is sizeable and obliterates the cistern of the cerebellopontine angle (CPA) or grows substantially during the follow-up, in principal, an intervention is indicated. In most centres, the choice is between microsurgical resection for any tumour size and radiosurgery for small-sized and medium-sized tumours or stereotactic radiotherapy for tumours over 25–30 mm diameter. Numerous case series and non-systematic reviews have been summarised recently by Arthurs et al.4
Understandably, owing to inherent limitations of case series, these reviewers did not arrive at firm conclusions. In this study, we limit our search for the best practice to comparative, controlled trials on interventions for vestibular schwannoma in a systematic and qualitative way.
Methods
PubMed and Embase were searched in November 2011 for controlled intervention studies on vestibular schwannomas. We imposed no restrictions on the kind of intervention or patient characteristics. We performed Boolean searches using the following keywords (‘vestibular schwannoma’ OR ‘acoustic neuroma’ NOT neurofibromatoses) and (management OR therapy OR treatment OR intervention) and (‘controlled trial’ OR ‘controlled study’ OR ‘clinical trial’) or (comparative OR comparison OR compared) (see online supplementary appendix 1). No language, publication status or other search restriction was imposed. The retrieved articles were screened by title and if necessary by abstract. Eventually 13 full text articles were examined. The reference lists of studies meeting the eligibility criteria were checked. We also searched the Cochrane Central Register of Controlled Trials without finding further studies. The six eligibility criteria included controlled, intervention study on a newly diagnosed, solitary vestibular schwannoma reporting on clinical outcome.
The two neurosurgeons of our team appraised the articles for inclusion and assessed the risk of bias in the individual studies. The quality was assessed by judging criteria that were considered relevant by the team. The assessment is based on the Sign-50 quality criteria for cohort studies. (Our criteria are enlisted in table 1) (http://www.ahrq.gov/clinic/epcix.htm: AHRQ Publication No. 02-E016, April 2002, http://www.sign.ac.uk/guidelines/fulltext/50/annexc.html: checklist and notes on cohort studies, annexe C).9 We abstracted the primary clinical outcome data: mortality, treatment failure (that is second intervention necessary), function of cranial nerves 7 and 8, other intervention-associated complications and the data on quality of life. These outcome measures are the most important to the patient. Secondary outcome measures, being duration of hospital stay and time off work were also addressed. Table 1 on risk of bias and table 3 on outcome measures served as a predefined format for data extraction. Disagreements between the two reviewers were resolved by consensus.
Table 1.
Checklist on cohort studies based on SIGN 50 comparing microsurgery (MS) and radiosurgery (RS) for solitary vestibular schwannoma
| Authors and publication year | Pollock 200610 | Myrseth 200911 | Pollock 199512 | Myrseth 200515 | Regis 200214 | Karpinos 200213 |
|---|---|---|---|---|---|---|
| Design | Prospective consecutive predefined inclusion criteria | Prospective consecutive predefined inclusion criteria | Retrospective consecutive matched controls | Retrospective consecutive matched controls | Retrospective non-consecutive matched controls | Retrospective consecutive matched controls |
| Allocation to treat arm | Preference patient | Preference patient | Preference patient and surgeon | Preference patient | 2 hospitals, preference by surgeon/patient | Miscellaneous criteria by surgeon |
| Same primary endpoint: intervention-associated morbidity | Yes | Yes | Yes | Yes | Yes | Yes |
| Selection of subjects | ||||||
| Source population: adult, solitary VS<30 mm, no previous intervention | Yes | Yes | Yes | Yes | Yes | No |
| Eligibility criteria: proven growth or predefined cisternal size | No | Yes | No | No | No | No |
| Exclusion criteria NOT more strict for MS because of age and comorbidity | Yes | No | No | No | No | No |
| Participation rate NOT lower for MS because of specific RS referral | Yes | No | No | No | No | No |
| Same baseline cranial nerve deficits | Yes | Yes | Yes | Yes | No | Yes |
| Consecutive series and loss to follow-up <10% | Yes | Yes | Yes | Yes | No | No |
| Adequate analysis drop outs | Yes | Yes | No | Yes | No | No |
| Outcome assessment | ||||||
| Prespecified endpoint | Yes | Yes | Yes | Yes | Yes | Yes |
| Mortality addressed | Yes | Yes | No | Yes | Yes | Yes |
| Blinded outcome measurement | Yes | No | No | No | No | No |
| Same measure new cranial nerve deficit | Yes | Yes | Yes | Yes | Yes | Yes |
| Same measure quality-of-life scores | Yes | Yes | Yes | Yes | Yes | No |
| Repeated outcome measurement | Yes | Yes | Yes | Yes | Yes | No |
| Confounding variables | ||||||
| NOT substantial larger tumour size in MS arm | Yes | Yes | Yes | Yes | Yes | No |
| NOT substantial higher age in RS arm | No | Yes | No | No | No | No |
| NOT less fit patients in RS arm | Yes | No | No | No | No | No |
| One single intervention in each arm | Yes | Yes | Yes | Yes | Yes | No |
| Statistical analysis | ||||||
| Statistical measure of precision | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall assessment | ||||||
| Number of relevant ‘no’ | 0 | 0 | 0 | 0 | 3 | 6 |
| Overall judgement | ++ | ++ | + | + | − | − |
| No commercial funding | Yes | Yes | Yes | Yes | Yes | Yes |
| No relevant bias, outcome owing to intervention | Yes | Yes | Yes | Yes | No | No |
| Outcome applicable to source population | Yes | Yes | Yes | Yes | No | No |
Yes: well covered or adequately addressed, increasing confidence that outcome is caused by the interventions.
No: poorly or not addressed or not reported; cause for bias. Bold: possible relevant bias, decreasing confidence.
++, All or most of the criteria have been fulfilled. Where they have not been fulfilled the conclusions of the study or reviews are thought to be very unlikely to alter.
+, Some of the criteria have been fulfilled. Those criteria that have not been fulfilled or not adequately described are thought unlikely to alter the conclusions.
−, Few or no criteria fulfilled. The conclusions of the study are thought likely or very likely to alter.
Table 3.
Outcome of the six controlled studies on vestibular schwannoma; all comparing microsurgery (MS) and radiosurgery (RS)
| Author publication ear | Therapy FU (no.) | Follow-up (range) | Mortality (%) | 2nd ther. (%) | Facial intact* (%) | % useful hearing† | Other complications‡ | Hospital days | Work resume (%) | QoL tests§ | QoL (% results) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pollock 200610 | MS 36 RS 46 |
3.5 year mean (1–5.2 year) |
0 0 |
0 4 |
83 98 |
5 63 |
33 11 |
? ? |
? ? |
DHI, HS, HSQ |
↓ =* |
| Myrseth 200911 | MS 28 RS 60 |
≥2 year | 0 0 |
18 2 |
82 100 |
0 68 |
14 0 |
12,5 2.5 |
100 93 |
SF36, GBI | SF36= GBI ↑ |
| Pollock 199512 | MS 40 RS 47 |
3 year median (2.1–4 year) |
0 0 |
0 0 |
78 91 |
14 75 |
38 13 |
9,5 1.4 |
? ? |
ANSPQ | ↓ 45 ↓ 26 |
| Myrseth 200515 | MS 86 RS 103 |
5.9 year mean (1–14.2 year) |
1 0 |
6 5 |
80 95 |
5 32 |
47 4 |
? ? |
? ? |
SF36, GBI | ↓ =* |
| Regis 200214 | MS 110 RS 97 |
≥3 year | 1 0 |
9 3 |
67 100 |
36 50 |
41 8 |
23 3 |
66 99 |
Pellet | ↓ 39 ↓ 9 |
| Karpinos 200213 | MS 18 RS 49 |
4 year median (0.3–7 year) |
0 0 |
0 4 |
60 97 |
40 44 |
48 5 |
2–16 1–2 |
88 94 |
None | – – |
Bold: significantly better.
*Percentage preserved, House-Brackmann grade 1–2.
†Percentage preserved, AAO-HNS class A–B or Gardner–Robertson grade I–II.
‡Percentage complications as new trigeminal deficit, haemorrhage, cerebrospinal fluid (CSF) leakage, meningitis, wound infection, CSF-shunt needed.
§Quality of life (QoL) from questionnaires as Dizziness Handicap Inventory, Headache Survey, Health Status Questionnaire, ShortForm36, Glasgow Benefit Inventory, Acoustic Neuroma Association Patient Questionnaire, Pellet Questionnaire.
Results
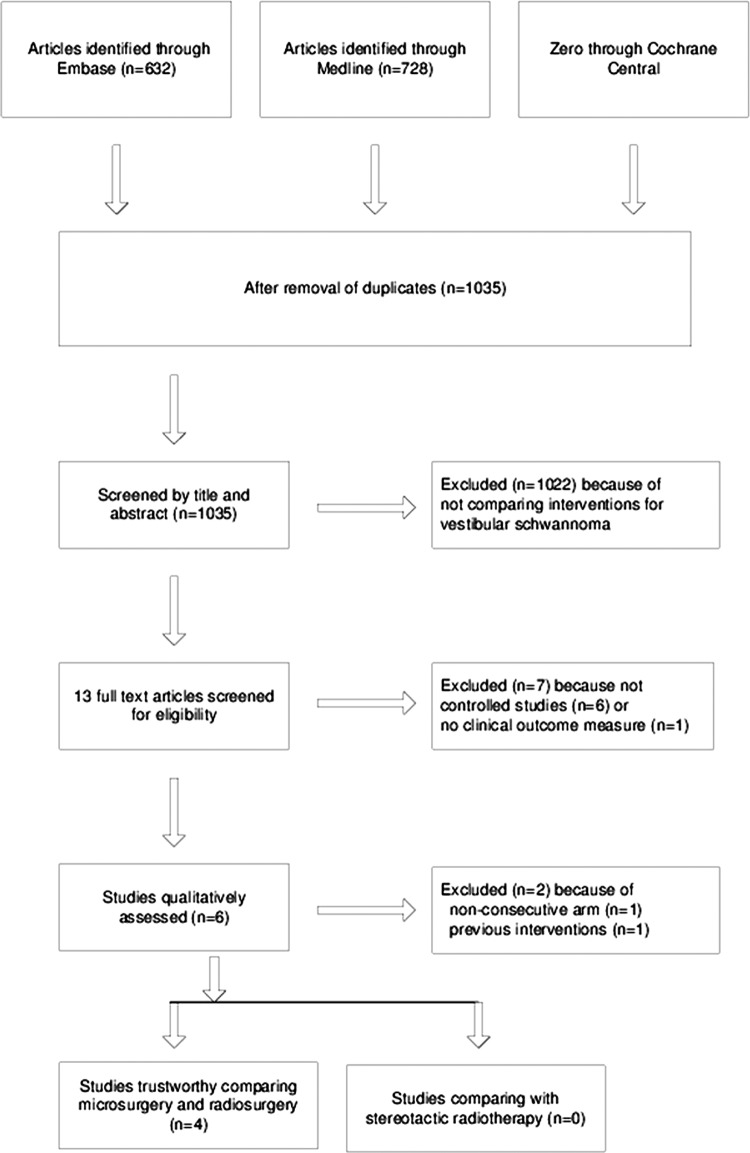
No randomised clinical trials on solitary vestibular schwannoma were found. Only two studies—both comparing microsurgical excision with radiosurgery—showed up that had a controlled, prospective design with predefined inclusion criteria.10 11 The search retrieved another four retrospective cohort studies with a matched control group, all comparing again microsurgery and radiosurgery.12–15 We identified no controlled studies involving fractionated stereotactic radiotherapy (figure 2).
Figure 2.
Flow diagram of study selection.
Four main quality items were assessed: selection of subjects, outcome measure, known confounders and statistical analysis (table 1). At the inception, in five out of six studies all patients were at the same stage of the disease having minor symptoms, tumour size limited to 30 mm extension into the CPA and no earlier intervention. ne exception is the study of Karpinos et al,13 which included recurrent tumours. The indication for an intervention was clearly defined in only one study.11 In the other studies, just having a vestibular schwannoma seemed sufficient to initiate an intervention, be it excision or radiosurgery. Baseline patient characteristics were quite similar in the treatment arms within the studies (table 2). Only the average age was higher in all radiosurgery arms. Specific allocation to the radiosurgery arm because of comorbidity or high age was permitted in all but the study of Pollock et al.10 These are known hazards for a favourable outcome. If imbalance was present, the higher risk patients were in the radiosurgery arms. There was minimal or no loss to follow-up in all but one study.13 After summation of the number of items that downgrade the confidence in outcome (bold No in table 1), four studies remained that showed trustworthy association between interventions and outcomes. 10–12 15 The outcomes are specified in table 3. There was 1% mortality in two microsurgery arms.14 15 After radiosurgery, there was no mortality and no surgical or anaesthetic complications, but better facial function, better hearing preservation and better quality of life.
Table 2.
Patients’ preintervention characteristics; only sporadic vestibular schwannomas
| Author publication year | Intervention included (no.) | Male: Female | Age (years) | n. trigeminal deficit % | n. facial deficit (%)† | Useful hearing (%)‡ | Tumour size§ (mean mm) | Previous treatment (%) |
|---|---|---|---|---|---|---|---|---|
| Pollock 200610 | MS: 36 RS: 46 |
19:17 27:19 |
48 54 |
0 0 |
0 0 |
61 65 |
14 12 |
No No |
| Myrseth 200911 | MS: 28 RS: 60 |
12:16 36:24* |
53 58 |
? ? |
0 0 |
44 42 |
18 16 |
No No |
| Pollock 199512 | MS: 40 RS: 47 |
18:22 23:24 |
51 62* |
10 6 |
5 2 |
12 4 |
>20 mm: 18% >20 mm: 29% |
No No |
| Myrseth 200515 | MS: 86 RS: 103 |
? ? |
50 60* |
20 12 |
1 1 |
2 10 |
>20 mm: 32% >20 mm: 17% |
No No |
| Regis 200214 | MS: 110 RS: 100 |
M 35% M 46% |
52 61 |
55 20 |
? 2 |
? 49 |
KoosIII: 55%§ KoosIII: 34% |
No No |
| Karpinos 200213 | MS: 23 RS: 73 |
6:17 23:50 |
45 62* |
30 17 |
26 10 |
30 24 |
>40 mm: 17* >40 mm: 3% |
26 14 |
MS: microsurgery, RS: radiosurgery.
*Significant (p<0.05).
†Percentage preserved, House-Brackmann grade 1–2.
‡Useful hearing: AAO-HNS class A–B or Gardner–Robertson grade I–II.
§KoosIII: tumour occupying the cerebellopontine cistern without brainstem displacement.
Discussion
Microsurgery and radiosurgery are equally effective interventions for vestibular schwannomas as demonstrated by numerous case series that were recently reviewed.4 While taking into account patients’ individual preferences, ideally the choice of treatment should be based on high-quality evidence from well-conducted clinical trials. We found evidence of greater clinical effectiveness of radiosurgery compared with that of microsurgery in medium-sized tumours.
Systematic reviews of randomised clinical trials—preferably double-blinded—are considered the gold standard of evidence-based practice. Regarding vestibular schwannomas, however, we most probably will have to do without randomised studies. Indeed, Myrseth et al11 failed to go on with their randomised trials, because patients were reluctant to accept chance to decide whether they would undergo surgery or radiosurgery.11 Next best evidence is obtained from well-designed non-randomised controlled trials.16 17 Next to the value of well-conducted randomised trials, the value of high-quality observational studies is validated by the remarkable similar results, which were observed when comparing specific treatments through both randomised and observational trials.18–20 Such observational studies may provide trustworthy information on the risks of the intervention, on adverse events and ultimately on the quality of life of patients. Overall, these patients are more similar to the general disease population than those complying with the strict inclusion and exclusion criteria of a randomised clinical trial. Such high quality of observational studies is achieved by studying the same intervention by the same outcome measures in well-matched patient population without dropouts. Based on Sign-50, this is the basic thought behind the assessment of quality of individual studies in table 1.
Selection of subjects
All retrieved controlled studies compared the same two interventions and consistently pointed to radiosurgery as being the best intervention for their research question. Some studies, however, provide more confidence to have unbiased results, as elucidated in table 1. A major risk of bias of all observational studies is that the compared groups are substantially unequal in their initial susceptibility to the outcome. In five studies the selection bias is reasonably controlled, since the compared groups are very similar except for the interventions under study. Only in the study by Karpinos et al13 the source population differed due to inclusion of patients having had earlier surgery for the same disease. In addition, this study had an unacceptably high loss to follow-up of over 20%. These two serious sources of bias prevented a favourable overall good quality judgement. In one study pertinent bias arose, because of non-consecutive inclusion in the microsurgery arm.14
Only Myrseth et al11 clearly defined the starting point of an intervention. Nevertheless, confounding by indication between the various studies appears unlikely, since major adverse events, like disabling neurological deficits, do not occur in the natural history of vestibular schwannomas smaller than 30 mm. It is very implausible that any of the major adverse events occur in the absence of an intervention. Therefore, the risk that an adverse outcome occurs by chance instead of being related to the intervention is not realistic and we assigned no relevance to the potential confounder of being at various points in the disease progression (non-bold No, table 1).
Outcome assessment
All but one study reported on the same clinical outcome measures, which are: failure because a second intervention was needed, function preservation of the involved cranial nerves, more general complications and quality of life. The exception is the study by Karpinos et al, who did not report on quality of life. All used established classifications of facial motor function and useful hearing.
Only one group managed a blinded outcome measurement.10 Taking into account that a troublesome outcome—when occurring—is quite clear-cut in this disease, non-blinded outcome measurement did not depreciate our trust that the reported outcome is true and caused by the specific intervention. Typically, repeated measurements increase this trust further.
Confounding variables
A previous treatment for the same disease induces relevant bias, because of different baseline characteristics and an inherent higher risk for adverse events. As mentioned already, this applied to the study of Karpinos et al,13 because the results from first and second interventions were not separated in their report. Frail patients were in all but the study of Pollock et al10 inclined to end up in the radiosurgery arm. In general higher age, comorbidity and larger tumours are drawbacks for a good outcome. In those studies showing significant imbalance of these variables the potential disadvantage, however, was at the side of radiosurgery, which nevertheless produced the best outcome in all studies.12 13 15 As these imbalances work in favour of microsurgery, we considered them not relevant (non-bold Nos in table 1).
The overall assessment of study quality gave confidence in four studies, because no relevant biases were identified. Quite importantly, all four consistently showed a significant advantage for radiosurgery over microsurgical excision, when directly compared in a controlled manner (table 2).
One might argue that a weakness of some of the four trustworthy studies is the relative small numbers and short follow-up. However, patients’ outcome in the assessed comparative studies is in accordance with the long-term outcome in sizeable contemporary radiosurgery series as summarised in online supplementary appendix 2.21–25 Radiosurgery for vestibular schwannoma is a day case with 2% (median) of patients requiring additional treatment; less than 1% (median) experienced some facial neuropathy and trigeminal neuropathy occurred in 5% (median). It has no direct mortality and the risk of incapacitating complications is negligible or non-existing. The comprehensive review of Arthurs et al showed that after microsurgery less than 2% of patients required additional treatment. The rates of facial nerve palsy are as high as 10–30%, varying with tumour size.4 These numbers are of the same range in the comparative studies on tumours limited to a size of 3 cm in table 2. Not mentioned in any detail by Arthurs et al are other surgical morbidities, which are not trivial at all, being between 14% and 47% in the comparative studies. Major adverse events like mortality and discharge to long-term care may occur after microsurgery in about 0.5% and 1.2%, respectively.26
Not addressed in the comparative studies is the risk of secondary cancer after radiation for a benign tumour causing mortality. Indeed, radiation-associated tumours do occur after sufficient follow-up of 5–20 years. So far, 12 cases of radiosurgery-associated malignant tumours have been reported worldwide.27 Based on model calculations the probability of a malignant tumour after radiosurgery is estimated at 1 per 1000.28 Distinctively, the hospital-based study mentioned herein before depicted 2643 surgeries in 265 US hospitals for vestibular schwannoma and showed a 3-month mortality of 0.5%.26 If radiosurgery is not employed too enthusiastically owing to its low threshold, but on proper indication, the risk of death by a radiation-induced tumour is not relevant in comparison to the (few) possible direct disasters of microsurgery. Undeniably, the mortality is much smaller and, if it occurs, it may do so many years later in a patient’s life.
Looking for the best practice, one should realise indeed that the results of various health-related quality-of-life studies after surgery called for modesty. Deterioration of the well-being of the patient proved difficult to avoid, even in elective surgery of relatively small tumours.29–31 In addition, the comparative studies showed deterioration in the quality of life as high as in 30–45% of patients operated on (table 3). Once an intervention is considered necessary, we conclude based on this systematic review of controlled studies, that radiosurgery is the best practice for patients with solitary vestibular schwannoma up to 30 mm in cisternal extension.
Supplementary Material
Acknowledgments
The authors acknowledge Bernard Pauw and Cees Avezaat for having initiated and stimulated evidence-based practice in our Working Party on cerebellopontine angle tumours.
Footnotes
Contributors: All authors participated in the conception, design and interpretation of data. JW and AD conducted literature searches and data extraction. JW prepared the initial draft and led the preparation of the manuscript. All authors were involved in drafting and reviewing the manuscript and approved the final version.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol 2006;8:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tos M, Stangerup SE, Caye-Thomasen P, et al. What is the real incidence of vestibular schwannoma? Arch Otolaryngol Head Neck Surg 2004;130:216–20 [DOI] [PubMed] [Google Scholar]
- 3.Lin D, Hegarty JL, Fischbein NJ, et al. The prevalence of "incidental" acoustic neuroma. Arch Otolaryngol Head Neck Surg 2005;131:241–4 [DOI] [PubMed] [Google Scholar]
- 4.Arthurs BJ, Fairbanks RK, Demakas JJ, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev 2011;34:265–77; discussion 77–9 [DOI] [PubMed] [Google Scholar]
- 5.Hajioff D, Raut VV, Walsh RM, et al. Conservative management of vestibular schwannomas: third review of a 10-year prospective study. Clin Otolaryngol 2008;33:255–9 [DOI] [PubMed] [Google Scholar]
- 6.Stangerup SE, Caye-Thomasen P, Tos M, et al. The natural history of vestibular schwannoma. Otol Neurotol 2006;27:547–52 [DOI] [PubMed] [Google Scholar]
- 7.Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope 2005;115:450–4 [DOI] [PubMed] [Google Scholar]
- 8.Herwadker A, Vokurka EA, Evans DG, et al. Size and growth rate of sporadic vestibular schwannoma: predictive value of information available at presentation. Otol Neurotol 2005;26:86–92 [DOI] [PubMed] [Google Scholar]
- 9.West S, King V, Carey TS, et al. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ) 2002(47):1–11 [PMC free article] [PubMed] [Google Scholar]
- 10.Pollock BE, Driscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery 2006;59:77–85; discussion 77–85 [DOI] [PubMed] [Google Scholar]
- 11.Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery 2009;64:654–61; discussion 61–3 [DOI] [PubMed] [Google Scholar]
- 12.Pollock BE, Lunsford LD, Kondziolka D, et al. Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotactic radiosurgery (published erratum appears in Neurosurgery 1995 Feb;36(2):427). Neurosurgery 1995;36:215–24 [DOI] [PubMed] [Google Scholar]
- 13.Karpinos M, Teh BS, Zeck O, et al. Treatment of acoustic neuroma: stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys 2002;54:1410–21 [DOI] [PubMed] [Google Scholar]
- 14.Regis J, Pellet W, Delsanti C, et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg 2002;97:1091–100 [DOI] [PubMed] [Google Scholar]
- 15.Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery 2005;56:927–35; discussion 27–35 [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP. When are observational studies as credible as randomised trials? Lancet 2004;363:1728–31 [DOI] [PubMed] [Google Scholar]
- 17.Vandenbroucke JP. What is the best evidence for determining harms of medical treatment. CMAJ 2006;174:645–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000;342:1878–86 [DOI] [PubMed] [Google Scholar]
- 19.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papanikolaou PN, Christidi GD, Ioannidis JP. Comparison of evidence on harms of medical interventions in randomized and nonrandomized studies. CMAJ 2006;174:635–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman WA, Bradshaw P, Myers A, et al. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg 2006;105:657–61 [DOI] [PubMed] [Google Scholar]
- 22.Hempel JM, Hempel E, Wowra B, et al. Functional outcome after gamma knife treatment in vestibular schwannoma. Eur Arch Otorhinolaryngol 2006;263:714–18 [DOI] [PubMed] [Google Scholar]
- 23.Chopra R, Kondziolka D, Niranjan A, et al. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2007;68:845–51 [DOI] [PubMed] [Google Scholar]
- 24.Regis J, Roche PH, Delsanti C, et al. Modern management of vestibular schwannomas. Prog Neurol Surg 2007;20:129–41 [DOI] [PubMed] [Google Scholar]
- 25.Fukuoka S, Takanashi M, Hojyo A, et al. Gamma knife radiosurgery for vestibular schwannomas. Prog Neurol Surg 2009;22:45–62 [DOI] [PubMed] [Google Scholar]
- 26.Barker FG, II, Carter BS, Ojemann RG, et al. Surgical excision of acoustic neuroma: patient outcome and provider caseload. Laryngoscope 2003;113:1332–43 [DOI] [PubMed] [Google Scholar]
- 27.Schmitt WR, Carlson ML, Giannini C, et al. Radiation-induced sarcoma in a large vestibular schwannoma following stereotactic radiosurgery: case report. Neurosurgery 2011;68:E840–6; discussion E46 [DOI] [PubMed] [Google Scholar]
- 28.Niranjan A, Kondziolka D, Lunsford LD. Neoplastic transformation after radiosurgery or radiotherapy: risk and realities. Otolaryngol Clin North Am 2009;42:717–29 [DOI] [PubMed] [Google Scholar]
- 29.da Cruz MJ, Moffat DA, Hardy DG. Postoperative quality of life in vestibular schwannoma patients measured by the SF36 Health Questionnaire. Laryngoscope 2000;110:151–5 [DOI] [PubMed] [Google Scholar]
- 30.Martin HC, Sethi J, Lang D, et al. Patient-assessed outcomes after excision of acoustic neuroma: postoperative symptoms and quality of life. J Neurosurg 2001;94:211–16 [DOI] [PubMed] [Google Scholar]
- 31.Betchen SA, Walsh J, Post KD. Self-assessed quality of life after acoustic neuroma surgery. J Neurosurg 2003;99:818–23 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.