Abstract
Objective
There are data suggesting that blood product transfusions increase the risk of developing acute lung injury (ALI) in adults, and may be associated with increased mortality in adults with ALI. A possible association between transfusions and adverse outcomes of pediatric patients with ALI has not been studied previously. We tested the hypothesis that blood product transfusions to pediatric patients with ALI within the first 72 hours of the diagnosis would be associated with increased mortality and prolonged mechanical ventilation.
Design
An epidemiologic database of pediatric ALI prospectively gathered from July 1996 to May 2000 was analyzed.
Setting
Children were enrolled from both a tertiary referral hospital and a major community children's hospital.
Patients
Three hundred fifteen patients who met the 1994 American European Consensus Committee definition of ALI between the ages of 36 weeks corrected gestational age and 18 years.
Main Outcome Measure
Mortality in the pediatric intensive care unit.
Results
Multivariate analyses indicated that the transfusion of fresh-frozen plasma (FFP) was associated with increased mortality, independent of the severity of hypoxemia (Pao2/Fio2), presence of multiple organ system failure or disseminated intravascular coagulation (odds ratio = 1.08, 95% confidence interval = 1.00–1.17, p = 0.04). FFP transfusion was analyzed as a continuous variable, so that for each milliliter of FFP transfused per kilogram patient body weight per day, the odds of death increased by 1.08. There was a trend toward an association of the transfusion of FFP with a fewer number of days of unassisted ventilation (regression coefficient = −0.21, 95% confidence interval = −0.42–0.01, p = 0.06).
Conclusions
The transfusion of FFP is associated with an increased risk of mortality in children with ALI. The association between FFP and mortality in children with ALI should be investigated further.
Keywords: transfusion, fresh-frozen plasma, transfusion-related acute lung injury, acute lung injury, acute respiratory distress syndrome, platelets, red blood cells, critical care, pediatric, children
Multiple transfusions are a known risk factor for the development of the acute respiratory distress syndrome (ARDS) in critically ill adult patients (1, 2). Several recent studies have investigated a possible association of red blood cell, platelet, and fresh-frozen plasma (FFP) transfusions with the development of acute lung injury (ALI) or with increased mortality in patients with ALI/ARDS. A large, randomized controlled trial of red blood cell transfusions in the intensive care unit suggested that adult patients who received a liberal strategy of red blood cell transfusions tended to have an increase in 30-day mortality, and an increased risk of developing ARDS (3). A prospective observational study of critically ill adults at risk for ARDS suggested that red blood cell transfusions were a risk factor for developing ARDS as well as for increased mortality in patients who did develop ARDS (4). A retrospective analysis of risk factors associated with the development of ALI/ARDS in critically ill adults identified that transfusions of FFP, platelets, or red blood cells were associated with an increased risk of developing ALI/ARDS, with the greatest risk being associated with FFP transfusions (5). In addition, FFP was implicated as a risk factor associated with the development of ALI in mechanically ventilated critical care adult patients (6).
ALI is also an important problem in children, as recognized by the original description of ARDS in 1967 (7), as well as by a recent epidemiologic study of 328 children with ALI (8). Because red blood cell, platelet, and FFP transfusion may be associated with increased mortality in adult patients with ALI/ARDS, it is important to identify whether or not there is an association between outcomes and blood product transfusion in children with ALI/ARDS.
To determine whether blood product transfusions were associated with adverse outcomes in pediatric patients with ALI/ARDS, we analyzed a large prospective database of children with ALI/ARDS (8). The epidemiology and clinical risk factors associated with death and prolonged mechanical ventilation in this cohort has been previously published (8). We tested the hypothesis that transfusions of packed red blood cells, platelets, and/or FFP within the first 72 hours after the diagnosis of ALI/ARDS were associated with increased mortality.
Materials and Methods
Pediatric intensive care patients who met the 1994 American European Consensus Committee definition of ALI (the acute onset of bilateral infiltrates on chest radiograph with the Pao2/Fio2 <300 and the absence of clinical evidence for left atrial hypertension) at any time during admission to the pediatric intensive care unit (PICU) were prospectively enrolled into the pediatric ALI database. The diagnosis of ALI was made in each case by the principal investigator.
Patients were enrolled from both an academic hospital, the University of California San Francisco Children's Hospital, from July 1996 to July 1998, and from a major community children's hospital, the Children's Hospital and Research Center at Oakland, from July 1996 to May 2000. Patients were excluded if they were less than 36 weeks gestational age or greater than 18 years of age at the onset of ALI, or if they had echocardiographic evidence of left atrial hypertension or intracardiac shunt. Patients were excluded from the blood transfusion analysis if they received an exchange transfusion or plasmapheresis within the first 72 hours after the diagnosis of ALI because the number of blood products or fluids administered to these patients differed enough from the rest of the cohort that their inclusion could potentially skew the results. Patients who had preexisting ALI at an outside hospital before transfer to a study-site hospital were also excluded because it was not possible to accurately identify when the diagnosis of ALI was made. Only those blood transfusions that were administered within the first 72 hours after diagnosis of ALI were included in the blood transfusion analysis to decrease the impact of patient dropout from the analysis secondary to death or discharge from the PICU.
At the time the diagnosis of ALI was made, demographic, diagnostic, and therapeutic information was obtained for each patient. The first Pao2/Fio2 <300 defined each patient's baseline Pao2/Fio2. The worst laboratory values and clinical findings recorded for each patient for the first 24 hours after diagnosis of ALI were used to assess baseline organ system dysfunction. Nonpulmonary end-organ dysfunction was divided into the following groups: neurologic, cardiovascular, hepatic, gastrointestinal, renal, and hematologic. Definitions for each category were derived from published, peer-reviewed standards (9–12). Hematologic dysfunction included thrombocytopenia (platelet count <75,000 per mm3), neutropenia (an absolute neutrophil count <1000 per mm3), anemia (hemoglobin of <5 g/dL), disseminated intravascular coagulation (DIC) (presence of three of the following: thrombocytopenia, low fibrinogen, or elevated prothrombin time, partial thromboplastin time, or d-dimers), or clinical evidence of inappropriate bleeding (epistaxis, purpura, or petechiae).
All medical therapies were recorded on a daily basis, including the volume and type of all blood product transfusions. The practice of leukocyte reduction of blood products was not uniform between the two participating children's hospitals. During the study period, the blood products from Oakland Children's Hospital were routinely leukocyte reduced whereas those from the University of San Francisco Children's Hospital were not.
The primary outcome was mortality in the PICU. The secondary outcome was the duration of unassisted ventilation, defined as the number of days the patient was alive and not mechanically ventilated in the 28 days after the onset of ALI, as defined in previous studies (13). All patients who died while still mechanically ventilated were assigned a value of zero; all patients not requiring mechanical ventilation were assigned a value of 28.
The Institutional Review Boards at Children's Hospital and Research Center at Oakland and the Committee on Human Research at the University of California Medical Center at San Francisco approved the study.
Statistical Analysis
The primary outcome was all-cause mortality in the PICU. Univariate assessment of clinical risk factors associated with mortality was completed using chi-squared and logistic regression analyses. Linear regression was used to test the association of transfusions with the duration of unassisted ventilation. Statistical analyses to evaluate for the presence of interactions between variables were also carried out. All variables with a p value <0.1 and with clinical relevance were included in backward, stepwise multivariate models. Predictor variables for the multivariate analyses included age, sex, ethnicity, diagnosis associated with ALI, medical history, air leak, adjusted exhaled tidal volume, hematologic failure, DIC, thrombocytopenia, neutropenia, red cell, platelet, or FFP transfusions, peak inspiratory pressure, positive endexpiratory pressure, Pao2/Fio2, static respiratory compliance, pH, base excess, mean airway pressure, and presence of organ system failure (2 or more nonpulmonary organ system failures vs. 0–1 organ system failure). The Pao2/Fio2 ratio is presented per 20-point decrease for greater clinical relevance.
A p value of <0.05 was considered statistically significant. Multivariate analyses were confirmed with the Hosmer-Lemeshow goodness of fit test. Statistical analyses were completed using Stata6 software Release 6.0 (Stata Press, College Station, TX).
Results
A total of 328 children were enrolled in the pediatric ALI database. Six patients were excluded from this analysis because they received an exchange transfusion, six were excluded because they had preexisting ALI at an outside hospital before transfer to a study-site hospital, and one patient was excluded for receiving plasmapheresis. Thus, 315 patients were included in the blood transfusion analysis. Of the 315 patients, 154 had a blood product transfusion, either in isolation or in combination with the transfusion of other blood products. One hundred fortyfive patients received red cells, 53 received platelets, and 40 received FFP within 72 hours of the diagnosis of ALI.
The primary diagnoses associated with ALI were pneumonia, aspiration, sepsis, near drowning, cardiac disease, and other (Table 1). Patients who received a blood product transfusion had a greater tendency to have more organ system failure than those who were not transfused (p < 0.01) at the onset of ALI. Blood product transfusions were more frequent in those patients who had sepsis; 88% of patients who had sepsis received a blood product transfusion, compared with 35% of patients who had pneumonia. The mean Pao2/Fio2 at the onset of ALI for those patients who received a blood product transfusion was 159 ± 72, compared with 164 ± 77 for those who were not transfused (p = 0.58).
Table 1. Characteristics of patients with ALI who were transfused with a blood product compared with those who were not transfused.
| Baseline Characteristics | No Transfusions | With Transfusions | p |
|---|---|---|---|
| Number | 163 | 152 | |
| Age in yrs, median (interquartile range) | 3.13 yrs (0–17.7 yrs) | 3.60 yrs (0–17.4 yrs) | 0.54 |
| Male, n (%) | 92 (56) | 86 (57) | |
| Medical history, n (%) | <0.001 | ||
| Premature at birth | 23 (14) | 19 (13) | |
| Genetic or neurologic abnormality | 51 (31) | 27 (18) | |
| Oncologic process | 4 (2) | 22 (14) | |
| History of pulmonary disease | 24 (15) | 9 (6) | |
| Other medical history | 47 (29) | 50 (33) | |
| No significant medical history | 14 (9) | 25 (16) | |
| Ethnicity, n (%) | 0.56 | ||
| White | 66 (40) | 55 (37) | |
| African American | 31 (19) | 31 (21) | |
| Hispanic/Latino | 30 (18) | 28 (19) | |
| Asian | 9 (6) | 15 (10) | |
| Other | 27 (17) | 20 (13) | |
| Diagnosis at ALI onset, n (%) | <0.01 | ||
| Pneumonia | 75 (46) | 40 (26) | |
| Aspiration | 30 (18) | 19 (13) | |
| Sepsis | 5 (3) | 35 (23) | |
| Near drowning | 20 (12) | 8 (5) | |
| Cardiac disease | 11 (7) | 12 (8) | |
| Other | 22 (14) | 38 (25) | |
| Pao2/Fio2 at ALI onset, mean ± sd | 164 ± 77 | 159 ± 72 | 0.58 |
| Mechanically ventilated at onset of ALI, n (%) | 112 (69) | 119 (78) | 0.06 |
| ≥2 nonpulmonary OSF at onset of ALI, n (%) | 32 (20) | 93 (61) | <0.01 |
| Unadjusted PRISM III score at onset of ALI | 8.1 ± 8.0 | 12.7 ± 8.5 | <0.01 |
| Total fluid balance, mean ± sda | 27.7 ± 54.0 | 43.5 ± 70.1 | 0.03 |
| Outcomes | |||
| Ventilator-free days, mean ± sd | 17 ± 11 | 12 ± 10 | <0.01 |
| PICU mortality, n (%) | 28 (17) | 41 (27) | 0.04 |
ALI, acute lung injury; OSF, organ system failure; PRISM, Pediatric Risk of Mortality; PICU, pediatric intensive care unit.
Total fluid in (mL/kg patient weight)—total fluid out (mL/kg patient weight) over the first 72 hrs following the diagnosis of ALI.
The mean fluid balance over the first 72 hours following the diagnosis of ALI was higher in those patients who received a blood product transfusion compared with those who did not (43.5 mL/kg vs. 27.7 mL/kg, respectively; p = 0.03). In addition, the mean fluid balance was higher in those patients who received FFP compared with those who did not (73.4 mL/kg vs. 29.7 mL/kg, respectively; p < 0.001). The transfusion of FFP was weakly correlated with the mean fluid balance (r = 0.17, p = 0.002).
The overall mortality of the patients with ALI in the PICU was 22%. Patients who received a blood product transfusion had a higher PICU mortality than those who were not transfused (27% vs. 17%, respectively, p = 0.04) and fewer ventilator-free days (12 ± 10 vs. 17 ± 11, respectively, p < 0.01). In the parent study, baseline Pao2/Fio2 and nonpulmonary organ system dysfunction were strong independent predictors of patient mortality (8).
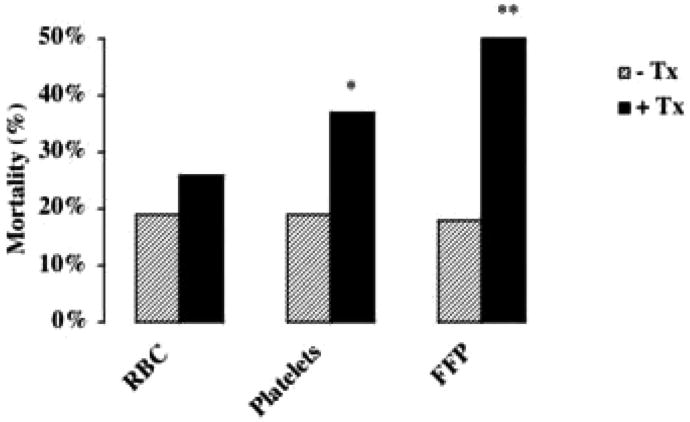
The possible association of mortality with each type of blood product transfused was assessed using the chi-square test. By univariate analysis, transfusions of platelets or FFP were significantly associated with increased mortality. Although there was a trend for increased mortality with red blood cell transfusions, this finding was not statistically significant (Fig. 1).
Figure 1.
The association of blood product transfusion with mortality: univariate analysis. *p < 0.005, **p < 0.001. RBC, red blood cells; FFP, fresh-frozen plasma; Tx, those patients who did not receive the indicated blood product; +Tx, those patients who did receive the indicated blood product.
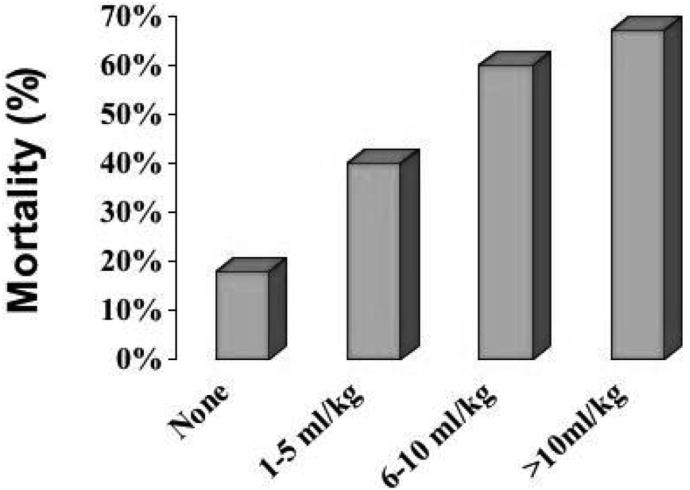
Stepwise, backward logistic regression analyses of the association of red blood cell or platelet transfusions with mortality indicated that neither red blood cell (odds ratio = 1.85, 95% confidence interval = 0.79–4.29, p = 0.15) nor platelet (odds ratio = 1.85, 95% confidence interval = 0.63–5.46, p = 0.26) transfusions were independently associated with mortality. The transfusion of FFP, however, was associated with mortality, independent of the Pao2/Fio2, presence of multiple organ system failure or DIC (odds ratio = 1.08, 95% confidence interval = 1.00–1.18, p = 0.04) (Table 2). FFP transfusion was analyzed as a continuous variable, so that for each milliliter of FFP transfused per kilogram of patient body-weight per day, the odds of death increased by 1.08. On the basis of these results, a dose-response curve was generated for the transfusion of FFP per patient bodyweight in 5 mL increments of FFP (Fig. 2).
Table 2. Fresh-frozen plasma, organ system failure, and mortality: Multivariate analysis.
| Risk Factor | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Fresh-frozen plasmaa | 1.08 | 1.00–1.18 | 0.04 |
| Organ system dysfunction | 10.23 | 4.89–21.34 | <0.001 |
| Pao2/Fio2 per 20-point decrease | 1.12 | 1.03–1.23 | 0.01 |
| Disseminated intravascular coagulation | 0.74 | 0.28–1.90 | 0.53 |
Fresh-frozen plasma is presented per mL/kg weight/24 hrs.
Figure 2.
The association of mortality with the amount of fresh-frozen plasma transfused: univariate analysis. The amount of fresh-frozen plasma transfused is depicted in 5 mL increments per patient body weight (kg).
Stepwise, backward linear regression of FFP transfusion and the duration of unassisted ventilation was also performed. The transfusion of FFP was predictive of fewer days of unassisted ventilation, independent of the Pao2/Fio2, presence of multiple organ system failure, or DIC (r = −.21, 95% confidence interval = − 0.43–0.01,p = 0.06) (Table 3).
Table 3. Fresh-frozen plasma and days of unassisted mechanical ventilation: Multivariate analysis.
| Risk Factor | r | 95% Confidence Interval | p |
|---|---|---|---|
| Fresh-frozen plasma | −.21 | −0.43 to 0.01 | 0.06 |
| Organ system dysfunction | −8.60 | −10.97 to −6.23 | <0.001 |
| Pao2/Fio2 per 20-point decrease | −1.12 | −0.97 to 0.81 | 0.01 |
| Disseminated intravascular coagulation | 0.62 | −3.30 to 4.54 | 0.76 |
Fresh-frozen plasma is presented per mL/kg weight/24 hrs.
In our primary analysis, we used multiple organ system failure to adjust for severity of illness. We did not use the Pediatric Risk of Mortality III (PRISM III) score for several reasons. First, the unadjusted PRISM III score is not validated for the prediction of mortality, and the PRISM III scores of our patients are unadjusted. Second, Pao2 is imbedded within both the diagnostic criteria of ALI and PRISM III score and it would be inappropriate to make a risk adjustment with a metric already included in the definition of ALI. Third, the PRISM III scores were calculated when the patients met criteria for the diagnosis of ALI, and not upon admission to the intensive care unit, for which the score is validated. Sixty-five percent of the patients included in the analysis met criteria for ALI on the day of admission to the PICU, whereas the remaining 35% did not.
In an alternate analysis, we incorporated the PRISM III score (Table 4). In this model, the odds of death per milliliter of FFP transfused was the same as in the previous model (Table 2), and there was a trend toward statistical significance (odds ratio = 1.08, 95% confidence interval = 0.98–1.19, p = 0.09).
Table 4. Fresh-frozen plasma, PRISM III Score, and mortality: Multivariate analysis.
| Risk Factor | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Fresh-frozen plasmaa | 1.08 | 0.98–1.19 | 0.09 |
| PRISM III | 1.19 | 1.13–1.24 | <0.001 |
| Disseminated intravascular coagulation | 0.62 | 0.20–1.88 | 0.40 |
PRISM III, Pediatric Risk of Mortality III.
Fresh-frozen plasma is presented per mL/kg weight/24 hr.
No statistically significant interactions between the transfusions of FFP, red blood cells, or platelets with hematologic failure, DIC, thrombocytopenia, or sepsis were identified. There was also no statistically significant interaction between the transfusion of FFP and oncologic diagnosis.
Discussion
The association between blood product transfusions and the outcomes of pediatric patients with ALI has not been previously analyzed. We hypothesized that blood product transfusions were associated with increased mortality in pediatric patients with ALI/ARDS independent of the severity of illness.
By univariate analysis, there was a trend suggestive of an association between red blood cell transfusions and mortality in pediatric patients with ALI/ARDS, but this finding was not statistically significant. This is consistent with the results of a recent randomized controlled trial of critically ill children stratified to either a liberal or a restrictive red blood cell transfusion strategy that did not find a difference in the development or worsening of organ dysfunction or in 28-day mortality between the two groups (14).
Both platelet and FFP transfusions were significantly associated with increased mortality on univariate analysis. On multivariate analysis, the transfusion of FFP alone was associated with increased mortality, independent of the presenting oxygenation defect as measured by the Pao2/Fio2, or the presence of multiorgan system failure or DIC. In addition, there was a trend for an association of FFP transfusion with prolonged mechanical ventilation, independent of the Pao2/Fio2, presence of multiorgan system failure, or DIC.
The association of FFP transfusion with mortality suggests that there may be factors in the donor plasma that are deleterious to the host. The passive transfusion of donor antibodies (to human leukocyte antigen class I or II antigens, granulocytes, or monocytes), lipids, or cytokines may modify the host response, as hypothesized in transfusion-related acute lung injury (TRALI). TRALI is thought to occur when these donor factors activate recipient neutrophils sequestered in the pulmonary vascular bed, culminating in an injured capillary endothelium, and the fulminant permeability edema characteristic of ARDS (15).
Interestingly, FFP is the most implicated blood product in TRALI-related fatalities (16). A randomized controlled trial of FFP transfusion in adult patients in the intensive care unit suggested that critically ill patients might incur under-recognized forms of lung injury in association with transfusion (17). Although, we do not have direct evidence that the transfusion of FFP in our patients actually worsened underlying ALI, it is interesting to note that the transfusion of FFP was predictive of fewer days of unassisted mechanical ventilation, a pulmonary morbidity.
Although TRALI is the leading cause of transfusion-related fatalities in the United States (18), look-back studies of implicated blood donors indicate that it is significantly under-recognized and under-reported (19). Because TRALI is a clinical diagnosis, defined by the development of ALI/ARDS within 6 hours of the transfusion of a plasma-containing blood product, it is difficult to recognize in patients with preexisting ALI/ARDS, as acknowledged by a recent consensus panel on a working diagnosis of TRALI (20). The pediatric patients with ALI in our study who had worse outcomes in association with transfusion of FFP may represent a population of patients who develop TRALI but remain unrecognized by the current limitations in the definition of TRALI.
In addition to TRALI, fluid overload, bacterial contamination, and transfusion reactions are potential effects associated with transfusion that may account in part for the adverse outcomes in patients who receive a transfusion. Fluid overload is probably an under-recognized effect of transfusions (21) that could exacerbate underlying lung edema in patients with ALI. In our study, the patients who received an FFP transfusion had a higher mean fluid balance than those who did not, and the transfusion of FFP was weakly correlated with the mean fluid balance. The finding that patients who received an FFP transfusion had a more positive mean fluid balance is not wholly unexpected, because FFP transfusions were included in the fluid balance calculations. However, it is also possible that a deleterious effect of FFP transfusion could be mediated by fluid overload.
Hemolytic transfusion reactions and bacterial contamination are potential effects of transfusions that could confound the interpretation of our results. However, the incidence of both of these events is low (22) so that their contribution to our results, if any, would be minimal. Finally, febrile nonhemolytic transfusion reactions and immunomodulatory effects of transfusions are hypothesized to occur primarily due to the coincidental transfusion of host leukocytes (22). As such, neither of these phenomena would explain why FFP, an acellular product, had the strongest association with mortality in the pediatric patients with ALI.
The indications for FFP transfusion given to the pediatric patients in this cohort were varied, given that this was not a controlled trial. Common practice by the pediatric intensivists at our institutions involves the administration of FFP for coagulopathy with or without bleeding, DIC, and volume resuscitation if the patient has been exposed to the unit previously. A recent analysis of transfusion practices in a Canadian teaching hospital suggests that many physicians do not follow expert guidelines regarding the transfusion of FFP; only 32.4% of 225 orders for FFP transfusion were found to be consistent with Canadian guidelines (23). In the critical care setting, FFP is often given for abnormal coagulation studies in the absence of bleeding, hypovolemia, and prophylactically before invasive procedures (24). Furthermore, a recent systematic review of randomized controlled trials of FFP indicates that most of the clinical guidelines for transfusion of FFP are not supported by scientific evidence (25).
The finding that the transfusion of FFP is associated with worse outcomes in pediatric patients with ALI underscores the need for more rigorous evaluation of appropriate indications for FFP in critically ill children. It would be helpful if blood banks mandated that ordering physicians state the indication for transfusion of FFP so that look-back studies evaluating outcomes of transfusion based on indication could be done.
A potential limitation of our study is that it is a retrospective analysis of a prospectively gathered database. However, the variables were defined and recorded before the analysis began, limiting the likelihood of selection bias or sampling error. It is also possible that the blood transfusions were a surrogate for the patients' underlying severity of illness. We attempted to correct for this by the inclusion of multiple organ system dysfunction in the multivariate analysis as an assessment of severity of illness, but recognize that this may not capture more subtle differences among patients. The PRISM III score, a recognized marker of pediatric critical illness severity, was not used in this study because it is not validated for the day of diagnosis of ALI. Another potential limitation is that the analyses of the individual blood products included patients who may have received more than one type of blood product, complicating our efforts to determine the effects of transfusion of one blood product in isolation. Our inclusion of these patients does, however, reflect real-world practice, as many critically ill children receive transfusions with more than one type of blood product. Finally, the observational study design dictates that while we can determine associations between blood product transfusions and the outcomes of pediatric patients with ALI, we cannot infer causal relationships when associations are found.
Conclusions
The transfusion of FFP is associated with an increased risk for mortality in pediatric patients with ALI. The previously undocumented association between FFP and mortality in children with ALI needs to be investigated further.
Acknowledgments
We acknowledge the Neonatal Pediatric Research Group for their assistance in data collection.
Supported, in part, by the Children's Hospital and Research Center at Oakland's Pediatric Clinical Research Center, NIH RR 1271, and by NHBLI P50HL74005 and RO1 HL51856. Dr. Flori is funded by NIH RR 15543. Dr. Liu is funded by NIH Roadmap for Medical Research 8 K12 RR023262. Dr. Matthay is funded by R01 HL 51856. The funding sources had no involvement in the study design, data collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
The authors have not disclosed any potential conflicts of interest.
For information regarding this article, ChurchG@peds.ucsf.edu
Contributor Information
Gwynne D. Church, Department of Pediatrics, University of California, San Francisco, CA.
Michael A. Matthay, Department of Medicine and Anesthesia and the Cardiovascular Research Institute, University of California, San Francisco, CA.
Kathleen Liu, Department of Medicine, University of California, San Francisco, CA.
Meredith Milet, Department of Translational Research, Children's Hospital and Research Center Oakland, Oakland, CA.
Heidi R. Flori, Department of Pediatric Critical Care, Children's Hospital and Research Center Oakland, Oakland, CA.
References
- 1.Pepe PE, Potkin RT, Reus DH, et al. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144:124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 2.Hudson LD, Milberg JA, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 3.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 4.Ng Gong M, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: Potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 5.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131:1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- 6.Gajic O, Rana R, Mendez JL, et al. Acute lung injury after blood transfusion in mechanically ventilated patients. Transfusion. 2004;44:1468–1474. doi: 10.1111/j.1537-2995.2004.04053.x. [DOI] [PubMed] [Google Scholar]
- 7.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 8.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 9.Davis S, Furman D, Costarino A. Adult respiratory distress syndrome in children: Associated disease, clinical course, and predictors of death. J Pediatr. 1993;123:35–45. doi: 10.1016/s0022-3476(05)81534-3. [DOI] [PubMed] [Google Scholar]
- 10.Proulx F, Gauthier M, Nadeau D, et al. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;226:1025–1031. doi: 10.1097/00003246-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Suchyta M, Clemmer T, Orme J, et al. Increased survival of ARDS patients with severe hypoxemia. Chest. 1991;99:51–55. doi: 10.1378/chest.99.4.951. [DOI] [PubMed] [Google Scholar]
- 12.Schnapp L, Chin D, Szaflarski N, et al. Frequency and importance of barotrauma in 100 patients with acute lung injury. Crit Care Med. 1995;232:272–278. doi: 10.1097/00003246-199502000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Network TA. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 15.Looney MR, Gropper MA, Matthay MA, et al. Transfusion-related acute lung injury: A review. Chest. 2004;126:249–258. doi: 10.1378/chest.126.1.249. [DOI] [PubMed] [Google Scholar]
- 16.Holness L, Knippen MA, Simmons L, et al. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–188. doi: 10.1016/j.tmrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Palfi M, Berg S, Ernerudh J, et al. A randomized controlled trial of transfusion-related acute lung injury: Is plasma from multiparous blood donors dangerous? Transfusion. 2001;41:317–322. doi: 10.1046/j.1537-2995.2001.41030317.x. [DOI] [PubMed] [Google Scholar]
- 18.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion- acute lung injury: Statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 19.Kopko PM, Marshall CS, Mackenzie MR, et al. Transfusion-related acute lung injury: Report of a clinical look-back investigation. JAMA. 2002;287:1968–1971. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 20.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: Definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 21.Popovsky MA. Breathlessness and blood: A combustible combination. Vox Sang. 2002;83:147–150. doi: 10.1111/j.1423-0410.2002.tb05290.x. [DOI] [PubMed] [Google Scholar]
- 22.Goodnough LT. Risks of blood transfusion. Crit Care Med. 2003;31:678–686. doi: 10.1097/01.CCM.0000100124.50579.D9. [DOI] [PubMed] [Google Scholar]
- 23.Lauzier F, Cook D, Griffith L, et al. Fresh frozen plasma transfusion in critically ill patients. Crit Care Med. 2007;35:1655–1659. doi: 10.1097/01.CCM.0000269370.59214.97. [DOI] [PubMed] [Google Scholar]
- 24.Gajic O, Dzik WH, Toy P. Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: Benefit or harm? Crit Care Med. 2006;34:S170–S173. doi: 10.1097/01.CCM.0000214288.88308.26. [DOI] [PubMed] [Google Scholar]
- 25.Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–152. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]