Abstract
RATIONALE
Tobacco smoke contains nicotine and many other compounds that act in concert on the brain reward system. Therefore, animal models are needed that allow the investigation of chronic exposure to the full spectrum of tobacco smoke constituents.
OBJECTIVES
The aim of these studies was to investigate if exposure to tobacco smoke leads to nicotine dependence in rats.
METHODS
The intracranial self-stimulation procedure was used to assess the negative affective aspects of nicotine withdrawal. Somatic signs were recorded from a checklist of nicotine abstinence signs. Nicotine self-administration sessions were conducted to investigate if tobacco smoke exposure affects the motivation to self-administer nicotine. Nicotinic receptor autoradiography was used to investigate if exposure to tobacco smoke affects central α7 nicotinic acetylcholine receptor (nAChR) and non-α7 nAChR levels (primarily α4β2 nAChRs).
RESULTS
The nAChR antagonist mecamylamine dose-dependently elevated the brain reward thresholds of the rats exposed to tobacco smoke and did not affect the brain reward thresholds of the untreated control rats. Furthermore, mecamylamine induced more somatic withdrawal signs in the smoke exposed rats than in the control rats. Nicotine self-administration was decreased 1 day after the last tobacco smoke exposure sessions and was returned to control levels 5 days later. Tobacco smoke exposure increased the α7 nAChR density in the CA2/3 area and the stratum oriens and increased the non-α7 nAChR density in the dentate gyrus.
CONCLUSION
Tobacco smoke exposure leads to nicotine dependence as indicated by precipitated affective and somatic withdrawal signs and induces an upregulation of nAChRs in the hippocampus.
Keywords: Tobacco, nicotine, dependence, withdrawal, rats
INTRODUCTION
Tobacco addiction is a chronic disorder that is characterized by a loss of control over smoking, the appearance of withdrawal symptoms upon smoking cessation, and relapse after periods of abstinence (American Psychiatric Association 2000). During the last decades, tobacco addiction research has focused on the role of nicotine in tobacco addiction. It has been suggested that nicotine is one of the main components of tobacco smoke that leads to and maintains smoking (Bardo et al. 1999; Crooks and Dwoskin 1997; Stolerman and Jarvis 1995). The positive reinforcing effects of nicotine (e.g., mild eurphoria) are at least partly mediated by the activation of neuronal nicotinic acetylcholine receptors (nAChRs). The nAChRs are ligand-gated ion channels and are composed of 5 subunits (α2–10 or β2–4)(Mineur and Picciotto 2008). Smoking leads to an increase in [3H]-nicotine and [3H]-epibatidine binding in the human brain (Benwell et al. 1988; Perry et al. 1999). Furthermore, exposure to tobacco smoke increases central [3H]-nicotine binding in rats (Yates et al. 1995). Increased nicotine binding is indicative of an increase in α4β2 nAChRs and an increase in epibatidine binding is indicative of an upregulation of a wide variety of nAChRs with the exception of the α 7 nAChRs (Houghtling et al. 1995; Zoli et al. 1998). At this point in time, there is no evidence that smoking leads to an upregulation of α7 nAChRs in humans (Breese et al. 2000). However, it has been reported that chronic nicotine administration increases α 7 nAChR levels in mice and rats (Marks et al. 1983; Pauly et al. 1991; Rasmussen and Perry 2006).
Rats readily acquire nicotine self-administration in operant conditioning chambers and blockade of nAChRs with non-selective nAChRs antagonists decreases the self-administration of nicotine (Corrigall et al. 1994; Corrigall and Coen 1989; Donny et al. 1999; Watkins et al. 1999). Discontinuation of chronic nicotine administration or the administration of nAChR antagonists to nicotine dependent rats leads to a deficit in brain reward function and somatic withdrawal signs (Bruijnzeel and Markou 2004; Epping-Jordan et al. 1998; Harrison et al. 2001). Preclinical research suggests that chronic subcutaneous administration of nicotine (3.2 mg/kg/day of nicotine base) rapidly leads to the development of nicotine dependence in rats. The nAChR antagonists dihydro-beta-erythroidine and mecamylamine have been shown to precipitate affective and somatic withdrawal signs in rats 6 days after the onset of nicotine administration (Bruijnzeel et al. 2007; Epping-Jordan et al. 1998). Although the aforementioned studies indicate that nicotine has positive and negative reinforcing properties, accumulating evidence suggests that there are other compounds in tobacco smoke that contribute to the tobacco addiction. 1) Nicotine and partial nAChR agonists only slightly improve smoking cessation rates (Eisenberg et al. 2008). 2) Nicotine by itself is not abused in humans and the majority of smokers do not prefer a nicotine-spray above placebo (Perkins et al. 1997). 3) There are compounds in tobacco smoke that may act in concert with nicotine to potentiate brain reward function (Fowler et al. 2003; Talhout et al. 2007). Acetaldehyde is one of the compounds in tobacco smoke that may contribute to the development of a tobacco addiction. Acetaldehyde is self-administered by rodents and induces conditioned place preference (Brown et al. 1979; Myers et al. 1982; Smith et al. 1984). Furthermore, acetaldehyde potentiates the positive reinforcing effects of nicotine (Belluzzi et al. 2005). Tobacco smoke also contains high concentrations of norharman and harman, which inhibit MAO-A and MAO-B and have antidepressant-like effects in rodents (Aricioglu and Altunbas 2003; Farzin and Mansouri 2006; Herraiz and Chaparro 2005; Totsuka et al. 1999). Positron emission tomography (PET) studies indicate that smoking inhibits MAO-A and MAO-B in the human brain (Fowler et al. 1996; Fowler et al. 1998). In order to develop novel pharmacotherapies for tobacco addiction, animal models are needed that allow the investigation of the long-term effects of exposure to all addictive compounds in tobacco smoke.
At this point in time very little is known about the effects of tobacco smoke exposure on the brain cholinergic systems. The aim of these experiments was to investigate if exposure to tobacco smoke leads to the development of nicotine dependence in rats. The rats were chronically exposed to tobacco smoke in order to allow the development of nicotine dependence and the development of tolerance to the aversive effects of nicotine (Foulds et al. 1997; Okoli et al. 2007). The first experiment investigated the effect of the nAChR antagonist mecamylamine on brain reward function in tobacco smoke exposed rats and control rats by using a discrete trial intracranial self-stimulation (ICSS) procedure. This procedure was used as it provides a quantitative measure of the emotional aspects of drug withdrawal (Bruijnzeel et al. 2006; Schulteis et al. 1995; Wise and Munn 1995). The second experiment investigated the effect of tobacco smoke exposure on nicotine self-administration. The third experiment investigated the effect of exposure to tobacco smoke on operant responding for food pellets. In the fourth experiment, quantitative nAChR autoradiography was used to investigate if chronic exposure to tobacco smoke affects [125I]-α-bungarotoxin (α7 nAChRs) and [125I]-epibatidine (non-α7 nAChRs) binding in the brain (Houghtling et al. 1995; McGehee and Role 1995). This experiment was conducted because the upregulation of nAChRs is a hallmark feature of the development of nicotine dependence (Dani and Heinemann 1996). The receptor binding studies focused on the hippocampus and cortex as previous research suggests that these brain areas are most sensitive to smoke and nicotine-induced changes in nAChR levels (Benwell et al. 1988; Pauly et al. 1991).
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles River, Raleigh, NC) weighing 250–300 gram at the beginning of the experiments were used. Animals were single-housed in a temperature and humidity-controlled vivarium and maintained on a 12 hour light-dark cycle (lights off at 6 PM). All testing occurred at the end of the light cycle. Food and water were available ad libitum in the home cages. All subjects were treated in accordance with the National Institutes of Health guidelines regarding the principles of animal care. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) and approved by the University of Florida Institutional Animal Care and Use Committee.
Drugs
Nicotine hydrogen tartrate salt, mecamylamine hydrochloride, and pentobarbital sodium salt were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA) and dissolved in sterile saline (0.9% sodium chloride). Research cigarettes (3R4F) were purchased from the University of Kentucky, College of Agriculture, Reference Cigarette Program.
Surgical Procedures
For experiment 1, the rats were prepared with an 11 mm electrode in the medial forebrain bundle as described previously (Bruijnzeel et al. 2007). The rats were anesthetized with an isoflurane/oxygen vapor mixture and placed in a stereotaxic frame with the incisor bar set 5.0 mm above the interaural line. The electrodes were implanted in the medial forebrain bundle by using the following coordinates: anterior posterior (AP) −0.5 mm, medial lateral (ML) ±1.7 mm, dorsal ventral (DV) −8.3 mm from dura. For experiment 2, the rats were prepared with a chronic catheter in the right jugular vein as described previously (Zislis et al. 2007). At the beginning of the surgery the rats were anesthetized with an isoflurane/oxygen vapor mixture. The catheters consisted of silastic tubing (length 13.5 cm, 0.51 mm inside diameter × 0.94 mm outside diameter, Dow Corning, Midland, MI) that was connected to an 22 gauge stainless steel guide cannula, which was molded onto a durable polyester fiber mesh (Plastics One, Roanoke, VA). The tubing was passed subcutaneously from the mid scapular region to the ventral thorax/lower part of the neck, inserted into the jugular vein (4.0 cm), and secured with silk suture thread. After the implantation of the catheters, the animals were allowed to recover for 7 days.
Apparatus
Food training and drug self-administration sessions were conducted in twelve operant conditioning chambers that were located inside sound-attenuating chambers (Med Associates, St. Albans, VT). One side of the operant conditioning chambers was equipped with an active and an inactive lever, and above each lever was a cue light. Data collection and test sessions were controlled by a microcomputer. Delivery of the nicotine solution was controlled by a syringe pump (Model A, Razel Scientific Instruments, Stamford, CT). Tygon tubing (0.508 mm inside diameter × 1.524 mm outside diameter, Saint-Gobain Performance Plastics, Valley Forge, PA) connected a 10 ml syringe, which was placed in the pump, to the backmount of the rat. A protective metal spring covered the tubing. One side of the spring was connected to a stainless steel swivel (Instech Laboratories, Plymouth Meeting, PA) and the other side of the spring was connected to the backmount.
Experimental procedures
Tobacco smoke exposure
The rats were exposed to tobacco smoke in their home cages (whole body exposure) and the rats were not restrained during the tobacco smoke exposure sessions. Four cages could be exposed to tobacco smoke simultaneously. Tobacco smoke was generated using a microprocessor-controlled cigarette smoking-machine (model TE-10, Teague Enterprises, Davis, CA), originally described by Teague and colleagues (Teague et al. 1994). Tobacco smoke was generated by burning filtered Kentucky 3R4F reference cigarettes(Reference Cigarette Program, University of Kentucky, Lexington, KY) using a standardized smoking procedure (35 cm3 puff volume, 1 puff per minute, 2 seconds per puff). The smoking machine produced a mixture of approximately 10% mainstream smoke and 90% sidestream smoke. Exposure conditions were monitored for carbon monoxide (CO) and total suspended particulate matter. CO levels were assessed using a continuous CO analyzer that accurately measures CO levels between 0 – 2000 parts per million (Monoxor II, Bacharach, New Kensington, PA USA). Total suspended particle matter in the exposure chambers was determined by measurement of samples collected from the chamber onto pre-weighed filters. Most of the food was removed from the home cages immediately prior to the tobacco smoke exposure sessions and returned after the tobacco smoke exposure sessions. A few food pellets were left in the cage during the tobacco smoke exposure sessions and these pellets were disposed immediately after the tobacco smoke exposure sessions. Water was freely available during the smoke exposure sessions.
Food training
Prior to the onset of food training, the rats were food deprived for 48 hours (5 gram lab chow/day). After the onset of food training, the rats were fed 17–20 gram (80–95% of baseline ad libitum calories) of lab chow per day, at least 1 hour after the end of the food training session. During the first training session, the rats received one 45-mg chocolate-flavored food pellet (Bio-Serv, Frenchtown, NJ) every 20 seconds for 30 minutes with no requirement to respond on the active lever. After this session, the rats had to respond on the active lever to receive food pellets. Instrumental training started on a fixed-ratio 1, time-out 1 second (FR1 TO1-s) schedule of reinforcement and the training sessions lasted 1 hour. The training schedule was progressively changed according to the following sequence: FR1 TO1, FR1 TO10, FR1 TO20, FR2 TO20, FR5 TO20-s. The rats had to reach the criterion of 100 pellets earned during a daily 1 hour session before training at the next level started. Food training continued until the subjects earned 100 food pellets in a daily 1-hour session on an FR5 TO20-s schedule of reinforcement. Food training typically required 7–9 days. After the completion of the food training sessions, all rats were fed 20 gram of lab chow per day (95% of baseline ad libitum calories) 1 hour after the end of testing.
Nicotine self-administration
After successful completion of food training and catherization surgeries, the rats were allowed to self-administer nicotine at the 0.03 mg/kg/infusion (base) dose by switching the delivery of a food pellet for the delivery of a nicotine infusion as described previously (Bruijnzeel and Markou 2003; Zislis et al. 2007). The operant conditioning chambers were equipped with two retractable levers. Responding on the active lever resulted in the delivery of a nicotine infusion and responding on the inactive lever was recorded but had no scheduled consequences. The delivery of an infusion (0.1 ml/infusion over a 5.6 second time-period) was earned by responding five times on the active lever (FR5 TO20-s). The initiation of the delivery of an infusion was paired with a cue light, which remained illuminated throughout the time-out period (initiated simultaneously with the initiation of delivery of a nicotine infusion). The active lever was retracted during the time-out period.
Intracranial self-stimulation procedure
Rats were trained on a modified discrete-trial ICSS procedure (Kornetsky and Esposito 1979), as described previously (Markou and Koob 1992). The twelve operant conditioning chambers were all housed in sound-attenuating chambers (Med Associates, Georgia, VT). The subjects were trained to turn the wheel on a FR1 schedule of reinforcement. Each quarter turn of the wheel resulted in the delivery of a 0.5 second train of 0.1 millisecond cathodal square-wave pulses at a frequency of 100 Hz. After the successful acquisition of responding for stimulation on this FR1 schedule, defined as 100 reinforcements within 10 minutes, the rats were trained gradually on a discrete-trial current-threshold procedure. Each trial began with the delivery of a non-contingent electrical stimulus, followed by a 7.5 second response window during which the animal can respond to receive a second contingent stimulus that is identical to the initial non-contingent stimulus. A response during this 7.5 second response window was labeled a positive response, while the lack of a response was labeled a negative response. During a 2 second period immediately after a positive response, additional responses had no consequences. The inter-trial interval (ITI) that followed either a positive response or the end of the response window (in the case of a negative response), had an average duration of 10 seconds (ranging from 7.5 to 12.5 seconds). Responses that occurred during the ITI resulted in a further 12.5 second delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the ITI and delay periods induced by time-out responses were gradually increased until animals performed consistently at standard test parameters. The subjects were subsequently tested on the current-threshold procedure in which stimulation intensities varied according to the classical psychophysical method of limits. A test session consisted of four alternating series of descending and ascending current intensities starting with a descending series. Blocks of three trials were presented to the subject at a given stimulation intensity, and the intensity was altered systematically between blocks of trials by 5 μA steps. The initial stimulus intensity was set 40 μA above the baseline current-threshold for each animal. Each test session typically lasted 30–40 minutes and provided two dependent variables for behavioral assessment: brain reward thresholds and response latencies.
Threshold
The current threshold for a descending series was defined as the midpoint between stimulation intensities that supported responding (i.e., positive responses on at least two of the three trials), and current intensities that failed to support responding. The threshold for an ascending series was defined as the midpoint between stimulation intensities that did not support responding and current intensities that supported responding for two consecutive blocks of trials. Thus, four threshold estimates were recorded, and the mean of these values was taken as the threshold for each subject on each test session.
Response Latency
The time interval between the beginning of the non-contingent stimulus and a positive response was recorded as the response latency. The response latency for each test session was defined as the mean response latency on all trials during which a positive response occurred.
Somatic withdrawal signs
Rats were observed for 10 minutes in Plexiglas observation chambers (25.4 × 25.4 × 45.7; L x W x H) as described previously (Cryan et al. 2003; Rylkova et al. 2008). Prior to the test sessions, the rats were habituated to the observation chambers by placing them in the chambers for 5 minutes on two consecutive days. During the test sessions the rats were observed blindly by an experienced observer for 10 minutes and the frequency of the following signs was recorded based on a checklist of nicotine abstinence signs: body shakes, chews, cheek tremors, escape attempts, eye blinks, foot licks, gasps, genital licks, head shakes, ptosis, scratches, teeth chattering, writhes and yawns (Malin et al. 1992). Multiple successive counts of any sign required a distinct pause between episodes. Ptosis, if present continuously, was counted once per minute.
Plasma nicotine and cotinine levels
A validated high-performance liquid chromatography-tandem mass spectrometry (HPLC/MS/MS) method was used to determine plasma nicotine and cotinine levels. Plasma proteins, which could interfere with the HPLC/MS/MS analysis, were precipitated by adding 150 μL methanol to 100 μL plasma. This mixture was vortexed for 30 seconds and then centrifuged at 1500 g for 15 minutes. The clear supernatant (100 μL) was carefully transferred into series 200 Perkin Elmer auto sampler vials for HPLC/MS/MS analysis. Nicotine and cotinine were separated by reversed phase chromatography using a Prodigy 5u, 100 × 4.6 mm, C18 column (Phenomenex, Torrance, CA) that was fitted with a C18 pre-column and an isocratic mobile phase composed of 10 mM ammonium acetate buffer in 75% methanol delivered at 1 mL/min by a series 200 Perkin Elmer HPLC pump (Waltham, MA). The injection volume was 10 μL and the chromatographic run time was 4 minutes. The column eluent was directed to the mass spectrometer by atmospheric pressure ionization (API) source. The mass spectrometer (API 4000 LC-MS-MS system, Applied Biosystems/MDS SCIEX, Foster City, CA) was operated in electro spray positive ion mode (ESI+) and quantitation was performed using multiple-reaction monitoring (MRM). The MRM transitions that were used for the quantification of nicotine and cotinine were m/z 163.1 > 132.0 and m/z 177.1 > 146.1, respectively. High purity nitrogen was used as curtain and collision gas and zero grade air was used as the source gas. The API source was operated at 300°C and the ion spray voltage was set at 5 kV. Data acquisition and quantitation were performed using Analyst software version 1.4.2 (Applied Biosystems/MDS SCIEX, Foster City, CA). During the sample analyses quality control samples were interspaced with test samples to ensure the accuracy and reliability of the assay procedure.
Nicotinic receptor autoradiography
The animals were euthanized by decapitation and then the brains were removed and frozen in isopentane that was chilled in dry ice to − 30 °C. The brains were stored at −70 °C until further processing. Brains were sliced using a cryostat (Lecia CM1850, Nussloch, Germany) to make a series of 16-μm thick sections, which were mounted onto gelatin, chromium potassium sulfate, and poly-L-lysine coated slides. Alpha7 nAChRs were measured using [125I]-α-bungarotoxin autoradiography, as previously described (Sparks and Pauly 1999). A ligand concentration of 2.5 nmol [125I]Tyr54-α-bungarotoxin (specific activity 102.9 Ci/mmol; Perkin-Elmer Life Sciences, Boston, MA) was used for section incubations. Non-α7 nAChR density was assessed using [125I]-epibatidine autoradiography (100 nM incubation concentration, specific activity 2200 Ci/mmol; Perkin-Elmer Life Sciences, Boston, MA)(Perry and Kellar 1995). RayMax Beta High Performance Autoradiography Film (ICN Biomedicals, Aurora, Ohio) was used to visualize the areas of ligand binding. Radioactive rat brain tissue standards were included with each film X-ray cassette in order to determine the response of the film to the increasing amounts of radioactivity. Exposure time was optimized for each ligand: 7 days for [125I]-α-bungarotoxin and 3 days for [125I]-epibatidine. All films were processed using Kodak D-19 developer. Binding data were analyzed using NIH image v1.59 on a Power Macintosh connected to a Sony XC-77 CCD camera via a Scion LG-3 frame-grabber. Molar quantities of bound ligand were determined by constructing a standard curve from radioactivity tissue standards fitted to a third degree polynomial.
Experimental Design
Experiment 1: Tobacco smoke exposure and intracranial self-stimulation
After recovery from the electrode implantations, the rats were trained on the ICSS procedure (See Figure 1 for a schematic overview). The tobacco smoke exposure sessions started when stable baseline brain reward thresholds were achieved (defined as less than 10% variation within a 5 day period). Half of the rats were exposed to tobacco smoke (n = 10) and the other rats (n = 10) were placed on a cart in the laboratory during the tobacco smoke exposure sessions. Rats were exposed to tobacco smoke for 4 hours per day for 28 consecutive days. The rats were continuously exposed to tobacco smoke during the tobacco smoke exposure sessions. During the first week, the rats were gradually introduced to tobacco smoke. The exposure duration was gradually increased according to the following sequence: Day 1–2, 1 cigarette at time for 1 hour (5 cigarettes per hour); Day 3–4, 2 cigarettes at a time for 2 hours (10 cigarettes per hour); Day 5 and onward, 4 cigarettes at at time for 4 hours (20 cigarettes per hour). From week 2 to 4, the average total suspended particulate matter and CO levels were 111 ± 5 mg/m3 and 402 ± 23 ppm, respectively. Tobacco smoke exposure conditions were based on previous studies by Pinkerton and colleagues (Smith et al. 2005; Zhong et al. 2005). Tobacco smoke exposure sessions were conducted between 1:00 PM and 6:00 PM. Brain reward thresholds and response latencies were assessed immediately prior to the tobacco smoke exposure sessions from day 1 – 19. ICSS parameters were assessed immediately prior to the tobacco smoke exposure session and immediately after the tobacco smoke exposure session on day 20. The ICSS parameters were assessed immediately after the rats were exposed to tobacco smoke from day 21 – 28. The nAChR antagonist mecamylamine was used to investigate the effects of precipitated withdrawal on brain reward thresholds and response latencies. Mecamylamine (1, 3 mg/kg, sc) was administered 5 minutes before the rats were placed in the ICSS test chambers. There was a 48-hour interval between each mecamylamine injection. This time interval allowed the reestablishment/maintenance of nicotine dependence. The serum elimination half-life of mecamylamine is approximately 1 hour (Debruyne et al. 2003).
Figure 1.
Experimental protocols for experiment 1–4. The thick black lines indicate tobacco smoke exposure. Abbreviations: ICSS, intracranial self-stimulation; Food, food responding; IVSA, intravenous self-administration; with, withdrawal.
Experiment 2: Tobacco smoke exposure and nicotine self-administration
Rats were trained to respond for food pellets in operant conditioning chambers and after the response requirements were met they were prepared with chronic intravenous catheters. After at least one week of recovery, the rats were allowed to self-administer nicotine for 8 consecutive days under a FR5 TO20-s schedule of reinforcement (1-hour sessions). Then about one half of the rats were exposed to tobacco smoke (n = 8) and the other rats (n = 10) were placed on a cart in the laboratory during the tobacco smoke exposure sessions. Tobacco smoke exposure sessions were conducted as described under experiment 1. Rats were exposed to tobacco smoke for 4 hours per day for 28 consecutive days with the exception of day 22 during which nicotine self-administration was investigated. The average total suspended particulate matter and CO levels were 96 ± 6 mg/m3 and 389 ± 24 ppm, respectively (week 1 not included). Blood samples (500 μl) were collected via the intravenous catheter on day 12, 20, and 28 immediately after exposure to tobacco smoke. Thirteen days after the onset of the tobacco smoke exposure regimen it was investigated if the rats were nicotine dependent. The nicotinic acetylcholine (nAChR) receptor antagonist mecamylamine (1 mg/kg, sc) was administered 5 minutes before the behavioral observations. The rats were observed for 10 minutes in Plexiglas observation chambers. In order to investigate if exposure to tobacco smoke affected nicotine self-administration, the rats were allowed to self-administer nicotine for 3 hours on day 22 (3-weeks of smoke exposure) and for 3 hours on day 29 (4-weeks of smoke exposure). These nicotine self-administration sessions were conducted 24 hours after the rats were exposed to tobacco smoke. In order to investigate the long-term effects of tobacco smoke exposure on nicotine self-administration, the rats were also allowed to self-administer nicotine for 3 hours 5 days after the last tobacco smoke exposure session (day 33). The rats did not self-administer nicotine on day 2 – 4 post tobacco smoke exposure.
An additional experiment was conducted in order to rule out the possibility that plasma nicotine levels in the rats remained elevated after the tobacco smoke exposure sessions due to the ingestion of tobacco residues from their coat, bedding material, or cages. In a separate group of rats (n = 6), blood samples (500 μl) were collected via an intravenous catheter 0, 24, 48, and 72 hours after exposure to tobacco smoke. The rats were exposed to tobacco smoke for 6 days as described under experiment 1 and blood collections started immediately after the last tobacco smoke exposure session (Day 6; total suspended particulate matter 100 mg/m3, CO level 355 ppm).
Experiment 3: Tobacco smoke exposure and food responding
Rats (n = 20) were allowed to respond for chocolate-flavored food pellets under a FR5 TO20-s schedule of reinforcement for 1 week. Then half of the rats were exposed to tobacco smoke (n = 10) for 32 days and the other rats (n = 10) were placed on a cart in the laboratory during the smoke exposure sessions. The rats were exposed to tobacco smoke for 4 hours per day for 32 consecutive days. Tobacco smoke exposure sessions were conducted as described under experiment 1. From day 8 to 32, the average total suspended particulate matter and CO levels were 105 ± 3 mg/m3 and 493 ± 14 ppm, respectively. Food responding was investigated on day 22 (24 hours after tobacco smoke exposure) and on day 23 (40 minutes after tobacco smoke exposure). Food responding was again investigated on day 29 (24 hours after tobacco smoke exposure) and day 30 (40 minutes after tobacco smoke exposure). Then the rats were exposed to tobacco smoke for an additional 2 days and the long-term effects of tobacco smoke exposure on food responding were investigated (day 33 – 39). During this period the rats were not exposed to tobacco smoke and food responding was assessed for 7 days.
Experiment 4: Tobacco smoke exposure and [125I]-epibatidine and [125I]-α-bungarotoxin binding
One half of the rats were exposed to tobacco smoke (n = 6) and the other rats (n = 6) were placed on a cart in the laboratory during the tobacco smoke exposure sessions. Tobacco smoke exposure sessions were conducted as described under experiment 1. During the first week the rats were gradually introduced to tobacco smoke. During the second and the third week the average total suspended particulate matter and CO levels were 147 ± 4 mg/m3 and 271 ± 13 ppm, respectively. In order to investigate if tobacco smoke exposure leads to the development of nicotine dependence, the rats were injected with mecamylamine (1 mg/kg) on day 16 and the number of somatic signs was recorded. The rats were decapitated 4–6 hours after the last tobacco smoke exposure session on day 21 and the brains were removed and frozen.
Statistical analyses
Body weights (experiment 1–4) and ICSS parameters (brain reward thresholds and response latencies, experiment 1) over the course of the tobacco smoke exposure period were expressed as percentages of the values obtained on the day prior to the onset of tobacco smoke exposure. The body weights and ICSS parameters over the course of the tobacco smoke exposure period were analyzed by two-way repeated measures analysis of variance (ANOVA) with time (days of tobacco smoke exposure) as the within subjects factor and treatment (control or tobacco smoke) as the between subjects factor. The effect of mecamylamine on ICSS parameters (expressed as pre-tobacco smoke exposure values) was analyzed using two-way repeated-measures ANOVA with the dose of mecamylamine as the within subjects factor and treatment (control or tobacco smoke) as the between subjects factor. The effect of acute tobacco smoke exposure on brain reward thresholds and response latencies (experiment 1) in the chronically tobacco smoke exposed rats was analyzed using a one-way repeated measures ANOVA. The effects of mecamylamine on somatic signs (experiments 2 and 4) in the tobacco smoke exposed rats and the control rats were analyzed using the non-parametric Kruskal-Wallis test. The effects of tobacco smoke exposure on nicotine self-administration (experiment 2) and responding for food pellets (experiment 3) was analyzed by one-way ANOVA with treatment (control or smoke) as the between subjects factor. The effect of tobacco smoke exposure on [125I]-epibatidine and [125I]-α-bungarotoxin binding (experiment 4) was analyzed by one-way ANOVA with treatment (control or smoke) as the between subjects factor. For all the experiments, statistically significant interactions in the ANOVA were followed by the Newman-Keuls post hoc test. Statistical analyses were performed using SPSS for Windows version 16.0.
RESULTS
Experiment 1: Tobacco smoke exposure and intracranial self-stimulation
There were no differences in body weights between the tobacco smoke exposed rats and the control rats prior to the onset of tobacco smoke exposure [Table 1; t(18)=0.69, n.s.]. Exposure to tobacco smoke decreased body weight gain during the 28-day exposure period (Table 1; Time x Treatment: F27,486=112.02, P<0.0001).
Table 1.
Effect of tobacco smoke exposure on absolute body weights.
| Experiment | Pre | Post | ||
|---|---|---|---|---|
|
| ||||
| Control (g) | Tobacco | (g) Control (g) | Tobacco (g) | |
| Expt. 1 (28-days, n=10/group) | 465 ± 15 | 477 ± 8 | 545 ± 17 | 484 ± 9** |
| Expt. 2 (21-days, n=8–10/group) | 348 ± 5 | 354 ± 8 | 414 ± 6 | 382 ± 9** |
| Expt. 3 (32-days, n=10/group) | 282 ± 3 | 282 ± 3 | 407 ± 5 | 367 ± 9** |
| Expt. 4 (21-days, n=6/group) | 269 ± 7 | 268 ± 3 | 420 ± 17 | 354 ± 9** |
Asterisks (**P<0.01) indicate lower body weights compared to the control group. Data are expressed as means (± S.E.M.).
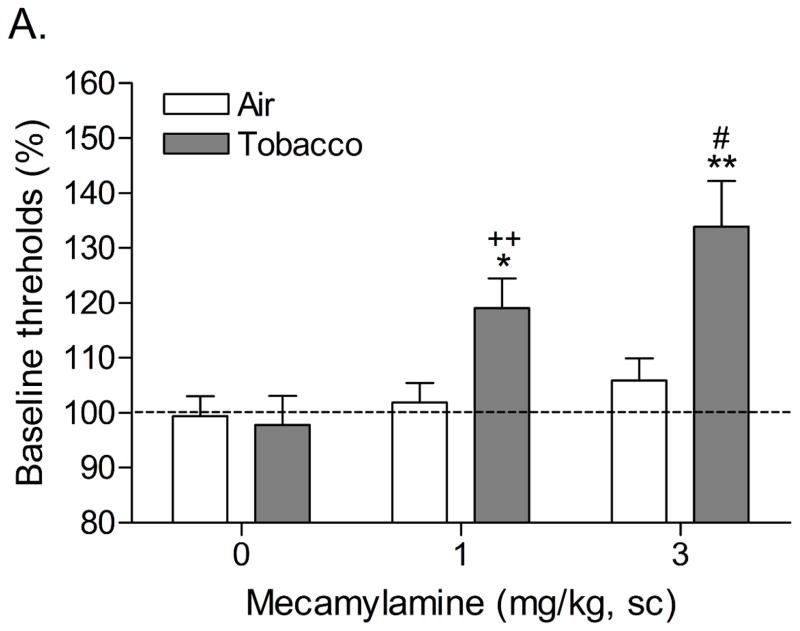
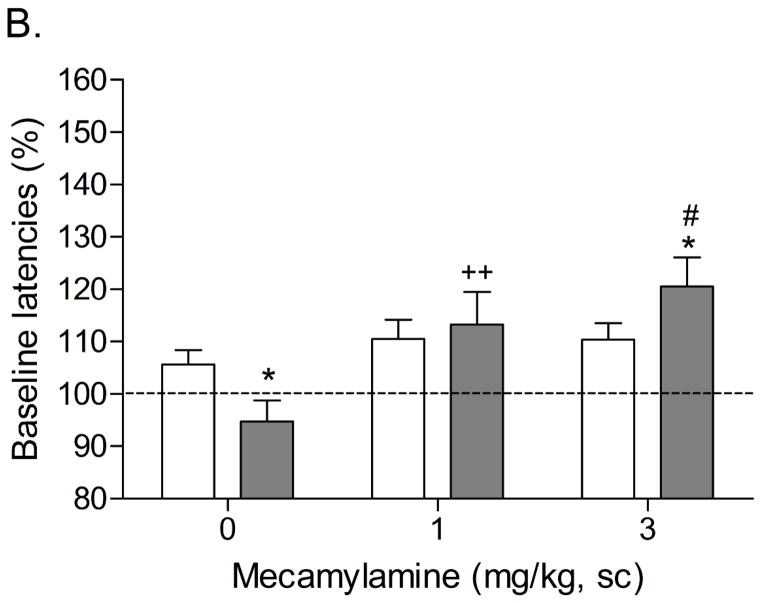
Mean (±S.E.M.) absolute brain reward thresholds before tobacco smoke exposure for the tobacco smoke group and the control group were 114.2 ± 5.6 and 117.0 ± 10.2 μA [t(18)=0.25, n.s.], respectively. Mean (±S.E.M.) absolute response latencies for the tobacco smoke group and the control group were 3.1 ± 0.1 and 3.3 ± 0.1 seconds [t(34)=0.18, n.s.], respectively. During the first 20 days of tobacco smoke exposure, brain reward thresholds and response latencies were assessed prior to the tobacco smoke exposure sessions. Chronic exposure to tobacco smoke did not affect the brain reward thresholds or the response latencies. The brain reward thresholds and response latencies were assessed immediately prior to tobacco smoke exposure and after tobacco smoke exposure on day 20. There was no difference in the brain reward thresholds before and after tobacco smoke exposure on day 20. However, tobacco smoke exposure decreased the response latencies (pre 3.14 ± 0.12 vs. post 2.89 ± 0.11 sec; F1,9=9.68, P<0.012), which is indicative of a stimulant-like effect. After day 20, the rats were tested immediately after the tobacco smoke exposure sessions. Systemic administration of the nAChR receptor antagonist mecamylamine elevated the brain reward thresholds of the rats chronically exposed to tobacco smoke and did not elevate the brain reward thresholds of the control rats (Figure 2A; Dose x Treatment interaction: F2,36=6.135, P<0.005). Newman-Keuls post-hoc comparisons indicated that the brain reward thresholds of the rats that were exposed to tobacco smoke and received 1 or 3 mg/kg of mecamylamine were elevated compared to those of the control rats. Mecamylamine increased the response latencies of the rats exposed to tobacco smoke and did not affect the response latencies of the control rats (Figure 2B; Dose x Treatment interaction: F2,36=9.675, P<0.0004). Newman-Keuls post-hoc comparisons revealed that exposure to tobacco smoke decreased the response latencies and this effect was reversed by the administration of 1 mg/kg of mecamylamine. Furthermore, the response latencies of the tobacco smoke - 3 mg/kg mecamylamine group were increased compared to those of the tobacco smoke - 1 mg/kg mecamylamine group.
Figure 2.
Effect of the nAChR antagonist mecamylamine on the brain reward thresholds (A) and response latencies (B) of rats exposed to tobacco smoke (n=10) and control rats (n=10). In figure 1A, asterisks (* P<0.05, ** P<0.01) indicate elevated brain reward thresholds compared to the corresponding control group. Pound signs (#P<0.05) indicate elevated brain reward thresholds compared to the tobacco smoke group treated with 1 mg/kg of mecamylamine. Plus signs (++ P<0.01) indicate elevated brain reward thresholds compared to the tobacco smoke group treated with vehicle. In figure 1B, asterisks (* P<0.05) indicate increased or decreased response latencies compared to the corresponding control group. Pound signs (#P<0.05) indicate increased latencies compared to the tobacco smoke group treated with 1 mg/kg of mecamylamine. Plus signs (++ P<0.01) indicate increased latencies compared to the tobacco smoke group treated with vehicle.
Experiment 2: Tobacco smoke exposure and nicotine self-administration
There were no differences in body weights between the tobacco smoke exposed rats and the control rats prior to the onset of tobacco smoke exposure [Table 1; t(16)=0.74, n.s.]. Similar to experiment 1, exposure to tobacco smoke decreased body weight gain (Table 1; Time x Treatment, F20,320=19.8, P<0.0001, body weights were recorded daily from week 1–3).
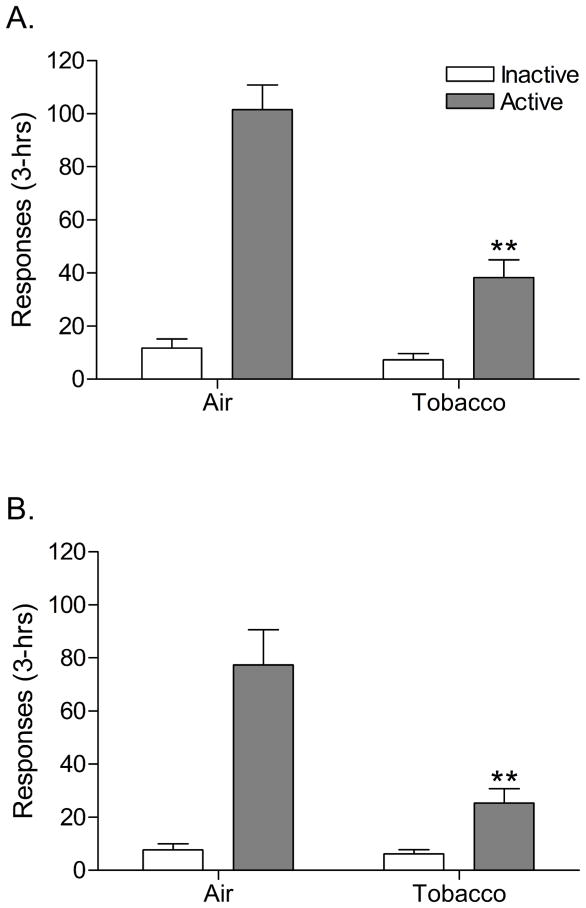
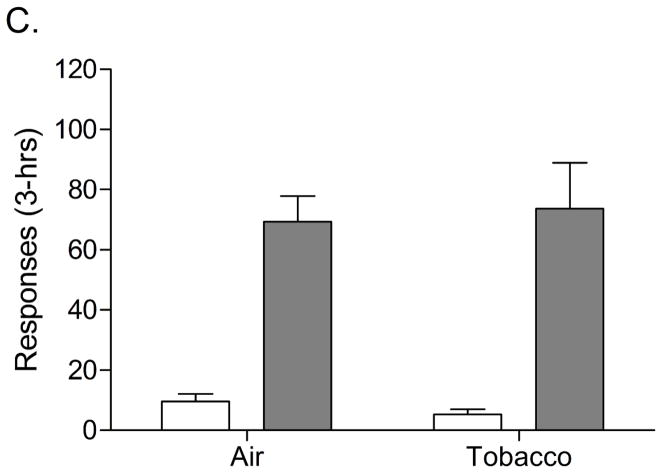
There was no significant effect of exposure time (day 12, 20, and 28) on plasma nicotine or cotinine levels (Table 2). This indicates that tobacco smoke exposure induced a reliable and consistent increase in plasma nicotine and cotinine levels. The nAChR antagonist mecamylamine induced more somatic withdrawal signs in the rats exposed to tobacco smoke than in the control rats (Table 3). This confirms that exposure to tobacco smoke leads to the development of nicotine dependence. Nicotine self-administration was investigated after 3 and 4 weeks of tobacco smoke exposure and five days after the last tobacco smoke exposure session. Prior to tobacco smoke exposure, the average number of responses on the active lever in the tobacco smoke group and the control group were 74.1 ± 8.6 (14.6 infusions) and 73.2 ± 8.8 (14.3 infusions), respectively [t(16)=0.07, n.s.]. The number of responses on the inactive lever in the tobacco smoke group and the control group were 7.8 ± 3.5 and 3.5 ± 1.3, respectively [t(16)=1.23, n.s.]. This indicates that there was no difference in responding for nicotine in the tobacco smoke group and the control group prior to the onset of tobacco smoke exposure. Three weeks after the onset of tobacco smoke exposure, nicotine self-administration was decreased in the tobacco smoke group compared to the control group as indicated by a decreased number of responses on the active lever (Figure 3A, Treatment, F1,17=27.60, P<0.0001). This suggests that three weeks of tobacco smoke exposure decreases the self-administration of nicotine. There was no difference in the number of responses on the inactive lever between the tobacco smoke group and the control group. Four weeks after the onset of tobacco smoke exposure the number of responses on the active lever was again decreased in the tobacco smoke group compared to the control group (Figure 3B, F1,17=10.84, P<0.005) and there was no difference in the number of responses on the inactive lever. Five days after the last tobacco smoke exposure session there was no difference in the number of responses on the active lever or the inactive lever between the tobacco smoke group and the control group (Figure 3C). This indicates that operant responding for nicotine recovers when the rats are not exposed to tobacco smoke.
Table 2.
Effect of tobacco smoke exposure on plasma nicotine and cotinine levels.
| Time (days) | Nicotine (ng/ml) | Cotinine (ng/ml) |
|---|---|---|
|
|
||
| 12 (n=8) | 111.2 ± 5.9 | 566.1 ± 35.2 |
| 20 (n=8) | 120.5 ± 5.2 | 572.0 ± 19.8 |
| 28 (n=8) | 121.3 ± 8.1 | 539.9 ± 50.3 |
Data are expressed as means (± S.E.M.). The rats were exposed to tobacco smoke for 4 hours per day and the blood samples were collected immediately after the tobacco smoke exposure sessions.
Table 3.
Effects of tobacco smoke exposure on mecamylamine-precipitated somatic withdrawal signs.
| Experiment 2 | Experiment 4 | |||
|---|---|---|---|---|
| Control (n=10) | Tobacco (n=8) | Control (n=6) | Tobacco (n=6) | |
| Abdominal const. | 0.2 ± 0.1 | 2.4 ± 0.4** | 0.5 ± 0.0 | 2.2 ± 1.3 |
| Eye blinks | 2.7 ± 0.7 | 2.0 ± 0.4 | 3.2 ± 1.1 | 8.8 ± 3.3 |
| Ptosis | 0.0 ± 0.0 | 2.2 ± 0.6** | 0.2 ± 0.0 | 2.7 ± 1.1* |
| Facial Fasc. | 0.5 ± 0.2 | 2.1 ± 0.7* | 0.7 ± 0.2 | 2.3 ± 1.9 |
| Yawns | 0.0 ± 0.0 | 1.6 ± 0.6** | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Shakes | 0.0 ± 0.0 | 2.6 ± 1.5* | 0.0 ± 0.0 | 4.0 ± 1.3** |
| Other signs | 0.2 ± 0.1 | 1.0 ± 0.4 | 0.0 ± 0.0 | 0.2 ± 0.0 |
| Total signs | 3.6 ± 0.8 | 13.9 ± 1.8** | 4.5 ± 1.2 | 20.2 ± 3.1** |
Asterisks (* P<0.05, ** P<0.01) indicate an increase in the number of somatic signs compared to the corresponding control group. Abdominal constrictions includes gasps and writhes; facial fasciculation includes cheek tremors, chews, and teeth chattering; shakes includes head shakes and body shakes; other signs includes escape attempts, foot licks, genital licks, scratches, and yawns. Data are expressed as means ± SEM.
Figure 3.
Nicotine self-administration (3-hrs) in rats chronically exposed to tobacco smoke (n=8) and control rats (n=10). Nicotine self-administration was investigated 1 day after tobacco smoke exposure after 3 weeks (Day 22, A) and 4 weeks (Day 29, B) of exposure to tobacco smoke (4 hours/day). A final self-administration session was conducted 5 days after the last tobacco smoke exposure session (Day 33, C). Asterisks (** P<0.01) indicate a decrease in responding on the active lever compared to the control group. Data are expressed as means ± SEM.
In a separate group of animals plasma nicotine and cotinine levels were determined from 0–72 hours after tobacco smoke exposure. The plasma nicotine level was 46.6 ± 3.7 ng/ml immediately after the last tobacco smoke exposure session and nicotine could not be detected in plasma at the 24 hour time or at any later time point. The plasma cotinine levels at the 0, 24, and 48 hour time points were 248.0 ± 17.9, 110.1 ± 6.0, 24.9 ± 1.7 ng/ml, respectively. At the 72 hour time point, cotinine could not be detected in 2 of the animals and in the other 4 animals the average cotinine level was 16.0 ± 0.2 ng/ml.
Experiment 3: Tobacco smoke exposure and food responding
Prior to the onset of the tobacco smoke exposure there was no difference in the body weights of the tobacco smoke group and the control group [Table 1; t(18)=0.18, n.s.]. Body weight gain was decreased in the tobacco smoke group compared to the control group during the 32-day tobacco smoke exposure period (Table 1; Time x Treatment interaction: F31,558=19.667, P<0.0001).
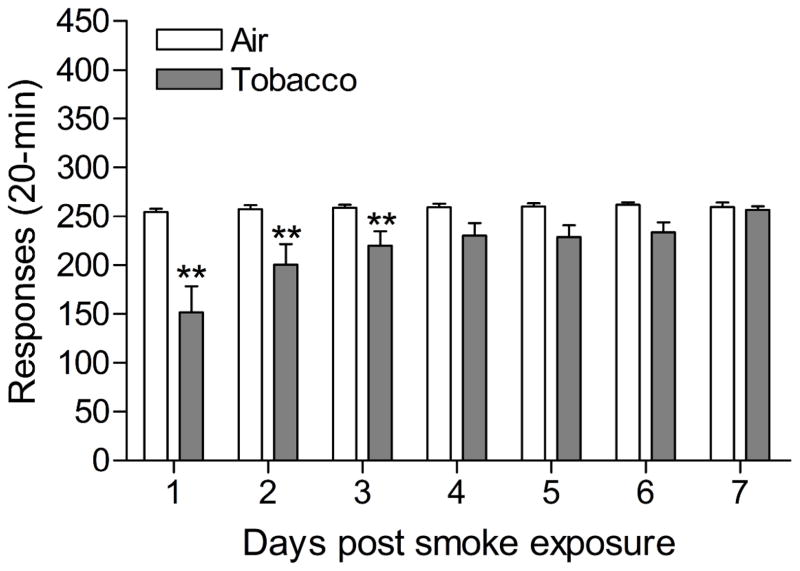
The mean (±S.E.M.) number of responses on the active lever for the tobacco smoke group and the control group immediately prior to the onset of the smoke exposure sessions were 240.7 ± 3.8 and 240.0 ± 9.2 [t(18)=0.071, n.s.], respectively. The mean (±S.E.M.) number of responses on the inactive lever for the tobacco smoke group and the control group prior to the onset of the tobacco smoke exposure sessions were 5.5 ± 1.0 and 3.3 ± 2.1 [t(18)=0.956, n.s.], respectively. After three weeks of tobacco smoke exposure, responding on active lever was decreased 40 minutes (Table 4; Treatment: F1,19=8.192, P<0.01) and 24 hours (Treatment: F1,19=27.674, P<0.0001) after the last smoke exposure session. Exposure to tobacco smoke did not affect responding on the inactive lever. After 4 weeks of tobacco smoke exposure, responding on active lever was again decreased 40 minutes (Table 4; Treatment: F1,19=7.348, P<0.014) and 24 hours (Treatment: F1,19=14.788, P<0.001) after the last tobacco smoke exposure session. Tobacco smoke exposure did not affect responding on the inactive lever. After the discontinuation of tobacco smoke exposure, responding on the active lever was decreased and then gradually increased compared to responding by the control rats (Figure 4; Time x Treatment interaction: F6,108=3.039, P<0.0001). Newman Keuls posthoc tests indicated that the rats exposed to tobacco smoke responded fewer times on the active lever than the control rats during the first three days after the discontinuation of tobacco smoke exposure. Discontinuation of tobacco smoke exposure did not affect responding on the inactive lever.
Table 4.
Effect of tobacco smoke exposure on responding for food pellets.
| Time | Active lever | Inactive lever | ||
|---|---|---|---|---|
| C (n=10) | Tobacco (n=10) | C (n=10) | Tobacco (n=10) | |
|
| ||||
| 3W/Day 22 – 24 hrs | 255.6 ± 3.9 | 148.0 ± 20.1** | 0.1 ± 0.1 | 4.7 ± 2.8 |
| 3W/Day 23 – 40 min | 256.0 ± 3.4 | 200.1 ±19.2** | 1.3 ± 0.9 | 2.4 ± 1.5 |
| 4W/Day 29 – 24 hrs | 256.0 ± 3.4 | 177.5 ± 20.1** | 0.4 ± 0.3 | 3.7 ± 1.9 |
| 4W/Day 30 – 40 min | 259.6 ± 3.6 | 202.5 ± 20.8* | 0.2 ± 0.1 | 0.2 ± 0.1 |
Data are expressed as means (± S.E.M.). Abbreviations: C, control group; 3W, 3 weeks of smoke exposure; 4W, 4 weeks of smoke exposure. Time (min/hrs) indicates duration after last smoke exposure session. Asterisks (**P<0.01, *P<0.05) indicate fewer responses on the active lever compared to the control group.
Figure 4.
Responding for food pellets (20-min) in rats chronically exposed to tobacco smoke (n=10) and control rats (n=10). Tobacco smoke exposure was discontinued after 32 days and food responding was recorded for 7 days post tobacco smoke exposure (Day 33 – 39). Asterisks (** P<0.01) indicate a decrease in responding on the active lever compared to the control group. Data are expressed as means ± SEM.
Experiment 4: Tobacco smoke exposure and [125I]-Epibatidine and [125I]-α-bungarotoxin binding
There were no differences in body weights between the tobacco smoke exposed rats and the control rats prior to the onset of tobacco smoke exposure [Table 1; t(10)=0.13, n.s.]. Exposure to tobacco smoke diminished body weight gain over the 21-day smoke exposure period (Table 1; Time x Treatment: F20,200=33.23, P<0.0001). Tobacco smoke exposure lead to the development of nicotine dependence as indicated by an increased number of mecamylamine precipitated somatic withdrawal signs in the rats exposed to smoke compared to the control rats (Table 3). Statistical analyses indicated that tobacco smoke exposure lead to a significant increase in the density of α7 nAChRs in the CA2/3 region (Treatment: F1,11=11.92, P<0.006) and the stratum oriens (Treatment: F1,11=10.04, P<0.01) and there was a trend towards an increase in the the hilus of dentate gyrus (Treatment: F1,11=4.80, P<0.053)(Table 5). Tobacco smoke exposure also lead to a significant increase in the density of non-α7 nAChRs in the dentate gyrus (Treatment: F1,11=14.12, P<0.004) and there was a trend towards an increase in the fasciculus retroflexus (Treatment: F1,11=4.84, P<0.052)(Table 6). Furthermore, tobacco smoke exposure lead to a significant decrease in the density of non-α7 nAChRs the thalamus (Treatment: F1,11=8.58, P<0.015).
Table 5.
Effects of passive exposure to tobacco smoke on [125I]-α-bungarotoxin binding.
| Brain region | Control (n=6) | Tobacco (n=6) |
|---|---|---|
| CA1 | 15.0 ± 1.1 | 14.8 ± 2.1 |
| CA2/3 | 52.0 ± 2.6 | 64.6 ± 2.5** |
| Dentate Gyrus | 39.6 ± 1.9 | 43.1 ± 4.0 |
| Hilus of dentate gyrus | 121.5 ± 2.8 | 130.9 ± 3.2 |
| Stratum oriens | 68.2 ± 2.7 | 79.8 ± 2.4** |
| Cortex layers 1–4 | 33.5 ± 2.3 | 30.3 ± 2.6 |
| Cortex layers 5–6 | 60.0 ± 3.0 | 63.6 ± 2.3 |
Data are expressed as means ± SEM. Asterisks (**P<0.01) indicate increased [125I]-α-bungarotoxin binding compared to the control group.
Table 6.
Effects of passive exposure to tobacco smoke on [125I]-epibatidine binding.
| Brain region | Control (n=6) | Tobacco (n=6) |
|---|---|---|
| Stratum oriens | 36.0 ± 2.5 | 38.3 ± 2.4 |
| Olfactory tubercle | 10.8 ± 2.3 | 13.0 ± 2.0 |
| Cortex layers 1–2 | 18.5 ± 4.1 | 25.2 ± 3.1 |
| Cortex layers 3–4 | 51.8 ± 5.9 | 49.1 ± 3.6 |
| Cortex layers 5–6 | 28.8 ± 4.3 | 36.6 ± 3.1 |
| Dentate gyrus | 20.3 ± 2.2 | 30.7 ± 1.7** |
| Medial habenula | 138.9 ± 4.0 | 123.0 ± 8.0 |
| Thalamus | 72.3 ± 2.8 | 58.7 ± 3.7* |
| Subiculum | 31.5 ± 4.2 | 35.4 ± 1.8 |
| Superior Colliculus | 91.4 ± 4.9 | 90.0 ± 2.6 |
| Medial geniculate n. | 62.7 ± 5.8 | 60.8 ± 2.0 |
| Substantia nigra | 55.0 ± 6.2 | 62.6 ± 2.0 |
| Fasciculus retroflexus | 83.7 ± 4.1 | 74.1 ± 1.6 |
Data are expressed as means ± SEM. Asterisks (**P<0.01, *P<0.05) indicate a significant difference compared to the control group.
DISCUSSION
The present results demonstrate that exposure to tobacco smoke leads to nicotine dependence. The nAChR antagonist mecamylamine dose-dependently elevated the brain reward thresholds of the rats exposed to tobacco smoke and did not affect the brain reward thresholds of the untreated control rats. Furthermore, mecamylamine induced more somatic signs in the rats exposed to tobacco smoke than in the control rats. To our knowledge, these are the first studies to report that exposure to tobacco smoke leads to the development of nicotine dependence in rats. The aforementioned results are in line with previous studies that reported that nAChR antagonists elevate the brain reward thresholds of nicotine treated rats and induce more somatic signs in nicotine treated rats than in control rats (Bruijnzeel et al. 2007; Epping-Jordan et al. 1998). It was also demonstrated that exposure to tobacco smoke temporarily decreased the self-administration of nicotine. Nicotine self-administration was decreased 1 day after the last tobacco smoke exposure session after 3 weeks and 4 weeks of tobacco smoke exposure. Five days after the last tobacco smoke exposure session there was no difference in nicotine self-administration between the tobacco smoke exposed rats and the control rats. Tobacco smoke exposure lead to a decreased growth rate. This is in line with clinical studies indicating that smoking reduces body weight gain (Grunberg 1985). Finally, tobacco smoke exposure increased α7 nAChR density in two hippocampal subregions, the CA2/3 region and the stratum oriens, and increased non-α7 nAChR density in the dentate gyrus of the hippocampus. These findings are in line with studies indicating that chronic nicotine administration increases α7 nAChR and non-α7 nAChR levels in the brains of rodents (Marks et al. 1983; Pauly et al. 1991; Zhang et al. 1994).
Blood samples were collected in order to determine plasma nicotine and cotinine levels. Nicotine and cotinine levels immediately after tobacco smoke exposure were 118 ng/ml and 559 ng/ml, respectively. These levels are in line with a previous study that reported that the plasma nicotine level is approximately 95 ng/ml and the cotinine level 790 ng/ml after 6 hours of exposure to tobacco smoke with a total suspended particulate matter of 87 mg/m3 (Anderson et al. 2004). Previous studies have shown that chronic subcutaneous administration of 3.2 mg/kg of nicotine base per day leads to the development of nicotine dependence as indicated by affective and somatic withdrawal signs (Bruijnzeel et al. 2007; Epping-Jordan et al. 1998). Chronic administration of 3.2 mg/kg of nicotine base per day leads to plasma nicotine and cotinine levels of 65 and 297 ng/ml, respectively (O’Dell et al. 2006). Therefore, the nicotine levels in the tobacco smoke exposed rats are somewhat higher then those in previous studies that demonstrated that chronic nicotine administration leads to the development of nicotine dependence. Plasma nicotine and cotinine levels in heavy smokers are approximately 35 and 300 ng/ml, respectively (Benowitz 1988; Lawson et al. 1998; Wall et al. 1988). The present findings indicate that plasma nicotine and cotinine levels that are the same or higher than those in heavy smokers can be obtained in rats by exposure to tobacco smoke. The present findings also indicate that nicotine is metabolized rapidly and that tobacco residues on the coat of the rats or the cages are not a significant source of nicotine. Nicotine was detected in plasma that was collected immediately after the tobacco smoke exposure session but nicotine could not be detected in plasma that was collected 24 hours after the tobacco smoke exposure session. Cotinine could be detected up to at least 48 hours after the tobacco smoke exposure session. The present findings are in line with previous studies that indicated that the half-lifes of nicotine and cotinine are approximately 1 and 5 hours, respectively (Ghosheh et al. 1999; Kyerematen et al. 1988).
In the present study, mecamylamine (3 mg/kg) elevated the brain reward thresholds of the tobacco smoke exposed rats by 32%. This is similar to the results of a previous study in which we reported that 3 mg/kg of mecamylamine elevates the brain reward thresholds of rats chronically treated with 3.2 mg/kg of nicotine base per day by 38% (Bruijnzeel et al. 2007). Tobacco smoke exposure decreased the response latencies (stimulant-like effect) of the rats in the ICSS test procedure. Mecamylamine dose-dependently increased the response latencies of the tobacco smoke exposed rats and did not affect the response latencies of the control rats (Figure 2B). A close look at the data indicates that a low dose of mecamylamine (1 mg/kg) reverses the tobacco smoke-induced decrease in response latencies and a high dose of mecamylamine (3 mg/kg) slightly but significantly increases the response latencies of the tobacco smoke exposed rats compared to those of the control rats. The observation that tobacco smoke decreases the response latencies is in line with previous studies that demonstrated that psychostimulants such as cocaine and amphetamine decrease the response latencies of rats in a similar ICSS test procedure (Kenny et al. 2003; Paterson et al. 2000). It is interesting to note that doses of nicotine (0.125 – 0.5 mg/kg of nicotine base, sc) that lower the brain reward thresholds of rats do not decrease the response latencies (Harrison et al. 2002). This pattern of results suggests that tobacco smoke exposure has a more pronounced stimulant-like effect on the response latencies than the acute administration of nicotine.
In the third experiment, the effect of tobacco smoke exposure on the self-administration of nicotine was investigated. It was shown that 1 day after the last tobacco smoke exposure session, after 3 weeks and 4 weeks of tobacco smoke exposure, nicotine self-administration was decreased (60–70% decrease in nicotine self-administration). A similar pronounced decrease in nicotine self-administration has been reported after the administration of the nAChR antagonist mecamylamine (Watkins et al. 1999) and after substituting nicotine by saline (Zislis et al. 2007). The present finding is in line with a study by Corrigall and colleagues that investigated the effects of chronic subcutaneous nicotine administration, 4 mg/kg of nicotine base per day, on the self administration of nicotine (Coen et al. 2009). It was shown that the subcutaneous administration of nicotine leads to a decrease in the self-administration of nicotine. Removal of the nicotine pumps only lead to a gradual increase in the self-administration of nicotine during the first week after minipump removal. Additional studies are needed to investigate the mechanisms that mediate the decrease in nicotine self-administration that can be detected for at least 1 day after the non-contingent administration of tobacco smoke or nicotine.
In a separate experiment the effect of chronic tobacco smoke exposure on brain α7 and non-α7 nAChR density was investigated. Smoking increases nAChR levels in the human brain and this is a hallmark feature of tobacco addiction (Benwell et al. 1988). In the present study we found that exposure to tobacco smoke increased [125I]-α-bungarotoxin binding (α7 nAChRs) in the CA2/3 region and stratum oriens and increased [125I]-epibatidine binding (non-α7 nAChRs) binding in the dentate gyrus. Tobacco smoke exposure also increased [125I]-epibatidine binding in cortex layers 1–2 (36% increase) and cortex layers 5–6 (27% increase), however, this difference did not reach statistical significance due to a large variation between animals. Chronic nicotine administration has been shown to significantly increase non-α7 nAChR levels in the cortex of rodents (Pauly et al. 1991; Perry et al. 1999; Wall et al. 2000; Zhang et al. 1994). There are major differences between the design of the present study and previous studies that investigated the effects of chronic nicotine administration on [3H]nicotine or [3H]/[125I]epibatidine binding in the rat brain. The majority of the studies that investigated the effect of chronic nicotine administration on rat brain [3H]nicotine or [3H]epibatidine binding administered nicotine (0.45 mg nicotine base/kg, sc) twice a day for 18 to 21 days (Wall et al. 2000; Zhang et al. 1994; Zhang et al. 2002). The injections with nicotine increased [3H]nicotine or [3H]epibatidine binding in cortical areas and the hippocampus including the dentate gyrus. Nguyen and colleagues reported an increase in [125I]epibatidine binding in cortical areas and the dentate gyrus after chronic, 14 – 17 days, continuous administration of nicotine (6 mg/kg/day, nicotine base)(Nguyen et al. 2003). The administration of 5 mg/kg of nicotine base per day leads to plasma nicotine levels of approximately 100 ng/ml (Trauth et al. 2000). This suggest that the administration of 6 mg/kg of nicotine base per day leads leads to plasma nicotine levels that are similar to those in the present tobacco smoke exposure experiment (110–120 ng/ml). Taken together, these studies suggest that chronic nicotine administration induces an upregulation of non-α7 nAChR levels in the cortex and hippocampus while chronic exposure to tobacco smoke only significantly increased non-α7 nAChR levels in the dentate gyrus of the hippocampus. It should be noted, however, that we investigated the effect of one level of tobacco smoke and one specific exposure period on non-α7 nAChR density in the rat brain. Therefore, it cannot be ruled out that different tobacco smoke exposure levels or a different exposure period might have led to a more pronounced upregulation of non-α7 nAChR in cortical brain areas.
The results of the present study indicated that exposure to tobacco smoke leads to an upregulation of α7 nAChRs in two hippocampal subregions: the CA2/3 region and stratum oriens. To our knowledge, this is the first study to demonstrate that exposure to tobacco smoke leads to an upregulation of α7 nAChRs in the hippocampus. Chronic nicotine administration (5 mg/kg/hr, nicotine base, 8–10 days) increases α7 nAChR levels in the hippocampus, but not in the cortex, of mice (Marks et al. 1983). These findings suggest that chronic exposure to tobacco smoke and chronic exposure to nicotine increases α7 nAChR levels in the hippocampus. Previous research has shown that α7 receptor agonists upregulate α7 nAChRs in human embryonic kidney (HEK) cells (Molinari et al. 1998). Therefore, α7 nAChR agonists in the tobacco smoke (e.g., anabasine) might have acted in concert with nicotine to mediate the tobacco smoke-induced upregulation of α7 nAChR in in the hippocampus (Kem et al. 1997; Maciuk et al. 2008). In vitro studies indicate that anabasine has a higher potency (EC50 of anabasine is 16.8 and EC50 of nicotine 47) at the rat α7 nAChR than nicotine (Kem et al. 1997). In addition, anabasine has a higher maximal efficacy at the rat α7 nAChR than nicotine (Kem et al. 1997). A study by Leonard and colleagues, in which homogenized human postmortem brain samples were used, suggests that smoking does not lead to an increase in α7 nAChR levels in the human brain (Breese et al. 2000). However, α7 nAChR receptor levels are extremely low in the human brain compared to the rodent brain and it was suggested that differences in α7 nAChR levels between smokers and non-smokers might have been detected with more sensitive methods such as [125I]-α-bungarotoxin receptor autoradiography (Breese et al. 1997; Breese et al. 2000).
Tobacco smoke exposure lead to a decrease in body weight gain in rats in all our experiments. This is in line with previous studies that reported that body weight gain is reduced in rats exposed to tobacco smoke (Anderson et al. 2004). Decreased body weight gain has also been reported in rats chronically exposed to nicotine (3.2–12 mg/kg/day of nicotine base) (Grunberg et al. 1984; Harrison et al. 2001). Food intake in the home cages was not recorded in the present experiments and therefore it is not known if the decrease in body weight gain was due to a decrease in food intake or due to metabolic changes. In a separate experiment we investigated the effect of smoke exposure on operant responding for food pellets. Responding for food pellets was decreased 40 minutes and 24 hours after tobacco smoke exposure and responding for food pellets remained decreased for three days after the last tobacco smoke exposure session. It is unlikely that operant responding for food was decreased due to tobacco smoke-induced sedative effects or motor impairments because tobacco smoke exposure decreased the response latencies in the ICSS test procedure (Figure 2B). A decrease in the response latency in the ICSS procedure is indicative of a stimulant-like effect (Harrison et al. 1999). The decrease in operant responding for food pellets may indicate that the tobacco smoke exposed rats have a decreased motivation to consume food and therefore have a decreased weight gain compared to the control rats. This is supported by a study by Schwid and colleagues that demonstrated that nicotine (3.4 mg/day, nicotine base) decreases food intake in the home cage and reduces body weight gain (Schwid et al. 1992). Experimental evidence indicates that nicotine also increases fat lipolysis and thereby reduces body weight gain independent of its effect on food intake (Grunberg et al. 1984; Schechter and Cook 1976). Therefore, a nicotine-induced increase in lipolysis may have contributed to the reduced weight gain in the smoke exposed rats.
Taken together, the present studies indicate that exposure to tobacco smoke leads to nicotine dependence as indicated by precipitated affective and somatic withdrawal signs, a short-term decrease in operant responding for nicotine, and an increase in α7 and non-α7 nAChR density in the hippocampus. These studies suggest that the rat tobacco smoke exposure model can be used to investigate the effects of tobacco smoke on the brain and to evaluate the efficacy of novel treatments for tobacco addiction.
Acknowledgments
This research was funded by a Flight Attendant Medical Research Institute Young Clinical Scientist Award (Grant nr. 52312) and a National Institute on Drug Abuse grant (DA023575) to A. Bruijnzeel. The authors would like to thank Ms. Deann Hopkins and Ms. Sara Cambron for technical assistance with the receptor binding studies.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Press; Washington, DC: 2000. text revision edn. [Google Scholar]
- Anderson KL, Pinkerton KE, Uyeminami D, Simons CT, Carstens MI, Carstens E. Antinociception induced by chronic exposure of rats to cigarette smoke. NeurosciLett. 2004;366:86–91. doi: 10.1016/j.neulet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Aricioglu F, Altunbas H. Harmane induces anxiolysis and antidepressant-like effects in rats. Ann NY Acad Sci. 2003;1009:196–201. doi: 10.1196/annals.1304.024. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988;319:1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, Sullivan B, Demasters BK, Freedman R, Leonard S. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387:385–398. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Brown ZW, Amit Z, Rockman GE. Intraventricular self-administration of acetaldehyde, but not ethanol, in naive laboratory rats. Psychopharmacology (Berl) 1979;64:271–276. doi: 10.1007/BF00427509. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M. Severe deficit in brain reward function associated with fentanyl withdrawal in rats. Biol Psychiatry. 2006;59:477–480. doi: 10.1016/j.biopsych.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology. 2004;47:572–579. doi: 10.1016/j.neuropharm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Coen KM, Adamson KL, Corrigall WA. Medication-related pharmacological manipulations of nicotine self-administration in the rat maintained on fixed- and progressive-ratio schedules of reinforcement. Psychopharmacology (Berl) 2009;201:557–568. doi: 10.1007/s00213-008-1321-6. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barre L. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J Pharm Sci. 2003;92:1051–1057. doi: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, Gervais A, O’Loughlin J, Paradis G, Rinfret S, Pilote L. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135–144. doi: 10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Farzin D, Mansouri N. Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test. Eur Neuropsychopharmacol. 2006;16:324–328. doi: 10.1016/j.euroneuro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton JA, Bell N, Swettenham J, Jarvis MJ, Russell MA. Mood and physiological effects of subcutaneous nicotine in smokers and never-smokers. Drug Alcohol Depend. 1997;44:105–115. doi: 10.1016/s0376-8716(96)01327-0. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Wolf AP, Warner D, Cilento R, Zezulkova I. Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition. J Addict Dis. 1998;17:23–34. doi: 10.1300/J069v17n01_03. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci US A. 1996;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li WK, Crooks PA. Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2′-(14)C]nicotine. Drug Metab Dispos. 1999;27:1448–1455. [PubMed] [Google Scholar]
- Grunberg NE. Nicotine, cigarette smoking, and body weight. Br J Addict. 1985;80:369–377. doi: 10.1111/j.1360-0443.1985.tb03008.x. [DOI] [PubMed] [Google Scholar]
- Grunberg NE, Bowen DJ, Morse DE. Effects of nicotine on body weight and food consumption in rats. Psychopharmacology (Berl) 1984;83:93–98. doi: 10.1007/BF00427430. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Parsons LH, Koob GF, Markou A. RU 24969, a 5-HT1A/1B agonist, elevates brain stimulation reward thresholds: an effect reversed by GR 127935, a 5-HT1B/1D antagonist. Psychopharmacology (Berl) 1999;141:242–250. doi: 10.1007/s002130050831. [DOI] [PubMed] [Google Scholar]
- Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Commun. 2005;326:378–386. doi: 10.1016/j.bbrc.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Houghtling RA, vila-Garcia MI, Kellar KJ. Characterization of (+/−)(−)[3H]epibatidine binding to nicotinic cholinergic receptors in rat and human brain. Mol Pharmacol. 1995;48:280–287. [PubMed] [Google Scholar]
- Kem WR, Mahnir VM, Papke RL, Lingle CJ. Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors. J Pharmacol Exp Ther. 1997;283:979–992. [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann NY Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kyerematen GA, Taylor LH, de Bethizy JD, Vesell ES. Pharmacokinetics of nicotine and 12 metabolites in the rat. Application of a new radiometric high performance liquid chromatography assay. Drug Metab Dispos. 1988;16:125–129. [PubMed] [Google Scholar]
- Lawson GM, Hurt RD, Dale LC, Offord KP, Croghan IT, Schroeder DR, Jiang NS. Application of serum nicotine and plasma cotinine concentrations to assessment of nicotine replacement in light, moderate, and heavy smokers undergoing transdermal therapy. J Clin Pharmacol. 1998;38:502–509. doi: 10.1002/j.1552-4604.1998.tb05787.x. [DOI] [PubMed] [Google Scholar]
- Maciuk A, Moaddel R, Haginaka J, Wainer IW. Screening of tobacco smoke condensate for nicotinic acetylcholine receptor ligands using cellular membrane affinity chromatography columns and missing peak chromatography. J Pharm Biomed Anal. 2008;48:238–246. doi: 10.1016/j.jpba.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem Pharmacol. 2008;75:323–333. doi: 10.1016/j.bcp.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari EJ, Delbono O, Messi ML, Renganathan M, Arneric SP, Sullivan JP, Gopalakrishnan M. Up-regulation of human alpha7 nicotinic receptors by chronic treatment with activator and antagonist ligands. Eur J Pharmacol. 1998;347:131–139. doi: 10.1016/s0014-2999(98)00084-3. [DOI] [PubMed] [Google Scholar]
- Myers WD, Ng KT, Singer G. Intravenous self-administration of acetaldehyde in the rat as a function of schedule, food deprivation and photoperiod. Pharmacol Biochem Behav. 1982;17:807–811. doi: 10.1016/0091-3057(82)90364-1. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- Okoli CT, Kelly T, Hahn EJ. Secondhand smoke and nicotine exposure: a brief review. Addict Behav. 2007;32:1977–1988. doi: 10.1016/j.addbeh.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Caggiula A, Wilson AS, Stiller RL. Acute reinforcing effects of low-dose nicotine nasal spray in humans. Pharmacol Biochem Behav. 1997;56:235–241. doi: 10.1016/s0091-3057(96)00216-x. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Perry DC, Kellar KJ. [3H]epibatidine labels nicotinic receptors in rat brain: an autoradiographic study. J Pharmacol Exp Ther. 1995;275:1030–1034. [PubMed] [Google Scholar]
- Rasmussen BA, Perry DC. An autoradiographic analysis of [125I]alpha-bungarotoxin binding in rat brain after chronic nicotine exposure. Neurosci Lett. 2006;404:9–14. doi: 10.1016/j.neulet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Rylkova D, Boissoneault J, Isaac S, Prado M, Shah HP, Bruijnzeel AW. Effects of NPY and the specific Y1 receptor agonist [D-His(26)]-NPY on the deficit in brain reward function and somatic signs associated with nicotine withdrawal in rats. Neuropeptides. 2008;42:215–227. doi: 10.1016/j.npep.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD, Cook PG. Nicotine-induced weight loss in rats without an effect on appetite. Eur J Pharmacol. 1976;38:63–69. doi: 10.1016/0014-2999(76)90201-6. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci US A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwid SR, Hirvonen MD, Keesey RE. Nicotine effects on body weight: a regulatory perspective. Am J Clin Nutr. 1992;55:878–884. doi: 10.1093/ajcn/55.4.878. [DOI] [PubMed] [Google Scholar]
- Smith BR, Amit Z, Splawinsky J. Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol. 1984;1:193–195. doi: 10.1016/0741-8329(84)90097-1. [DOI] [PubMed] [Google Scholar]
- Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci US A. 2005;102:2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Talhout R, Opperhuizen A, van Amsterdam JG. Role of acetaldehyde in tobacco smoke addiction. Eur Neuropsychopharmacol. 2007;17:627–636. doi: 10.1016/j.euroneuro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, Moneyhun JH. A sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhalation Toxicology. 1994;6:79–93. [Google Scholar]
- Totsuka Y, Ushiyama H, Ishihara J, Sinha R, Goto S, Sugimura T, Wakabayashi K. Quantification of the co-mutagenic beta-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods. Cancer Lett. 1999;143:139–143. doi: 10.1016/s0304-3835(99)00143-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Wall A, Gong ZH, Johnson AE, Meyerson B, Zhang X. Cross-tolerance in drug response and differential changes in central nicotinic and N-methyl-D-aspartate receptor binding following chronic treatment with either (+)- or (−)-nicotine. Psychopharmacology (Berl) 2000;148:186–195. doi: 10.1007/s002130050041. [DOI] [PubMed] [Google Scholar]
- Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of nonsmokers, passive smokers, and active smokers. Am J Public Health. 1988;78:699–701. doi: 10.2105/ajph.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Wise RA, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmacology (Berl) 1995;117:130–136. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- Yates SL, Bencherif M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochem Pharmacol. 1995;50:2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gong ZH, Nordberg A. Effects of chronic treatment with (+)- and (−)-nicotine on nicotinic acetylcholine receptors and N-methyl-D-aspartate receptors in rat brain. Brain Res. 1994;644:32–39. doi: 10.1016/0006-8993(94)90343-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tian JY, Svensson AL, Gong ZH, Meyerson B, Nordberg A. Chronic treatments with tacrine and (−)-nicotine induce different changes of nicotinic and muscarinic acetylcholine receptors in the brain of aged rat. J Neural Transm. 2002;109:377–392. doi: 10.1007/s007020200030. [DOI] [PubMed] [Google Scholar]
- Zhong CY, Zhou YM, Douglas GC, Witschi H, Pinkerton KE. MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis. 2005;26:2187–2195. doi: 10.1093/carcin/bgi189. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;58:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]