Abstract
This study examined responsiveness to acoustic stimuli among neurons of the basolateral amygdala. While recording from single neurons in awake mustached bats (Pteronotus parnellii), we presented a wide range of acoustic stimuli including tonal, noise, and vocal signals. While many neurons displayed phasic or sustained responses locked to effective auditory stimuli, the majority of neurons (n = 58) displayed a persistent excitatory discharge that lasted well beyond stimulus duration and filled the interval between successive stimuli. Persistent firing usually began seconds (median value, 5.4 s) after the initiation of a train of repeated stimuli and lasted, in the majority of neurons, for at least 2 min after the end of the stimulus train. Auditory-responsive amygdalar neurons were generally excited by one stimulus or very few stimuli. Most neurons did not respond well to synthetic stimuli including tones, noise bursts or frequency-modulated sweeps, but instead responded only to vocal stimuli (82 of 87 neurons). Furthermore, most neurons were highly selective among vocal stimuli. On average, neurons responded to 1.7 of 15 different syllables or syllable sequences. The largest percentage of neurons responded to a hiss-like rectangular broadband noise burst (rBNB) call associated with aggressive interactions. Responsiveness to effective vocal stimuli was reduced or eliminated when the spectrotemporal features of the stimuli were altered in a subset of neurons. Chemical activation of the medial geniculate body (MG) increased both background and evoked firing. Among 39 histologically localized recording sites, we saw no evidence of topographic organization in terms of temporal response pattern, habituation, or the affect of calls to which neurons responded. Overall, these studies demonstrate that amygdalar neurons in the mustached bat show high selectivity to vocal stimuli, and suggest that persistent firing may be an important feature of amygdalar responses to social vocalizations.
Keywords: bat, Pteronotus parnellii, persistent firing, acoustic communication, basolateral amygdala
INTRODUCTION
The amygdala, a collection of diverse and interconnected nuclei of the medial temporal lobe, is involved in establishing the biological relevance of sensory stimuli and in mediating many elements of emotional responses to those stimuli (Cardinal et al., 2002; Sah et al., 2003; Paré et al., 2004; Phelps and LeDoux, 2005; Heimer and Van Hoesen, 2006). Although one aspect of the amygdala’s role is to generate stereotypic responses to learned aversive stimuli, a broader perspective is that the amygdala participates in evaluating the biological significance or salience of a broad range of sensory stimuli. This includes stimuli with either positive or negative valence or affect (Heimer and Van Hoesen, 2006; Costafreda et al., 2008), particularly sensory stimuli associated with social interactions (Sander and Scheich, 2005; Ball et al., 2007; Gothard et al., 2007; Sergerie et al., 2008; Van Bavel et al., 2008; Andics et al., 2010). This study examines responses of neurons in the basolateral amygdala to social vocalizations and other acoustic signals.
Given the salience of acoustic communication in humans and some other animals, it is not surprising that the amygdala may play a significant role in the analysis of and response to social vocalizations. In humans, the amygdala is implicated in processing the prosodic features of speech. For example, increased amygdalar activation is associated with angry vs. neutral prosody (Sander et al., 2005; Wiethoff et al., 2009), speech containing either positive or negative emotions (Fecteau et al., 2007), the intensity of emotion in speech (Leitman et al., 2010), and the identity of voices (Andics et al., 2010). Further, the amygdala is involved in disorders that include an altered emotional response to speech, such as schizophrenia (Sanjuan et al., 2007; Escarti et al., 2010), autism (Gabis et al., 2008; Kim et al., 2010), and some forms of post-traumatic stress (Protopopescu et al., 2005; Shin et al., 2006).
In other species, early work showed that amygdalar neurons respond to social vocalizations (Sawa and Delgado, 1963; O’Keefe and Bouma, 1969; Jacobs and McGinty, 1972), but there have been few systematic studies of responses to social vocalizations by amygdalar neurons. In recent work on mustached bats (Naumann and Kanwal, 2011), big brown bats (Gadziola et al., 2012), and rats (Parsana et al., 2012), conspecific vocal signals evoked a variety of temporal response patterns among basolateral amygdalar neurons, but neurons generally showed stronger excitatory discharge in response to vocal signals with negative affect. Further, amygdalar neurons in each species showed some evidence of discrimination or selectivity among vocal calls. In big brown bats, high neuronal discriminability is based on the duration of response to different vocal stimuli. In most of these neurons, responses to some vocalizations extended well beyond the duration of the stimulus. This persistent firing may be a key feature of amygdalar responses. The present study examines responses to vocal stimuli in the amygdala of mustached bats, and shows that many amygdalar neurons express selective responses to vocal signals through persistent firing.
EXPERIMENTAL PROCEDURES
We describe auditory responses of amygdalar neurons obtained in 20 awake mustached bats (Pteronotus parnellii), captured in Trinidad and Tobago. All procedures were approved by the Institutional Animal Care and Use Committee of the Northeast Ohio Medical University (formerly, Northeastern Ohio Universities College of Medicine) and were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Surgery
Each bat was sedated with butorphanol (5 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA, USA), anesthetized with isoflurane (1.5–2.0%; Abbott Laboratories, North Chicago, IL, USA), and placed in a custom-made stereotaxic holder that we have used extensively in studies throughout the auditory system of this species (Wenstrup and Grose, 1995; Wenstrup, 1999; Portfors and Wenstrup, 2001; Marsh et al., 2006). Depilatory lotion was used to remove hair over the skull, and the skin was disinfected with betadine. A midline incision was made in the skin over the dorsal surface of the skull, and the underlying muscles were reflected laterally. A metal pin was then cemented onto the skull to secure the head during physiological experiments, and a tungsten wire was cemented through a small opening in the skull to serve as a ground for electrophysiological recordings. Using surface and stereotaxic coordinates, a small hole (<0.5 mm in diameter) was opened in the skull to expose cerebral cortex dorsal to the amygdala. For the basolateral amygdala, we used coordinates 5–6 mm rostral to the internucal crest, 3.2–3.6 mm lateral to the mid-sagittal crest, and 4.5–5.2 mm below the cortical surface.
After surgery, a local anesthetic (4% Lidocaine, Ferndale Laboratories, Inc., Ferndale, MI, USA) and an antibiotic (Neosporin, Pfizer, Morris Plains, NJ, USA) were applied to the surgical area and the bat was returned to the holding cage. Two or three days after surgery, physiological experiments were initiated.
Physiological experiments
To minimize distress, bats were lightly sedated with butorphanol (2.5 mg/kg, i.p.) before they were placed in a stereotaxic holder for physiological experiments. The apparatus was located within a heated, single-walled acoustic chamber. To allow time for the animals to fully recover from the sedative, physiological recording began at least one hour after the drug was injected. Recording sessions did not exceed 6 h/day and occurred no more than every other day.
Acoustic stimulation
Acoustic stimuli were computer synthesized or previously recorded and digitized at 250 kHz. All sounds were downloaded to a digital signal processor (Microstar DAP5216a, Microstar Laboratories, Bellevue, WA, USA), converted to analog signals (sampling rate 400 kHz for synthesized stimuli; 250 kHz for vocalizations), filtered (model FT6–2; Tucker-Davis Technologies, Alachua, FL, USA), attenuated (model PA5; Tucker-Davis Technologies, Alachua, FL, USA), and amplified (Parasound model HCA-10000A). Sounds were transmitted through an EMIT-B (Infinity, www.infinitysystems.com) tweeter that was placed 10 cm from the ear and 25° into the sound field contralateral to the recorded amygdala.
Speaker performance was tested with a calibrated microphone (Brüel and Kjaer, model 4135) placed 10 cm from the speaker and 0° azimuth. From 10 to 120 kHz the speaker performance showed a smooth decrease (approximately 3 dB/10 kHz). Distortion components were not detectable ~55 dB below the signal level. For sounds with multiple frequency components (i.e., communication calls, broadband noise, narrowband noise, and upward/downward sweeps), sound levels are shown in dB relative to maximum speaker output. For tonal signals, maximum output at 20 kHz and 60 kHz was 99 and 87 dB SPL, respectively.
Physiological recording
Neural recordings were obtained using glass micropipette electrodes filled with physiological saline (resistances typically 5–15 MΩ). The electrodes were stereotaxically inserted into the amygdala from the dorsal surface of the brain. Neuronal activity was amplified, bandpass filtered (600–6000 Hz), and digitized at a sampling rate of 40 kHz (Microstar DAP5216a). Custom-made software calculated the time of occurrence of spikes and displayed peri-stimulus time histograms (PSTHs), raster plots, and basic statistics of the neural responses in real time.
An extensive set of natural and artificial sounds was presented at each recording site. Stimuli included tones, combinations of tones, broadband noise (BBN), narrowband noise (NBN), frequency-modulated (FM) upward and downward sweeps, synthesized echolocation pulses, echoes, pulse–echo combinations, and social vocalizations. For each of these categories, initial tests were followed by more targeted, additional tests if an auditory response was detected. Stimuli were presented in test sequences consisting of repetitions of the same stimulus at a rate of 2 Hz.
Pure tones were presented at 82 combinations of frequency and sound level (i.e., 18–100 kHz in 1-kHz steps, 0.5-ms rise/fall) at levels 20 and 40 dB below maximum speaker output (78–47 and 58–27 dB SPL). BBN bursts (1–120 kHz, 20-dB attenuation) were presented at durations ranging from 11 to 61 ms in steps of 10 ms. NBN (bandwidths: 2 or 10 kHz) was presented at center frequencies between 25 and 95 kHz in steps of 10 kHz at 20- and 40-dB attenuation. In addition to these stimuli, 15 different communication calls [obtained from Drs. J. Kanwal (Kanwal et al., 1994) and C. Portfors] were presented to the animals at levels ranging from 0 to 80 dB below maximum speaker output in steps of 5 or 10 dB. To examine the influence of upward and downward FM sweeps, we presented sweeps (bandwidth = 10 kHz) over a range of center frequencies (95–25 kHz in steps of 10 kHz; 0.5-ms rise/fall). Additional tests of FM sweeps altered sweep duration (11–61 ms in steps of 10 ms) and/or sound level (in 10-dB steps). Pulse–echo sequences were generated to mimic recordings of sonar calls emitted by mustached bats. Each pulse and echo had four harmonic elements with relative levels based on previous work (Kawasaki et al., 1988; Vater et al., 2003). Simulated echoes were shifted up by 1–3 kHz and delayed after the pulse by 2–10 ms to mimic different Doppler shifts and sonar target distances.
To be considered responsive to a particular stimulus, a neuron was required to display, in at least two test sequences, a discharge significantly above background over 20 or more stimulus repetitions (see Data analysis). This conservative criterion was adopted to avoid false-positive responses in neurons that could display altered background discharge rates for reasons that appeared to be unrelated to acoustic stimuli. As a result, we did not consider further neurons that habituated rapidly to repeated acoustic stimuli.
Additional tests were performed on those neurons demonstrating responsiveness to sounds. For tones, BBN, and NBN, the sound level was varied in 10-dB steps and the duration of the stimulus was varied in 5-ms steps (11–101 ms). For neurons that responded to one or more communication calls, additional tests were performed if time permitted. For these tests, acoustic stimuli included: (1) time-reversed calls, (2) frequency-shifted calls, (3) calls with removal of specific temporal or spectral elements, and (4) combinations of communication calls. All manipulations of calls were performed using custom software created by D. Gans.
Drug application in medial geniculate body (MG)
In five animals, one or more excitatory drugs (glutamate (GLU); aspartate (ASP); bicuculline (BIC)) (GABAa receptor antagonist) were iontophoretically injected into MG in order to increase auditory excitation to the amygdala. In these animals, single neuron recordings were obtained from MG using a micropipette electrode (physiological saline) mounted on a five-barrel pipette (Havey and Caspary, 1980). The tip of the multibarrel pipette was broken to a diameter of 30 μm, and the unbroken tip of the single electrode extended 10–20 μm beyond the multibarrel pipette. To balance all currents used to apply or retain drugs, the center barrel of the multibarrel pipette was filled with physiological saline and connected to a sum channel (Dagan programmable current generator, model 6400). Other barrels were filled with a cocktail of L-glutamate (S)-2-aminopentanedioic acid (GLU) (500 mM, pH 8; Sigma, St. Louis, MO, USA) and aspartate (S)-(+)-aminosuccinic acid (ASP) (500 mM, pH 8; Sigma, St. Louis, MO, USA), or GABAa receptor antagonist BIC (10 mM, pH 3.0, Sigma, St. Louis, MO, USA). BIC was retained with negative current (−15 nA) and ejected using positive currents (+15 to +30 nA), while GLU and ASP were retained with positive current (+15 nA) and ejected using negative currents (−15 to −30 nA). Iontophoresis currents for drug application and retention were based on previous studies by Nataraj and Wenstrup (2005, 2006) and Bauer et al. (2000). Pilot tests established iontophoretic current levels that altered spike discharge of neurons in the MG.
For placement of multi-barreled pipettes in the mustached bat’s MG, we used stereotaxic coordinates developed in previous studies (Wenstrup and Grose, 1995; Wenstrup, 1999) but modified for this subspecies: 3.0–3.2 mm lateral from the midline, 2.2–3.0 mm in depth. Caudo-rostral position was 3.0 mm rostral to the center of the surface of the inferior colliculus. To confirm location within MG, auditory-evoked activity was recorded. Successful application of drugs was confirmed by alterations in these evoked responses. Location within MG, confirmed by observation of the multi-barreled pipette track in Nissl-stained material, was in the medial MG.
Data analysis
Most data were obtained from well-isolated single neurons defined by spikes of constant waveform and amplitude, with peak voltage exceeding background noise by a factor of five. When necessary, custom offline spike-sorting software (D. Gans) was used to segregate spikes into single units on the basis of waveform morphology.
To test whether spike counts differed significantly from background discharge, we used t-tests (paired or unpaired, depending on the type of trial). Background activity was measured in 20 or 32 samples of a 200-ms time window with no sound presentation. Spike counts that were significantly different from (greater or less than) the background discharge (p < 0.05) were defined as a response. As described above, we used a conservative criterion requiring that the response be observed in more than one test sequence for a given stimulus. Because background discharge was often low (<5 spikes/s), observation of suppressive effects of acoustic stimuli was infrequent. While we note suppressive effects when observed, we have refrained from population analyses.
In test sequences, spike discharge was obtained over 20 or more presentations of a stimulus in a repeated pattern, and analyzed in 2- or 3-ms bins. The first-spike latency, or beginning of a suppressive response, was calculated as the first bin after sound presentation in which the number of spikes per second was statistically different from baseline. After the response was initiated, the cessation of the response was calculated as the time in which the spike count was no longer statistically different from baseline. The duration of the excitatory or suppressive response was measured as the difference between the first-spike latency and the end of the response.
Some neurons displayed slowly acting response habituation (over several seconds), in which the spike count was initially significantly above background, but later changed to become no different than the background spike count. The “latency” of habituation was calculated as the time from initial response to the time at which a response ceased to meet statistical criterion for responsiveness. If the response returned, the duration of habituation was calculated from the beginning of the habituated response to the time when the response returned. To ensure that the loss of recording stability would not influence our observation of habituation, we required that the response recovered within 20 min.
Histological reconstruction
In each animal, up to four auditory responsive recording sites received iontophoretic deposits of neuronal tracers: biotinylated dextran amine (MW 10,000, 10% in saline, Molecular Probes, Eugene, OR, USA); FluoroGold (4% in saline; FluoroChrome, Inc., Englewood, CO, USA); FluoroRuby (tetramethylrhodamine dextran, MW 10,000, 10% in saline, Molecular Probes, Eugene, OR, USA); or fluorescein dextran (MW 10,000, 10% in saline, Molecular Probes, Eugene, OR, USA). Depending on the tracer, we used positive or negative current (5–7 μA; variable on/off) for 5–10 min. Because these deposits were also used for axoplasmic transport studies, no more than four sites were marked in each animal. To localize recording sites, we combined use of the stereotaxic coordinates with at least one tracer deposit in each recorded amygdala.
After tracer deposits, animals survived for 5–14 days, then were euthanized with an overdose of Fatal Plus (100 mg/kg, i.p., Vortech, Dearborn, MI, USA). Following loss of corneal and withdrawal reflexes, an animal was perfused with 0.1 M phosphate-buffered saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB). The brain was removed and stored overnight at 4 °C in 4% paraformaldehyde with 30% sucrose. After that time, brains were frozen, sectioned at 50 μm in the transverse plane, and collected into three series. One or two series (to be used for fluorescence) were mounted on gelatin-coated slides and coverslipped with DPX. One series was mounted on gelatin-coated slides and stained with cresyl violet for cytoarchitecture. In cases with BDA deposits, one series was treated with avidin–biotin–peroxidase and then stained with diaminobenzidine enhanced with nickel ammonium sulfate (Adams, 1981). The sections were then mounted on gelatin-coated slides, air-dried, and coverslipped with DPX.
Amygdalar borders and injection sites were plotted in every third section through the amygdala with a Zeiss Axioplan microscope and Neurolucida reconstruction system (MicroBrightField, Williston, VT, USA). Subdivisions of the amygdala are based on those described by Marsh et al. (2002). Once plotted, Adobe Illustrator was used to overlay injection sites onto one amygdalar series and add labels. These plots were used to analyze the dorso-ventral and medio–lateral distribution of auditory responses. Tracer deposit sites were photographed with a SPOT RT3 camera and SPOT Advanced Plus imaging software (version 4.7) mounted on a Zeiss Axio Imager M2 fluorescence microscope. Adobe Photoshop was used to adjust brightness and contrast globally.
RESULTS
This report describes auditory responses of 87 single neurons in the basolateral amygdala of awake mustached bats. A larger number of amygdalar neurons did not respond to any of the presented stimuli according to our criteria. Other neurons habituated so rapidly that their responses could not be evaluated. The results describe, in turn, the temporal features of auditory responses, their selectivity for vocal stimuli, their modulation by stimulation of the MG, and their distribution within the amygdala.
The background activity of most amygdalar neurons was low (mean ± standard deviation: 3.8 ± 3.2 spikes/s). Seventy-five percent of neurons had background discharge rates below 5.5 spikes/s. As we show in the next section, high “background” firing rates may reflect the prolonged effect of sensory input. We therefore excluded from the above statistics any neurons with background rates greater than 20 spikes/s.
Temporal features of auditory responses
When amygdalar neurons responded to sound, there were differences in several temporal features of their spike discharges. Three basic patterns were observed, sometimes within the same neuron for different stimuli: locked excitation (either tonic or phasic), persistent firing, and suppression. For many neurons, two or more of these patterns were observed in response to the same stimulus.
Locked excitatory responses
Neurons with locked excitatory responses displayed an increased discharge for a limited duration after stimulus onset. We observed this pattern in responses to 85 stimuli across 45 neurons. In some neurons the spiking pattern was phasic, consisting of a discharge of one or a few tightly locked spikes that did not exceed 5 ms (Fig. 1A). Eleven percent of locked excitatory responses (9 of 85 responses) displayed a phasic pattern. The remainder of locked excitatory responses (89%, 76 of 85 responses) displayed a more sustained pattern, with increased discharge lasting more than 10 ms (Fig. 1B). This group also included responses lasting longer than the stimulus duration. Sustained responses typically had discharge patterns that were not as precisely locked to the stimulus as occurred for phasic responses (c.f., Fig. 1A, B).
Fig 1.
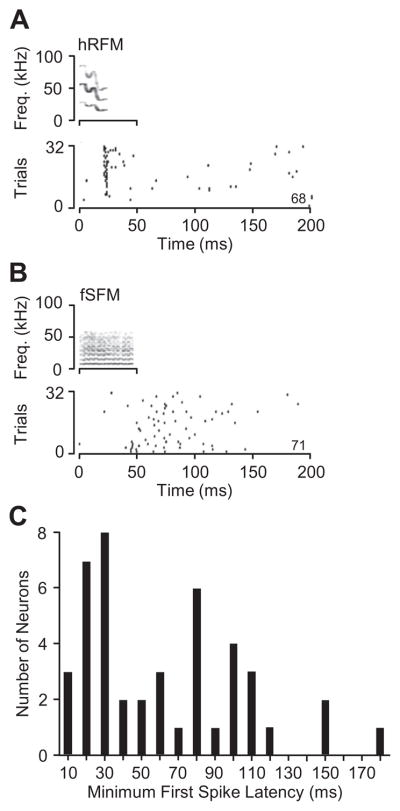
Locked responses to acoustic stimuli by amygdalar neurons. (A) Phasic response was tightly locked to the humped rippled frequency-modulated (hRFM) call. (B) Locked response to fixed sinusoidal frequency-modulated (fSFM) call was sustained, with more variable timing of first spike. Numbers at the lower right of each PSTH show spike count in response to 32 stimuli. (C) Minimum first-spike latencies for each neuron that displayed a locked response, across all stimuli. Abbreviations used in the figures: hRFM, humped rippled frequency-modulated; fSFM, fixed sinusoidal frequency-modulated.
For neurons that displayed locked excitatory responses, minimum first-spike latencies were broadly distributed (range, 10–178 ms; median, 54 ms) (Fig. 1C). For vocal stimuli, we were generally unable to identify the acoustic element that evoked excitation. As a result, some latency measurements may overestimate the true first-spike latency for many responses. Moreover, for responses that began after the end of a vocal stimulus, we could not determine whether the response was to stimulus onset or offset. However, for responses to artificial stimuli (tone and noise bursts), we were able to evaluate spike-timing issues by varying the duration of signals. Among the eight neurons tested in this manner, three were related to stimulus onset, with first-spike timing unaffected by changing sound duration. In four neurons, neural discharge was related to sound offset, with the latency of discharge increasing with increasing signal duration. The eighth neuron was duration-sensitive, responding to 51-ms signals but not to durations that differed by 10 ms or more.
Persistent firing
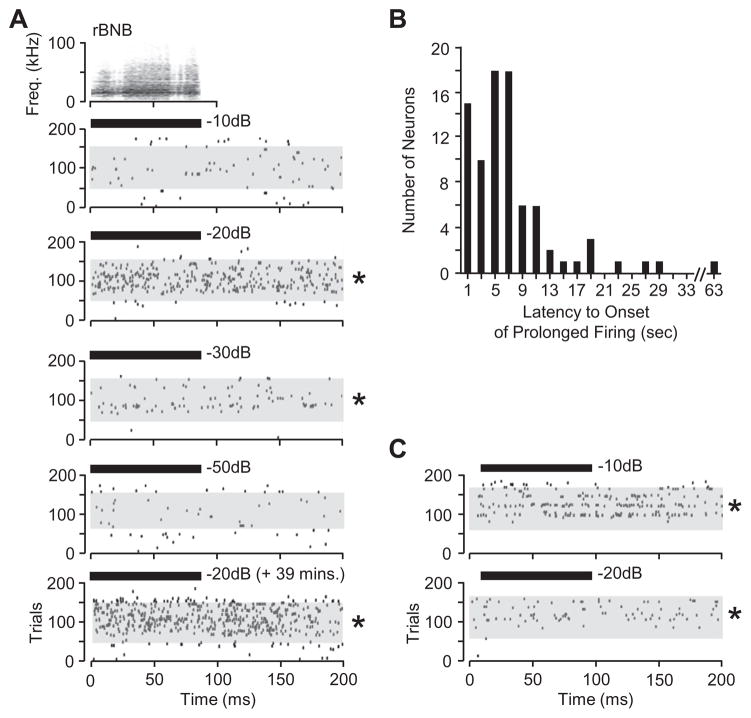
A striking pattern of spike discharge was observed in 67% of neurons (58 neurons, responses to 85 stimuli). After the onset of trials featuring repetitions of the same stimulus, spike discharge increased and remained elevated for hundreds of milliseconds or more (Fig. 2). To document this persistent firing pattern, we recorded 50 trials of background activity (recording window at 200 ms), followed by 100 trials during which a sound was presented, followed by another 50 trials with no sound. In Fig. 2A, the response to the rectangular broadband noise burst (rBNB) call was tested across a range of sound levels in 10-dB increments. During the first 50 trials, prior to acoustic stimulation, background discharge was very low, less than 1.2 spikes/s. When the rBNB call was presented at a low level (−50 dB) or at a high level (−10 dB), spike discharge during the 100 repetitions with sound was not significantly different from the background discharge. However, for intermediate sound levels (−30 and −20 dB), spike discharge increased significantly above background levels to 4.0 and 23.6 spikes/s, respectively (asterisks, Fig. 2A). This persistent firing response was consistent in tests obtained 39 min apart (Fig. 2A, lower raster).
Fig 2.
Persistent firing in amygdalar neurons. (A) Raster plots show persistent firing to the rBNB call only at intermediate sound levels (−20 and −30 dB). Asterisks show statistically significant changes in firing compared to background (i.e., the no-sound condition). Gray rectangles indicate trials in which the sound was presented. Black rectangle indicates the timing and duration of the rBNB call. The second test featuring the −20-dB stimulus (bottom panel) occurred 39 min after the initial −20-dB test. Sound was presented at a rate of 2/s. (B) Persistent firing pattern was initiated seconds or tens of seconds after the onset of the stimulus train. Each histogram bar represents the shortest latencies of onset of persistent firing for an individual neuron, across tests and across calls. (C) In a different neuron, persistent firing evoked by −10- and −20-dB rBNB stimuli remained constant after sound presentation and before the subsequent sound presentation. No significant differences were observed in spike rates calculated over different epochs of the recording window: 0–10 ms, 0–50 ms, 100–200 ms, 100–110 ms and 190–200 ms. This supports the audiovisual observations that persistent firing continued during the 300-ms interval between the end of one recording window and the beginning of the next window.
The temporal properties of this discharge pattern were distinctive compared to locked responses. First, onset of the increased discharge was often delayed, usually occurring seconds after the first presentation of the stimulus. Onset latency for persistent firing, referenced against the first sound in the 2/s train, ranged from 0.5 to 63 s (median 5.4 s) (Fig. 2B). Second, our qualitative and quantitative observations suggest that the persistent firing extended beyond our recording window (200 or 400 ms). For example, the discharge rate in Fig. 2A was high even at the beginning of each trace, before the stimulus would have evoked a locked increase in discharge rate. This is illustrated more clearly for the neuron in Fig. 2C that showed persistent firing to the rBNB call. When no sound was presented, this neuron had a spike rate of 0.6 spike/s. When the sound was presented at levels of −20 and −10 dB, the spike rate increased to 14.0 and 29.6 spikes/s, respectively. We compared the spike rate in the initial 10 ms of the recording window with the rates at later epochs. The discharge rates within the first 10 ms of the window, 14.8 and 31.3 spikes/s, respectively, were similar to those after the sound presentation. This result suggests that the elevated discharge rate remained high for the ~400 ms between the offset of one stimulus and the onset of the next. A third temporal feature of the persistent firing pattern was related to firing after the stimulus train ended. In 35 of the 85 responses featuring persistent firing, the increased discharge lasted between 1 and 120 s. However, in the majority of cases (45/85 responses), persistent firing extended beyond 120 s and lasted as long as 11 min beyond the last presented stimulus. These results suggest that some amygdalar neurons respond to optimal acoustic stimuli with an increased discharge that lasts for hundreds of milliseconds, seconds, or even minutes.
Suppressive responses
In 10 neurons we observed suppression of spiking activity, in either the background discharge, locked excitatory responses, or persistent firing. An example is shown in Fig. 3A. The background discharge of this neuron was 2.8 spikes/s (determined during 32 “no sound” traces of 200 ms). When the rBNB call was presented, suppression of background firing was followed by weakly locked firing. The suppression (0.6 spikes/s) occurred about 60 ms after presentation of the rBNB call and lasted for about 100 ms after stimulus offset. The locked response (7.5 spikes/s) began about 100 ms after sound offset (Fig. 3A). For the nine neurons that showed suppression of background discharge, the latency of suppression varied from 20 to 210 ms (median = 40 ms) after stimulus onset and lasted for 32–180 ms (median = 63 ms). We suspect that many more neurons may display sound-evoked suppression of spiking, but the low background discharge of most amygdalar neurons limited such observations.
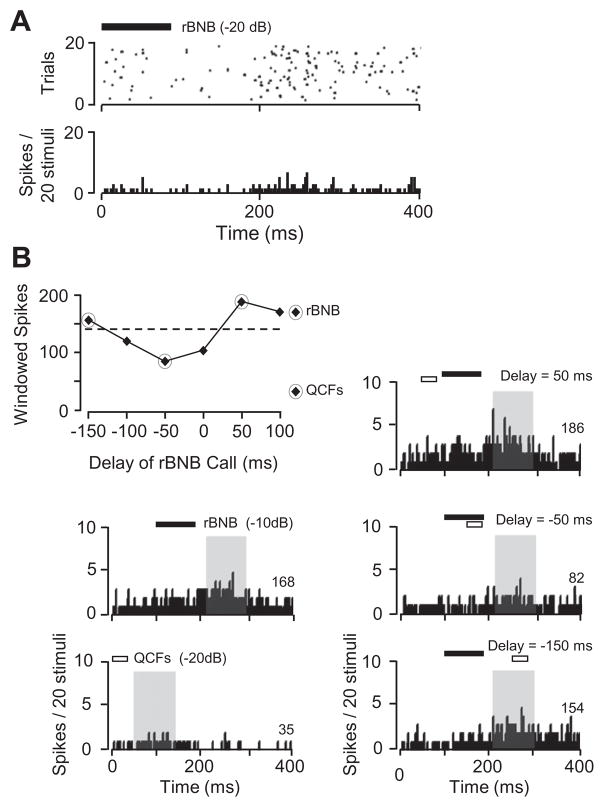
Fig 3.
Suppression of background and evoked firing by acoustic stimuli. (A) Raster plot and PSTH show that response to rBNB call includes suppression of background activity followed by a weak, locked discharge. (B) Excitatory discharge to one call was suppressed by a second call. PSTHs show robust firing to rBNB call and weak response to QCFs call when these were presented alone. Note difference in both locked and persistent spike patterns. The locked discharge to the rBNB called was suppressed by QCFs call that overlapped in time. The persistent discharge to the rBNB call was suppressed when the QCFs call was presented during or after the rBNB call. Delay function illustrates suppression of both locked and persistent firing as a function of the relative timing of the two calls. Data points falling below the dashed line have discharge rates significantly less than neuron’s response to the rBNB call alone. Spikes were counted within a 100-ms time window at the offset of the rBNB call (gray box). The number of spikes within this time window is shown at the right of each histogram.
Additional suppressive responses were revealed in two neurons during presentation of combinations of stimuli. In these tests, one stimulus evoked an excitatory response that was suppressed by a properly timed second stimulus. For the neuron in Fig. 3B, there were both locked and persistent firing responses to the rBNB call. There was a very weak, non-significant discharge to the quasi-constant frequency, short-duration (QCFs) call. However, when the QCFs call overlapped in time with the rBNB call, the increased discharge (persistent and locked) to the rBNB call was reduced or eliminated. No suppression was observed when the two signals did not overlap in time.
Response habituation
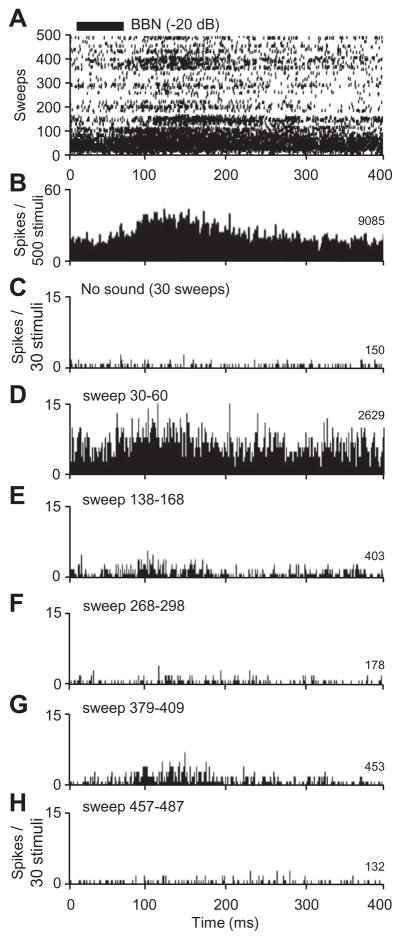
Although we were unable to study neurons that responded only to the first few presentations of a stimulus, many other neurons (n = 31) displayed habituation that had a slower time course. We defined habituation as the elimination of the statistically significant increase (for locked and persistent firing responses) or decrease (for suppressive responses) in firing activity compared to background discharge. The neuron in Fig. 4 displayed responses to a broadband noise burst that showed both habituation and recovery over 4 min (Fig. 4A). An overall PSTH shows both locked and persistent firing patterns (Fig. 4B). In a separate no-sound test (Fig. 4C), the background discharge was 11 spikes/s. After a few repetitions of the stimulus (2/s), the neuron displayed both locked and persistent firing (Fig. 4D). The persistent firing response habituated at sound presentation 124 (Fig. 4E), and the locked response habituated at trace 203 (Fig. 4F). The locked response returned between repetitions 365–440 (Fig. 4G), and then habituated again between repetitions 441–532 (Fig. 4H). After 10 min with no sound stimulation, both locked and persistent responses could be evoked by renewed sound stimulation.
Fig 4.
Response habituation in an amygdalar neuron. (A) Raster display of spike discharge during presentation of a broadband noise burst over 500 consecutive trials. This neuron displayed both locked and persistent response patterns. (B) PSTH of entire 500-trial recording. (C) No-sound test immediately preceding the recordings in (A) and (B). (D) PSTH shortly after sound presentation was initiated. (E) Habituation preferentially affected persistent responses. (F) Habituation affected both locked and persistent responses. (G) Partial dishabituation, (H) Renewed habituation. Black bar indicates the timing and duration of the broadband noise stimulus. Number of spikes in each histogram is shown at right.
To examine the time course of response habituation, 3–5 successive tests were performed in which the optimal stimulus was presented at 2/s for 4–10 min. Across the population, habituation began 7.5 s to 11.2 min after the onset of the stimulus train (median = 67 s) and lasted from 5 s to more than 23 min (median = 51 s). Most neurons with habituating responses displayed persistent discharge (45/49 responses), with fewer neurons displaying locked responses (10/49) or suppressive responses (1/49). In about one-third of tested neurons (10/31), the spike rate after recovery from habituation was lower than that of the original response. This decrease occurred only when there was no break in the repetitions of the sound (2/s). In all tested neurons, the evoked spike rate returned to pre-habituation levels when the sound stimulus was turned off for 10–30 min.
Selectivity of auditory responses
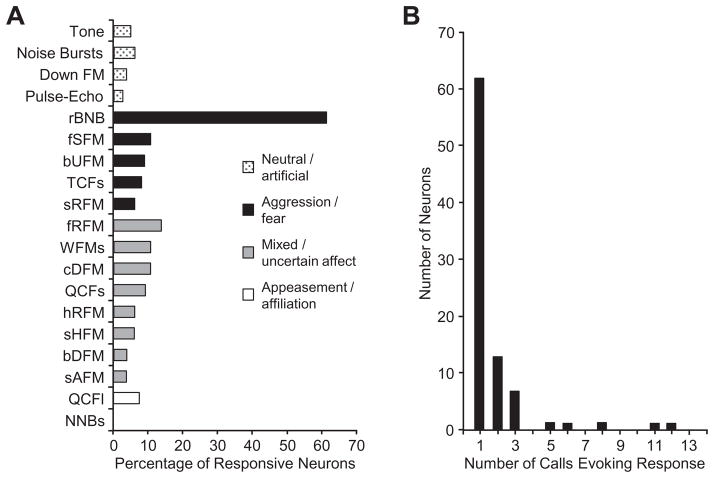
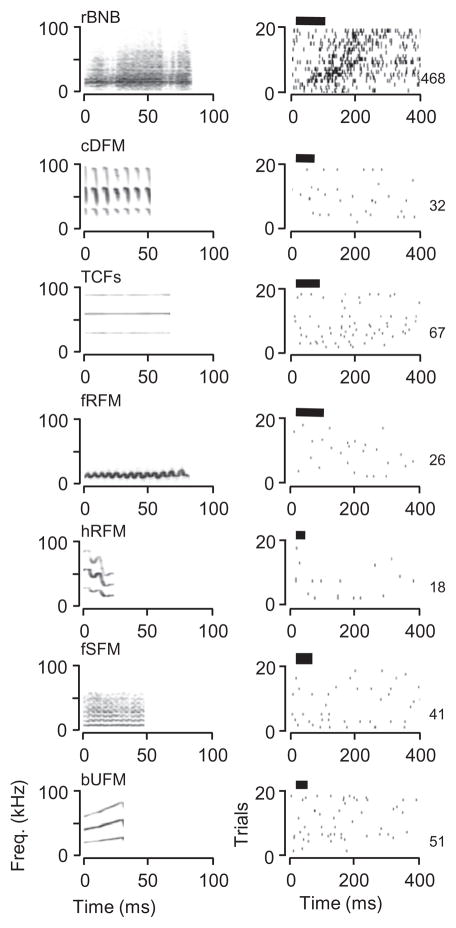
Most acoustically responsive neurons in the mustached bat’s amygdala were excited by a very narrow range of sounds. Of the 87 neurons in the sample, 58 (67%) displayed a repeatable, statistically significant response to only a single type of stimulus. In general, neurons were most likely to respond to social vocalizations (82/87 neurons), with very few neurons responding to tones, noise bursts, frequency-modulated sweeps, or sonar pulse–echo combinations (Fig. 5A). When neurons responded to social vocalizations, they displayed high selectivity, usually responding to a single type of vocal call (mean, 1.74 calls) (Fig. 5B). The largest number of responses was to the rBNB call, a noisy call associated with aggressive interactions (Fig. 5A, Clement et al., 2006). An example of a neuron’s selective response to the rBNB call is shown in Fig. 6. Both locked and persistent firing responses are evident. For other stimuli, there was no locked firing and the overall discharge was highly variable and not repeatable. In general, highly selective neurons responded well to the effective stimulus. Their median stimulus-evoked spike rate at the best sound level was 11.3 spikes/s, with 75% having a discharge rate of 7.5 spikes/s or higher. We used a response index measure to quantify the increase in response rate compared to background discharge. For neurons responsive to a single stimulus, the acoustically driven response increased to 470% of background activity. Three-quarters of evoked responses in these selective neurons were at least 250% above background spike rates.
Fig 5.
Amygdalar neurons responded preferentially and selectively to social vocalizations. (A) Percentage of 87 neurons that discharged in response to different acoustic stimuli. The rBNB syllable evoked responses from the largest percentage of amygdalar neurons. Overall, most acoustically responsive amygdalar neurons responded only to social vocalizations, with few responses to simple acoustic elements (tones, noise, or FM sweeps) or simulated sonar pulse–echo combinations. (B) Most acoustically responsive amygdalar neurons responded to only one syllable type. Abbreviations for mustached bat syllables (in order shown) based on Clement et al. (2006): rBNB, rectangular broadband NB; fSFM, fixed sinusoidal FM; bUFM, bent upward FM; TCFs, short, true CF; sRFM, stretched rippled FM; fRFM, fixed rippled FM; WFMs, short, wrinkled FM; cDFM, checked downward FM; QCFs, short quasi CF; hRFM, humped rippled FM; sHFM, short humped FM; bDFM, bent downward frequency modulated; sAFM, single arched FM; QCFI, long, quasi constant frequency; NNBs, short, narrowband noise burst.
Fig 6.
Amygdalar neuron responded to the rBNB call but not to other calls. The response to the rBNB call includes both locked and persistent elements. There was no evoked discharge to other calls. Background, or non-locked discharge varied in responses to other stimuli. The higher non-locked discharge in response to some stimuli (e.g., TCFs and NNBs calls) was not repeatable. All stimuli presented at an attenuation of 20 dB. For each stimulus type, we display spectrograms (left) and the rasters (right). For abbreviations of syllable types, see Fig. 5 caption.
One-third of neurons (29/87) responded to multiple stimuli. An example is shown in Fig. 7, which displays rasters associated with responses to six mustached bat vocal signals. Strong, locked responses that varied with sound level were obtained in response to eleven signals, many consisting of a spectrotemporally structured, relatively broadband syllable (Fig. 7A). Spike discharge to these calls began about 50 ms after stimulus onset, with median first-spike latencies ranging from 45 to 66 ms. Four other syllables, with both narrow and broad frequency bands, with short and long durations, did not evoke consistent responses (e.g., Fig. 7B). Moreover, the neuron did not respond to artificial stimuli (tones, noise, or frequency-modulated stimuli). Thus, even neurons that responded to multiple sounds displayed selectivity among acoustic stimuli.
Fig 7.
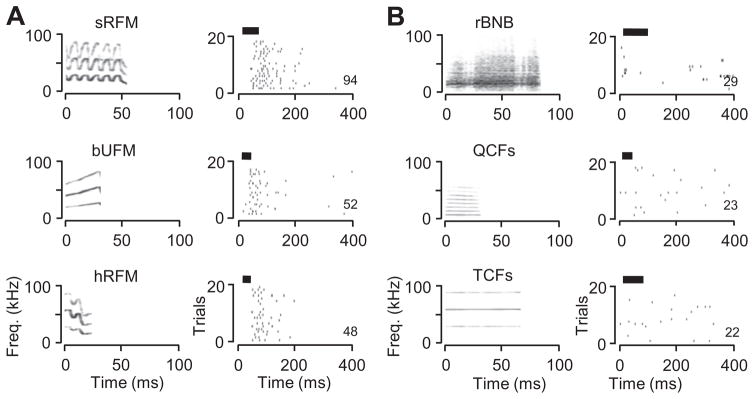
Amygdalar neuron responded to many but not all syllables. (A) Spectrograms and raster plots, associated with three syllables to which this neuron responded consistently. (B) Spectrograms and raster plots associated with three other vocalizations to which the neuron did not respond consistently. Each stimulus was presented at a level of 20 dB below maximum. Black bars represent the timing and duration of the stimulus. Numbers at lower right of each raster indicate total spikes in response to 20 stimuli.
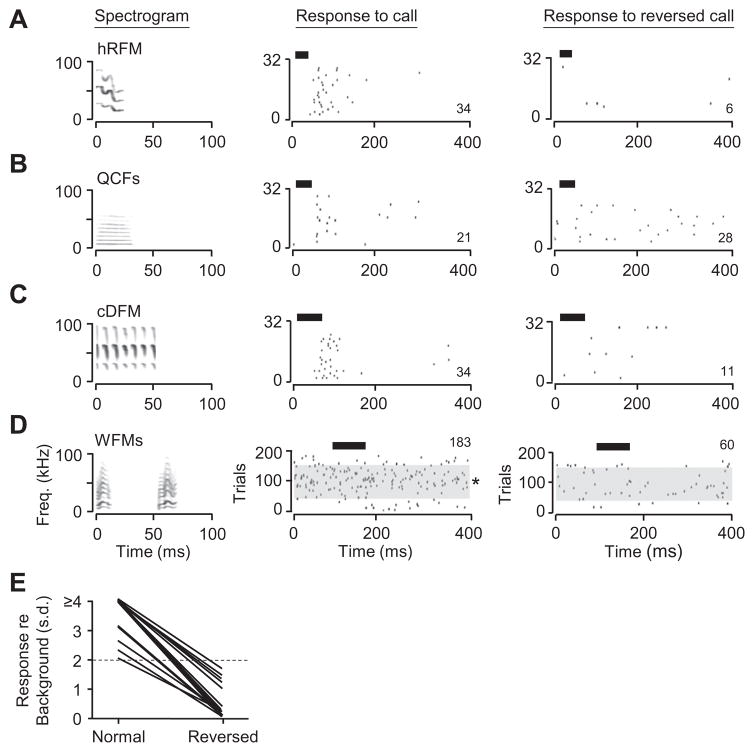
We explored several aspects of this response selectivity. To examine the dependence of auditory responses on the frequency–time structure of signals, we compared responses to normal and reversed calls (Fig. 8). Responses to three calls from one neuron were robust and well-locked (Fig. 8A–C), but responses to the reversed calls were always less when referenced to background discharge. In Fig. 8D, a different neuron showed a persistent discharge to the normal call, but no significant response when the call sequence and the elements of the call were reversed in time. From eight neurons, we compared responses to calls and reverse calls, totaling 18 pairs of calls. In each of the 18 pairs, there was a substantial reduction in response to the reversed call (Fig. 8E).
Fig 8.
Time-reversed social calls did not elicit strong responses in amygdalar neurons. (A–C) Responses of one neuron to three normal and time-reversed calls. For each call, the locked discharge was reduced. (D) Persistent spike discharge of a different neuron was substantially reduced when WFMs call pair was reversed. The raster shows spikes during 200 trials; see Fig. 2 for description. For the normal call, asterisk indicates that the response to stimulus presentation was statistically greater than during the no-sound trials (*p < 0.05, t-test). For the reversed call, there was no statistical difference in firing rate for sound vs. no-sound trials. All sounds in (A–D) were presented at 20-dB attenuation. (E) Response of neurons to normal and time-reversed calls. Response is computed as the number of standard deviations (s.d.) above baseline spiking activity. Values above two standard deviations (dashed line) represent our criterion for a response. In all cases, spike discharge was substantially reduced with time-reversal of calls.
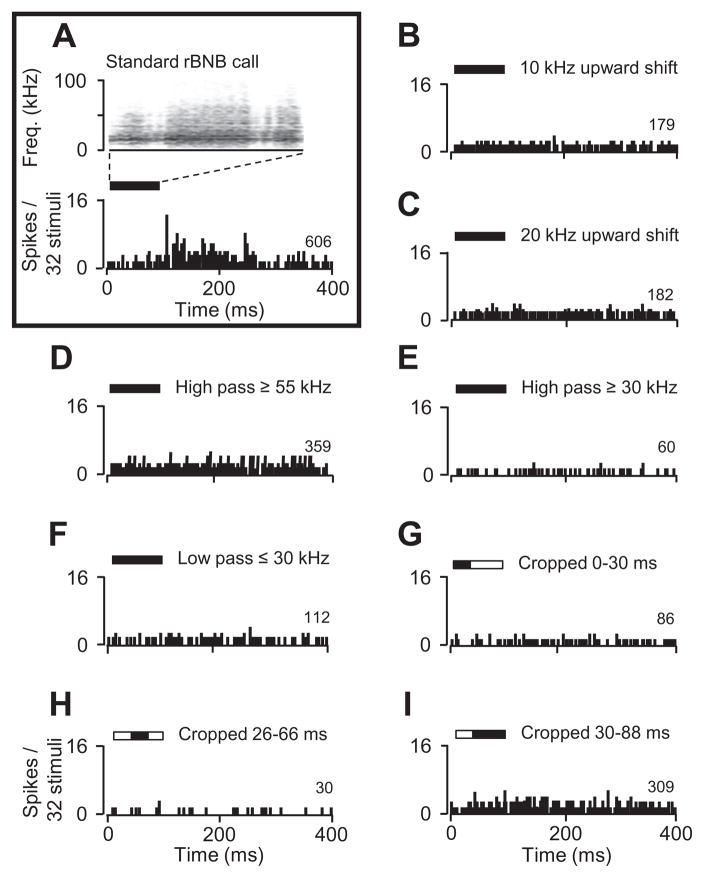
In 10 neurons, we examined responses to sounds in which spectral or temporal elements were shifted or eliminated. An example is shown in Fig. 9. This neuron displayed a locked response to the rBNB call with a latency of 107 ms (Fig. 9A). When the entire call was shifted upward by 10 or 20 kHz (Fig. 9B, C), the locked response was no longer evident. To examine whether the response depended on particular frequency bands, we presented frequency-filtered versions of the call. There were no stimulus-locked responses to these filtered versions (Fig. 9D–F). Finally, we presented versions of the call in which we eliminated early, late, or both early and late segments of the signal. None of these manipulated signals evoked a locked response, although the background discharge varied across stimuli (Fig. 9G–I). In eight of the 10 neurons tested in this way, there was no response to the altered signals. These results suggest that a complex set of temporal and spectral features is required for auditory responses by these amygdalar neurons.
Fig 9.
Alteration of the spectral or temporal properties of calls eliminated auditory responses in an amygdalar neuron. (A) Sound spectrogram and post-stimulus time histogram (PSTH) show a locked response to the rBNB call (boxed area). (B–I) Altered rBNB calls did not evoke locked responses, although background discharge varied. Solid black bars represent the timing and duration of the sound stimulus. Numbers at the lower right of each PSTH show spike count in response to 32 presentations.
Amygdalar responses during MG stimulation
We obtained recordings from 12 amygdalar neurons before and during iontophoretic application in MG of an excitatory drug mixture including GLU, ASP, and/or BIC. Auditory-evoked responses and histological reconstruction confirmed location of the multibarrel drug-application pipette in the medial MG, centered in the medial or dorsal divisions (e.g., Fig. 10A). The main result was that excitatory drug application in MG resulted in increased background and stimulus-evoked discharge, but stimulus-evoked discharge showed a greater increase. An example is shown in Fig. 10. In pre-drug testing, background activity (“No sound”) for this neuron was 113 spikes over 42 trials of 400-ms windows, or 6.7 spikes/s (Fig. 10B). This increased to 237 spikes (14.1 spikes/s) with GLU/ASP/BIC application in the medial MG (Fig. 10A, C). The auditory-evoked response also increased: the pre-drug persistent firing rate to the rBNB syllable was 14.0 spikes/s (Fig. 10B), but increased to 74.0 spikes/s during drug application in MG (Fig. 10C). Thus, the overall gain of the neuron (i.e., evoked spike rate/background rate) more than doubled, increasing from 2.1 to 5.3.
Fig 10.
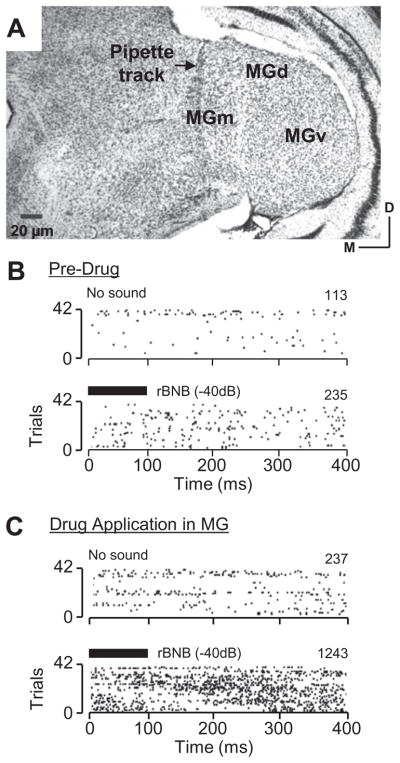
Drug application in the medial geniculate nuclei body (MG) alters the response of an amygdalar neuron to sound. (A) Nissl-stained section of MG shows track of a multibarrel pipette in the medial division of the MG. (B) Spike discharge of an amygdalar neuron before drug application in MG. Raster plots show background spiking (No Sound) and persistent firing response to rBNB syllable (black bar, −40 dB). (C) Spike discharge of same amygdalar neuron during GLU/ASP/BIC application in MG. Raster plots show background and rBNB-evoked spike discharge. Drug application increased both the background and evoked spiking. Number of spikes is shown in the upper right corner of each raster plot. Abbreviations: MGd, dorsal division of MG; MGm, medial division of MG, MGv, ventral division of MG.
In the tested sample, eight neurons responded to a single communication call while four neurons responded to multiple calls (total 21 responses to auditory stimuli). Upon drug application in MG, background spike rate more than doubled (pre-drug, 0.16 ± 0.17 spikes/s; drug application, 0.40 ± 0.67 spikes/s; paired t-test, p < 0.05, df = 40). Auditory-evoked responses increased by an even greater amount (pre-drug, 0.38 ± 0.49; drug application, 1.38 ± 2.98; paired t-test, p < 0.05). The increase in response gain with drug application (80.7% ± 120.1%) was also highly significant (paired t-test, p < 0.05).
Location of amygdalar recording sites
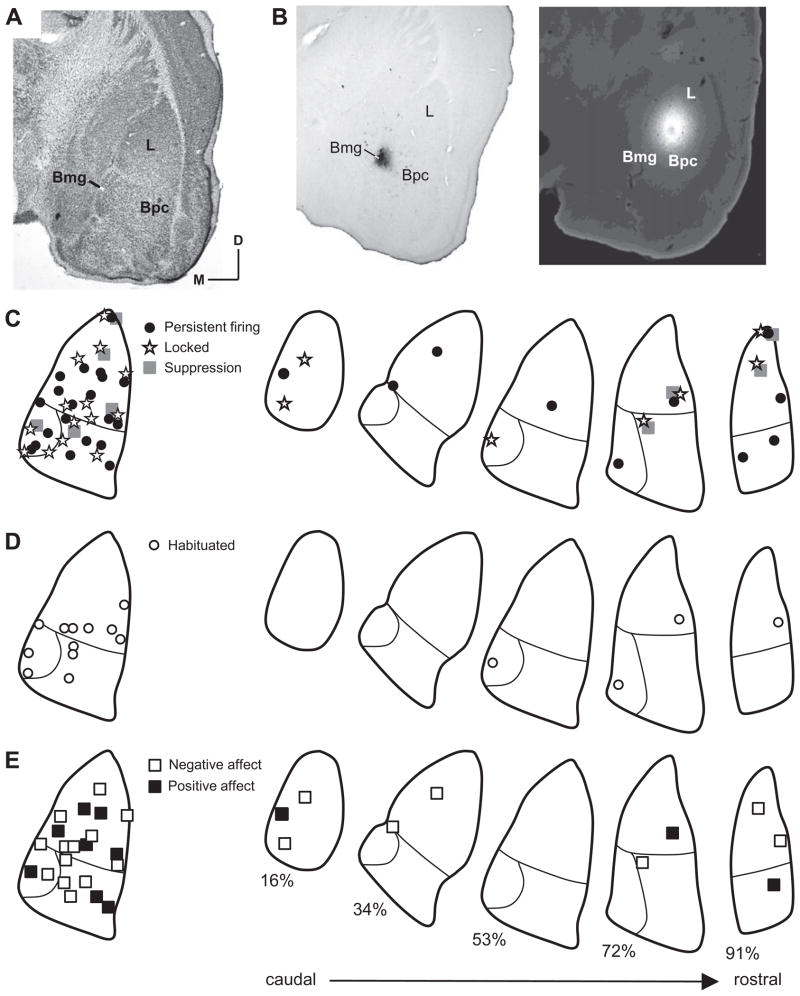
The locations of recording sites were assessed by stereotaxic coordinates and, in some cases, tracer deposits. For 39 recording sites, successful deposits were small enough to pinpoint location within an amygdalar subdivision (Fig. 11). Locked, persistent, and suppressive firing patterns were observed throughout the dorso-ventral, medio-lateral, and rostro-caudal extent of the lateral and basal nuclei (Fig. 11C). Thirteen of the 39 recording sites in Fig. 11C displayed two temporal response patterns. Of these, five had both locked and suppressive responses and are displayed as suppressive responses in Fig. 11C. Eight neurons with persistent firing responses in the lateral nucleus also had locked responses; these are plotted as persistent firing responses for figure clarity. Neurons with dual temporal patterns were observed at depths ranging from 4547 to 5102 μm, indicating their occurrence in both the lateral and basal amygdala.
Fig 11.
Auditory responses were observed throughout the lateral and basal nuclei of the amygdala. (A) Nissl-stained, coronal section through amygdala. This section is used in outlines at left in (C–E). Abbreviations: Bpc, parvicellular division of basal nucleus, Bmg, magnocellular division of the basal nucleus; D, dorsal; L, lateral nucleus; M, medial. (B) Recording sites marked by biotinylated dextran amine deposit in Bmg (left) and by Fluoro-Gold deposit in L (right). (C–E) Anatomical distribution of different categories of auditory responses, compressed onto a single section (left) and displayed throughout a rostro-caudal series of amygdalar sections matched to site location (right). Only responses localized by tracer deposits are plotted. (C) Distribution of neurons based on temporal discharge pattern. (D) Distribution of habituating responses. (E) Distribution based on affect of call as described previously (Clement et al., 2006). Numbers below sections indicate percent location within caudal-to-rostral series.
Tracer deposits marked the locations of 12 habituating responses. Habituating responses were observed in both the lateral and basal nuclei (open circles, Fig. 11D). During recording sessions, they were observed at depths ranging from 4547 to 5230 μm, indicating a wide distribution throughout the amygdala.
At recording sites showing selective responses to one call type, we plotted the emotional affect of the call (Clement et al., 2006). We observed no dorso-ventral, rostro-caudal, or medio-lateral pattern of responses. Neurons responded to calls with positive affect (e.g., appeasement, calming) ranged in depth from 4501 to 5102 μm (black squares, Fig. 11E). Similarly, neurons that responded to communication calls with negative affect (e.g., aggression, warning, fear) ranged in depth from 4499 to 5230 μm (white squares, Fig. 11E). Overall, we observed no clear clustering of the various response properties within the amygdala, but the numbers of localized recording sites precluded a definitive analysis.
DISCUSSION
This study describes two striking features of basolateral amygdalar responses to acoustic stimuli in the mustached bat. First, neurons were highly selective for particular social vocalizations, with most responding to only one or two syllables. Most responses were to syllables associated with aggression. Second, many neurons display a persistent firing pattern in response to the preferred stimulus, in which spike discharge remained elevated for seconds or minutes as a stimulus was repeated. This persistent discharge was not necessarily locked to individual stimulus presentations, and it could persist for seconds after a train of stimuli ended. These striking features have implications for both the extent to which the amygdala responds to vocal stimuli and the spike discharge associated with vocal stimulation.
Selectivity for vocal stimuli in the basolateral amygdala
Amygdalar neurons responded to few auditory stimuli. Less than 20% responded to synthesized tones, noise, or FM sweeps. Instead, nearly all auditory-responsive neurons (94%) displayed excitation to syllables found in social vocalizations but with high selectivity. On average, neurons responded to 1.7 of the 15 presented syllables, or 12%. Further, they responded poorly when syllables that evoked a response were modified temporally or spectrally. Most neurons responded to the rBNB call, a noisy, hiss-like syllable associated with aggression (Clement et al., 2006). In general, calls associated with predominantly negative affect evoked the largest proportion of responses. Each of these results corroborates, in a qualitative way, the findings of a recent study by Naumann and Kanwal (2011) in the mustached bat’s amygdala. Our results also support their finding that suppressive responses are an important element of amygdalar responses, but the low spontaneous rate of most neurons precluded more systematic observation in our study.
Some quantitative features of our results differed substantially from the Naumann and Kanwal study. Generally, their neurons were more responsive to acoustic stimuli. For instance, they report that 85% of neurons responded to tonal stimuli, vs. 5% in our study. Further they report that neurons responded to a greater percentage of vocalizations (26% of presented calls). These response differences are the likely result of several methodological differences. All of our tests were performed using a stimulus rate of 2/s, while Naumann and Kanwal report rates of either 1/s or 2/s; it is unclear when one or the other rate was used. Stimulus rate is known to affect amygdalar responses (Ben-Ari and La Salle, 1974; Bordi et al., 1993; Adams et al., 2011). Further, our study presented stimuli from the contralateral sound field, while Naumann and Kanwal presented sounds from directly in front of the animal. Some amygdalar neurons respond differently to sounds in the ipsilateral and contra-lateral sound fields (Brown and Buchwald, 1973). Finally, we used a more strict response criterion, and only a single variant of syllables. Each of these differences helps to explain the more restricted responsiveness to acoustic signals in our study. Despite these differences, both studies show that excitatory responses to vocal stimuli are highly selective and that these are generally associated with syllables linked to aggression or fear. However, the comparison indicates some of the methodological variables that may affect amygdalar auditory responses.
Our results show more dramatic differences in amygdalar responses to vocal stimuli compared to a recent study in awake big brown bats from our laboratory (Gadziola et al., 2012). In a paradigm similar to the present study, Gadziola and colleagues presented 11 syllables or syllable sequences of big brown bat social vocalizations in a repeated presentation sequence, but used a stimulus rate of 1/s. In big brown bats, neurons responded well to both artificial sounds (65%) and social vocalizations (86%). Further, most amygdalar neurons responded to most calls; on average, neurons responded to 9 of 11 calls (82%). Thus, big brown bat neurons did not show the spike-rate-based selectivity for particular social vocalizations that was evident in both mustached bat studies. Nonetheless, amygdalar neurons in big brown bats were highly discriminative of vocalizations. This discrimination was expressed most strongly in the duration of excitatory response, and to some extent in the duration of persistent discharge and spike rate. Although the use of different stimulus repetition rates likely accounts for some differences in results between our two studies (present study vs. Gadziola et al., 2012), we do not believe that it solely accounts for this difference. In the two species, neurons participate in different forms of a population representation of social vocalizations: the mustached bat representation is based on neurons that respond to very few stimuli, while the big brown bat representation is based on broadly responsive neurons that code vocal stimuli on the basis of both response duration and magnitude.
Beyond these studies, there has been little systematic study of selectivity for, or discrimination among, social vocalizations by amygdalar neurons. Early work provided anecdotal evidence that some amygdalar neurons respond well to social vocalizations (O’Keefe and Bouma, 1969; Jacobs and McGinty, 1972; Brothers et al., 1990). Recent work in rats shows differential responses of amygdalar neurons to sequences of “22-kHz” and “50-kHz” ultrasonic vocalizations (Parsana et al., 2012). These vocal sequences are associated with aversive and affiliative behaviors, respectively. The aversive, 22-kHz signal generally evoked increased discharge while the 50-kHz signal mostly evoked suppression. What is surprising is that neurons appear to show little distinction in their spike discharge between the 22-kHz vocal sequence and a pure tone at the same center frequency and overall duration. Results from amygdalar neurons in both bat species suggest a higher discriminability than in rats. Nonetheless, the common theme in the rat and bat studies is the tendency for increased discharge to result from aversive vocal stimuli. We do not, however, observe the clear distinction between increased discharge to aversive vocalizations and decreased discharge to affiliative vocalizations that others have reported (Naumann and Kanwal, 2011; Parsana et al., 2012).
In humans, the amygdalar response to the affective features of vocal stimuli is similarly not clear cut. Certainly, many fMRI studies have shown that amygdalar activation increases in response to prosodic features of speech associated with negative emotions (Sander et al., 2005; Wiethoff et al., 2009), as it does in response to facial expressions of negative emotions (Boll et al., 2011; Dima et al., 2011). However, other studies show that increased amygdalar activation is associated with speech containing either positive or negative emotions (Fecteau et al., 2007), with the intensity of emotion in speech (Leitman et al., 2010), and with the identity of voices (Andics et al., 2010). Further, the affective bias in amygdalar activation may depend on experience. Thus, among adults who are parents, the amygdala is activated more by sounds of crying rather than laughing infants, whereas the reverse is true among adults who are not parents (Seifritz et al., 2003). These studies support the broader perspective that the amygdala participates in evaluating the biological significance or salience of a broad range of sensory stimuli, with both positive and negative valence or affect (Heimer and Van Hoesen, 2006; Costafreda et al., 2008), particularly sensory stimuli associated with social communication (Sander and Scheich, 2005; Ball et al., 2007; Gothard et al., 2007; Sergerie et al., 2008; Van Bavel et al., 2008; Andics et al., 2010).
One way to reconcile these views is to recognize the multiple functions of amygdalar output. The coordination of emotional responses resulting from negative-affect stimuli—e.g., learned aversive sounds, angry prosody, fearful faces, or the smell of predators—is a hallmark of the central nucleus of the amygdala (Rolls, 2000; Cardinal et al., 2002; Sah et al., 2003; Price, 2003). There is little doubt that the circuitry involving the central amygdala is more closely tied to aversive emotional responses (Hopkins and Holstege, 1978; Krettek and Price, 1978; Pitkänen et al., 1997; Paré et al., 2004). On the other hand, another major function of the amygdala seems to be an analysis of stimulus salience, positive or negative, that results in modulation of sensory processing (Bjordahl et al., 1998; Kilgard and Merzenich, 1998; Ma and Suga, 2003; Chavez et al., 2009) through its direct projections (Amaral and Price, 1984; McDonald and Jackson, 1987; Kosmal et al., 1997; Marsh et al., 2002; Yukie, 2002) and indirect projections through the basal cholinergic fore-brain (Price et al., 1987; Pitkänen et al., 2000; Paré, 2003).
Persistent firing to vocal stimuli
A major finding of this study is that many amygdalar neurons display their selective response to acoustic and vocal signals through persistent discharge, i.e., the duration of firing that extends beyond the duration of the stimulus. This persistent firing usually occurred seconds or tens of seconds after the beginning of a train of acoustic stimuli and was not locked to individual stimulus presentations in any obvious way. Rather, firing continued for at least the interval between stimuli, thus lasting over 400 ms. Following the termination of a stimulus train, neurons continued to fire for periods of seconds to minutes. While these features suggest an independence from the acoustic stimulus, there was a clear dependence on both the stimulus type and sound level. Our conclusion is that this firing is the result of both the acoustic stimulus and some element of the context surrounding the stimulus (e.g., the animal’s emotional state) and represents a critical aspect of the output of neurons in the basolateral amygdala.
Although Naumann and Kanwal (2011) report many responses that feature some degree of persistent firing, they do not describe the dramatic persistent firing behavior that we report here. We propose that the difference may relate to the sequencing of stimuli. We used a repeated stimulus paradigm, in which the same stimulus was repeated for 20 or more repetitions. This preserves at least some of the affective context of the syllables. Naumann and Kanwal presented a sequence of syllables based on syllable acoustics, and then repeated the sequence. By presenting stimuli in such a sequence, Naumann and Kanwal reduced the context associated with particular vocal signals. If persistent firing depends on both stimulus and context as we hypothesize, then it would be less likely that amygdalar neurons display persistent firing using their paradigm. We believe these differing results show that syllable sequencing may be a critical factor in the responses of amygdalar neurons to vocal stimuli.
In the big brown bat, the duration of spike discharge underlies the ability of lateral amygdalar neurons to discriminate among vocal signals (Gadziola et al., 2012). This is based in part on persistent firing, which could extend for at least 250 ms beyond stimulus duration. Furthermore, manipulations of signal features that increased their salience, such as the modification of a weakly aggressive call by addition of a tonal ending, could substantially increase the duration of persistent firing. Thus, both studies indicate that persistent firing is a major feature of amygdala responses to social vocalizations. They reinforce our view that stimulus sequencing may have a major impact on amygdalar neuron discharge, particularly if the sequencing alters the salience of acoustic signals. Ultimately, an understanding of the role of persistent firing must be examined when syllables are presented in the appropriate acoustic context, i.e., within probabilistic sequences that can be associated with particular behaviors or emotional states.
Persistent firing functions in several neural systems to sustain representations of sensory stimuli for working memory (Frank and Brown, 2003; Major and Tank, 2004). Such firing is present in auditory responses of cortical (Pena et al., 1999; Romanski et al., 2005; Moshitch et al., 2006; Bendor and Wang, 2008; Campbell et al., 2010) and pontine (Miller and Covey, 2011) neurons. Among basolateral amygdalar neurons, it has been observed to auditory and other sensory stimuli (Bordi and LeDoux, 1992; Bordi et al., 1993; Maeda et al., 1993; Naumann and Kanwal, 2011), but it had not been related until recently to discrimination of acoustic stimuli (Gadziola et al., 2012) or to response selectivity (this study). Persistent firing likely plays a crucial role in memory operations associated with the amygdala (Pelletier et al., 2005; Egorov et al., 2006), but may also serve a more general function by transforming the time scale of acoustic stimuli to time scales appropriate for control of emotional expression and modulation of sensory processing.
In vitro studies of the amygdala, entorhinal cortex, and endopiform nucleus have reported similar firing patterns, termed graded persistent firing (Egorov et al., 2002, 2006; Frank and Brown, 2003). In these studies, depolarizing current in the presence of muscarinic agonists creates a persistent firing pattern that depends on the strength and duration of the depolarizing current. The persistent nature of the firing depends on a cholinergic input to maintain the increased firing rate (Frank and Brown, 2003; Egorov et al., 2006). If the persistent firing response that we observed has a similar mechanism, then it likely depends on the combination of auditory input from MG or auditory cortex (LeDoux et al., 1990; McDonald, 1998) and cholinergic inputs from the nucleus basalis (Emson et al., 1979; Carlsen et al., 1985).
A further aspect of our results relevant to persistent firing is the finding that chemical activation of the medial MG, a region that projects directly to the lateral amygdala, raised firing rates of amygdala neurons. Particularly intriguing is the observation that the sound-evoked, persistent firing rates increased more than background firing, and the increase in evoked firing was greater, in the presence of MG activation. This suggests that the auditory environment may establish a level of amygdala input that can enhance the gain of salient auditory stimuli. Because the MG has projections to both auditory cortex and amygdala, it is unclear whether direct MG projections, indirect higher order projections, or a combination of multiple amygdalar inputs are necessary to elicit this response.
There are several possible explanations of the fact that chemical stimulation of the medial geniculate influenced the background activity of non-auditory responsive neurons. (1) It is possible that we did not use appropriate auditory stimuli to elicit responses in these neurons. (2) The excitation may have influenced other multi-synaptic circuits that project to the amygdala (e.g., nucleus basalis of Meynert, hippocampus, auditory cortex, and/or association cortices). Combinations of excitatory input from these projections may have influenced the background activity. (3) The excitation from MG activation may have had a direct excitatory effect on local amygdalar circuits. (4) MG projections only activate amygdalar neurons when large numbers of MG projection neurons are activated simultaneously. The precise timing of combinations of inputs (sensory, memory, association) may then enable the amygdala to evaluate stimulus significance.
Response habituation in the amygdala
Although our experimental design precluded study of short-term adaptation effects acting on a time scale of a few seconds or less, we did observe habituation with time scales of seconds or minutes. In some neurons, the habituation waxed and waned, while in others the habituation continued until acoustic stimulation ended. Our interpretation of these effects is that the non-auditory elements of input to the amygdala, including those that may activate persistent firing, change over time.
Some amygdalar habituation is likely inherited from the auditory inputs. Several studies in the past decade have documented the presence of stimulus-specific adaptation in auditory responses. Repeated sounds evoke diminished responses while those that are novel evoke a stronger response (Ulanovsky et al., 2003; Pérez-González et al., 2005). The major auditory inputs to the amygdala, the medial and dorsal divisions of the MG and the auditory cortex, each show stimulus-specific adaptation (Ulanovsky et al., 2003; von der Behrens et al., 2009; Anderson et al., 2009; Antunes et al., 2010). This could well explain the poor response to artificial stimuli in our study. Moreover, the longer duration forms of habituation in our study may correspond to the longer time scales of adaptation observed in auditory cortical neurons (Ulanovsky et al., 2004).
Nonetheless, the major time-varying response feature that we observed among amygdalar neurons is very different from stimulus-specific adaption, in fact closer to its opposite. In response to repeated presentations of the same vocal stimulus, spike discharge increased and became persistent. Further, we believe that this effect only occurs when the stimulus is repeated, since stimulus presentation with an interleaved stimulus paradigm (Naumann and Kanwal, 2011) does not evoke the level of persistent discharge that we observed. These results are consistent, in our view, with the understanding that the amygdala analyzes stimulus salience. Novel stimuli (especially artificial stimuli) may be salient, but that salience is short-lived and is not likely to activate strongly the emotional response circuits of the amygdala unless the context surrounding the novel stimulus is appropriate. In contrast, emotion-laden vocalizations may become increasingly salient when they are repeated. Similarly, repeated artificial stimuli that have become aversive through conditioning evoke strong amygdalar responses (Quirk et al., 1995; Amano et al., 2011). Thus, the overall response is determined by context surrounding the immediate stimulus, but novelty is only one of several contextual factors. Others may include the temporal patterning of vocalizations, other sensory input, and an animal’s internal state. Habituation of seconds to minutes that we observed is likely to depend on internal state factors rather than novelty.
Overall functional view
The social acoustic environment of mustached bats seems to require high selectivity to its social calls in brain regions that establish the salience of acoustic stimuli. As highly social mammals, mustached bats roost in large groups (hundreds to thousands or more) within caves or other structures (Bateman and Vaughan, 1974). Like many neotropical species, these bats do not appear to display daily torpor (Bonaccorso et al., 1992). Unlike many other bats, these bats produce a nearly continuous stream of echolocation signals (Kanwal et al., 1994). As a result, these animals live in close quarters with conspecifics, engage socially for 12 or more hours a day, and are bombarded by high-amplitude echolocation signals of limited social salience. It is reasonable to expect that neurons in the mustached bat amygdala, a brain region that helps to establish the salience of acoustic stimuli, would not respond to most echolocation signals. Instead, amygdalar neurons appear to filter these signals out and respond predominantly to signals that have social salience, i.e., the signals indicating aggressive interactions with nearby bats as well as the signals indicating affiliative or appeasing interactions. Appropriate behavioral responses to these social signals are critical to an individual’s survival. The responses of basolateral amygdalar neurons in these situations contribute to the amygdala’s orchestration of emotional responses to these acoustically based social interactions, as well as providing the basis for altering auditory responsiveness to subsequent signals. The persistent firing of mustached bat amygdalar neurons to a particular salient call may serve to match the time scale of the auditory response to desirable emotional responses and modulation of subsequent auditory responses.
Acknowledgments
These studies were supported by research grants R01 DC00937 (J.J.W.) and National Research Service Award F32 DC007786 (D.C.P.) from the National Institute on Deafness and Other Communication Disorders of the U.S. Public Health Service. Communication calls were provided through research grant R01 DC04733 (C. Portfors, PI; J. Wenstrup and J. Kanwal, co-investigators) from the National Institute on Deafness and Other Communication Disorders. The authors are profoundly grateful to Don Gans (deceased) for his contributions to this work. We thank Marie Gadziola and Jasmine Grimsley for comments on the manuscript and sound analysis, Carol Grose for assistance in data analysis and histological reconstruction, the Wildlife Section of the Ministry of Agriculture, Land and Marine Resources of Trinidad and Tobago for permission to exports bats, and Brett Schofield for use of the Neurolucida reconstruction system.
Abbreviations
- ASP
aspartate
- BBN
broadband noise
- bDFM
bent downward frequency modulated
- BIC
bicuculline
- bUFM
bent upward frequency modulated
- cDFM
checked downward frequency modulated
- FM
frequency modulated
- fRFM
fixed rippled frequency modulated
- fSFM
fixed sinusoidal frequency modulated
- GLU
glutamate
- hRFM
humped rippled frequency modulated
- MG
medial geniculate body
- NBN
narrowband noise
- NNBs
short, narrowband noise burst
- PSTH
peristimulus time histogram
- QCFI
long, quasi constant frequency
- QCFs
short quasi constant frequency
- rBNB
rectangular broadband noise burst
- sAFM
single arched frequency modulated
- s.d
standard deviations
- sHFM
short humped frequency modulated
- sRFM
stretched rippled frequency modulated
- TCFs
short, true constant frequency
Contributor Information
D. C. PETERSON, Email: dcpet@iastate.edu.
J. J. WENSTRUP, Email: jjw@neomed.edu.
References
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Adams RB, Jr, Franklin RG, Jr, Kveraga K, Ambady N, Kleck RE, Whalen PJ, Hadjikhani N, Nelson AJ. Amygdala responses to averted vs. direct gaze fear vary as a function of presentation speed. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsr038. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Duvarci S, Popa D, Paré D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–15489. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Anderson LA, Christianson GB, Linden JF. Stimulus-specific adaptation occurs in the auditory thalamus. J Neurosci. 2009;29:7359–7363. doi: 10.1523/JNEUROSCI.0793-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andics A, McQueen JM, Petersson KM, Gal V, Rudas G, Vidnyanszky Z. Neural mechanisms for voice recognition. Neuroimage. 2010;52:1528–1540. doi: 10.1016/j.neuroimage.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Antunes FM, Nelken I, Covey E, Malmierca MS. Stimulus-specific adaptation in the auditory thalamus of the anesthetized rat. PLoS One. 2010;5:e14071. doi: 10.1371/journal.pone.0014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I. Response properties of human amygdala subregions: evidence based on functional MRI combined with probabilistic anatomical maps. PLoS One. 2007;2:e307. doi: 10.1371/journal.pone.0000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman GC, Vaughan TA. Nightly activities of mormoopid bats. J Mammal. 1974;55:45–65. [Google Scholar]
- Bauer EE, Klug A, Pollak GD. Features of contralaterally evoked inhibition in the inferior colliculus. Hear Res. 2000;141:80–96. doi: 10.1016/s0378-5955(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Le Gal la Salle G. Lateral amygdala unit activity: II. Habituating and non-habituating neurons. Electroencephalogr Clin Neurophysiol. 1974;37:463–472. doi: 10.1016/0013-4694(74)90087-x. [DOI] [PubMed] [Google Scholar]
- Bendor D, Wang X. Neural response properties of primary, rostral, and rostrotemporal core fields in the auditory cortex of marmoset monkeys. J Neurophysiol. 2008;100:888–906. doi: 10.1152/jn.00884.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Boll S, Gamer M, Kalisch R, Buchel C. Processing of facial expressions and their significance for the observer in subregions of the human amygdala. Neuroimage. 2011;56:299–306. doi: 10.1016/j.neuroimage.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Bonaccorso FJ, Arends A, Genoud M, Cantoni D, Morton T. Thermal ecology of moustached and ghost-faced bats, (Mormoopidae) in Venezuela. J Mammal. 1992;73:365–378. [Google Scholar]
- Bordi F, LeDoux J. Sensory tuning beyond the sensory system: an initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci. 1992;12:2493–2503. doi: 10.1523/JNEUROSCI.12-07-02493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux J, Clugnet MC, Pavlides C. Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: rates, discharge patterns, and responses to acoustic stimuli. Behav Neurosci. 1993;107:757–769. doi: 10.1037/0735-7044.107.5.757. [DOI] [PubMed] [Google Scholar]
- Brothers L, Ring B, Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res. 1990;41:199–213. doi: 10.1016/0166-4328(90)90108-q. [DOI] [PubMed] [Google Scholar]
- Brown KA, Buchwald JS. Acoustic responses and plasticity of limbic units in cats. Exp Neurol. 1973;40:608–631. doi: 10.1016/0014-4886(73)90099-x. [DOI] [PubMed] [Google Scholar]
- Campbell RA, Schulz AL, King AJ, Schnupp JW. Brief sounds evoke prolonged responses in anesthetized ferret auditory cortex. J Neurophysiol. 2010;103:2783–2793. doi: 10.1152/jn.00730.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Zaborszky L, Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol. 1985;234:155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Chavez CM, McGaugh JL, Weinberger NM. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiol Learn Mem. 2009;91:382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement MJ, Gupta P, Dietz N, Kanwal JS. A-udiovocal communication and social behavior in mustached bats. In: Kanwal JS, Ehret G, editors. Behavioral and neurodynamics for auditory communication. Cambridge, NY: Cambridge University press; 2006. pp. 57–84. [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Dima D, Stephan KE, Roiser JP, Friston KJ, Frangou S. Effective connectivity during processing of facial affect: evidence for multiple parallel pathways. J Neurosci. 2011;31:14378–14385. doi: 10.1523/JNEUROSCI.2400-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Unsicker K, von Bohlen und Halbach O. Muscarinic control of graded persistent activity in lateral amygdala neurons. Eur J Neurosci. 2006;24:3183–3194. doi: 10.1111/j.1460-9568.2006.05200.x. [DOI] [PubMed] [Google Scholar]
- Emson PC, Paxinos G, Le Gal La Salle G, Ben-Ari Y, Silver A. Choline acetyltransferase and acetylcholinesterase containing projections from the basal forebrain to the amygdaloid complex of the rat. Brain Res. 1979;165:271–282. doi: 10.1016/0006-8993(79)90559-6. [DOI] [PubMed] [Google Scholar]
- Escarti MJ, de la Iglesia-Vaya M, Marti-Bonmati L, Robles M, Carbonell J, Lull JJ, Garcia-Marti G, Manjon JV, Aguilar EJ, Aleman A, Sanjuan J. Increased amygdala and parahippocampal gyrus activation in schizophrenic patients with auditory hallucinations: an fMRI study using independent component analysis. Schizophr Res. 2010;117:31–41. doi: 10.1016/j.schres.2009.12.028. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Belin P, Joanette Y, Armony JL. Amygdala responses to nonlinguistic emotional vocalizations. Neuroimage. 2007;36:480–487. doi: 10.1016/j.neuroimage.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN. Persistent activity and memory in the entorhinal cortex. Trends Neurosci. 2003;26:400–401. doi: 10.1016/S0166-2236(03)00176-0. [DOI] [PubMed] [Google Scholar]
- Gabis L, Wei H, Azizian A, DeVincent C, Tudorica A, Kesner-Baruch Y, Roche P, Pomeroy J. 1H-magnetic resonance spectroscopy markers of cognitive and language ability in clinical subtypes of autism spectrum disorders. J Child Neurol. 2008;23:766–774. doi: 10.1177/0883073808315423. [DOI] [PubMed] [Google Scholar]
- Gadziola MA, Grimsley JM, Shanbhag SJ, Wenstrup JJ. A novel coding mechanism for social vocalizations in the lateral amygdala. J Neurophysiol. 2012;107:1047–1057. doi: 10.1152/jn.00422.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97:1671–1683. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- Havey DC, Caspary DM. A simple technique for constructing ‘piggy-back’ multibarrel microelectrodes. Electroencephalogr Clin Neurophysiol. 1980;48:249–251. doi: 10.1016/0013-4694(80)90313-2. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, McGinty DJ. Participation of the amygdala in complex stimulus recognition and behavioral inhibition: evidence from unit studies. Brain Res. 1972;36:431–436. doi: 10.1016/0006-8993(72)90750-0. [DOI] [PubMed] [Google Scholar]
- Kanwal JS, Matsumura S, Ohlemiller K, Suga N. Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J Acoust Soc Am. 1994;96:1229–1254. doi: 10.1121/1.410273. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Margoliash D, Suga N. Delay-tuned combination-sensitive neurons in the auditory cortex of the vocalizing mustached bat. J Neurophysiol. 1988;59:623–635. doi: 10.1152/jn.1988.59.2.623. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Lyoo IK, Estes AM, Renshaw PF, Shaw DW, Friedman SD, Kim DJ, Yoon SJ, Hwang J, Dager SR. Laterobasal amygdalar enlargement in 6- to 7-year-old children with autism spectrum disorder. Arch Gen Psychiatry. 2010;67:1187–1197. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- Kosmal A, Malinowska M, Woznicka A. Diversity of connections of the temporal neocortex with amygdaloid nuclei in the dog (Canis familiaris) Acta Neurobiol Exp (Wars) 1997;57:289–314. doi: 10.55782/ane-1997-1239. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Ragland JD, Laukka P, Loughead J, Valdez JN, Javitt DC, Turetsky BI, Gur RC. “It’s Not What You Say, But How You Say it”: a reciprocal temporo-frontal network for affective prosody. Front Hum Neurosci. 2010;4:19. doi: 10.3389/fnhum.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Suga N. Augmentation of plasticity of the central auditory system by the basal forebrain and/or somatosensory cortex. J Neurophysiol. 2003;89:90–103. doi: 10.1152/jn.00968.2001. [DOI] [PubMed] [Google Scholar]
- Maeda H, Morimoto H, Yanagimoto K. Response characteristics of amygdaloid neurons provoked by emotionally significant environmental stimuli in cats, with special reference to response durations. Can J Physiol Pharmacol. 1993;71:374–378. doi: 10.1139/y93-057. [DOI] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol. 2004;14:675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Marsh RA, Fuzessery ZM, Grose CD, Wenstrup JJ. Projection to the inferior colliculus from the basal nucleus of the amygdala. J Neurosci. 2002;22:10449–10460. doi: 10.1523/JNEUROSCI.22-23-10449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh RA, Nataraj K, Gans D, Portfors CV, Wenstrup JJ. Auditory responses in the cochlear nucleus of awake mustached bats: precursors to spectral integration in the auditory midbrain. J Neurophysiol. 2006;95:88–105. doi: 10.1152/jn.00634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Jackson TR. Amygdaloid connections with posterior insular and temporal cortical areas in the rat. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- Miller K, Covey E. Comparison of auditory responses in the medial geniculate and pontine gray of the big brown bat, Eptesicus fuscus. Hear Res. 2011;275:53–65. doi: 10.1016/j.heares.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshitch D, Las L, Ulanovsky N, Bar-Yosef O, Nelken I. Responses of neurons in primary auditory cortex (A1) to pure tones in the halothane-anesthetized cat. J Neurophysiol. 2006;95:3756–3769. doi: 10.1152/jn.00822.2005. [DOI] [PubMed] [Google Scholar]
- Nataraj K, Wenstrup JJ. Roles of inhibition in creating complex auditory responses in the inferior colliculus: facilitated combination-sensitive neurons. J Neurophysiol. 2005;93:3294–3312. doi: 10.1152/jn.01152.2004. [DOI] [PubMed] [Google Scholar]
- Nataraj K, Wenstrup JJ. Roles of inhibition in complex auditory responses in the inferior colliculus: inhibited combination-sensitive neurons. J Neurophysiol. 2006;95:2179–2192. doi: 10.1152/jn.01148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann RT, Kanwal JS. Basolateral amygdala responds robustly to social calls: spiking characteristics of single unit activity. J Neurophysiol. 2011;105:2389–2404. doi: 10.1152/jn.00580.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Bouma H. Complex sensory properties of certain amygdala units in the freely moving cat. Exp Neurol. 1969;23:384–398. doi: 10.1016/0014-4886(69)90086-7. [DOI] [PubMed] [Google Scholar]
- Paré D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol. 2003;70:409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parsana AJ, Li N, Brown TH. Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behav Brain Res. 2012;226:77–86. doi: 10.1016/j.bbr.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JG, Likhtik E, Filali M, Paré D. Lasting increases in basolateral amygdala activity after emotional arousal: implications for facilitated consolidation of emotional memories. Learn Mem. 2005;12:96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena JL, Perez-Perera L, Bouvier M, Velluti RA. Sleep and wakefulness modulation of the neuronal firing in the auditory cortex of the guinea pig. Brain Res. 1999;816:463–470. doi: 10.1016/s0006-8993(98)01194-9. [DOI] [PubMed] [Google Scholar]
- Pérez-González D, Malmierca MS, Covey E. Novelty detector neurons in the mammalian auditory midbrain. Eur J Neurosci. 2005;22:2879–2885. doi: 10.1111/j.1460-9568.2005.04472.x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Jolkkonen E, Kemppainen S. Anatomic heterogeneity of the rat amygdaloid complex. Folia Morphol (Warsz) 2000;59:1–23. [PubMed] [Google Scholar]
- Portfors CV, Wenstrup JJ. Responses to combinations of tones in the nuclei of the lateral lemniscus. J Assoc Res Otolaryngol. 2001;2:104–117. doi: 10.1007/s101620010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann NY Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Hofelt T, Swanson LW, Bjorkllund A, editors. Handbook of chemical neuroanatomy. Amsterdam: Elsevier; 1987. pp. 279–388. [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Précis of the brain and emotion. Behav Brain Sci. 2000;23:177–191. doi: 10.1017/s0140525x00002429. (discussion 192–233) [DOI] [PubMed] [Google Scholar]
- Romanski LM, Averbeck BB, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J Neurophysiol. 2005;93:734–747. doi: 10.1152/jn.00675.2004. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sander K, Scheich H. Left auditory cortex and amygdala, but right insula dominance for human laughing and crying. J Cogn Neurosci. 2005;17:1519–1531. doi: 10.1162/089892905774597227. [DOI] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, Vuilleumier P. Emotion and attention interactions in social cognition: brain regions involved in processing anger prosody. Neuroimage. 2005;28:848–858. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Lull JJ, Aguilar EJ, Marti-Bonmati L, Moratal D, Gonzalez JC, Robles M, Keshavan MS. Emotional words induce enhanced brain activity in schizophrenic patients with auditory hallucinations. Psychiatry Res. 2007;154:21–29. doi: 10.1016/j.pscychresns.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Sawa M, Delgado JM. Amygdala unitary activity in the unrestrained cat. Electroencephalogr Clin Neurophysiol. 1963;15:637–650. doi: 10.1016/0013-4694(63)90115-9. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shin RM, Tsvetkov E, Bolshakov VY. Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways. Neuron. 2006;52:883–896. doi: 10.1016/j.neuron.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Nelken Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Farkas D, Nelken Multiple time scales of adaptation in auditory cortex neurons. J Neurosci. 2004;24:10440–10453. doi: 10.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, Cunningham WA. The neural substrates of in-group bias: a functional magnetic resonance imaging investigation. Psychol Sci. 2008;19:1131–1139. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- Vater M, Kossl M, Foeller E, Coro F, Mora E, Russell IJ. Development of echolocation calls in the mustached bat, Pteronotus parnellii. J Neurophysiol. 2003;90:2274–2290. doi: 10.1152/jn.00101.2003. [DOI] [PubMed] [Google Scholar]
- von der Behrens W, Bäuerle P, Kössl M, Gaese BH. Correlating stimulus-specific adaptation of cortical neurons and local field potentials in the awake rat. J Neurosci. 2009;29:13837–13849. doi: 10.1523/JNEUROSCI.3475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup JJ. Frequency organization and responses to complex sounds in the medial geniculate body of the mustached bat. J Neurophysiol. 1999;82:2528–2544. doi: 10.1152/jn.1999.82.5.2528. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, Grose CD. Inputs to combination-sensitive neurons in the medial geniculate body of the mustached bat: the missing fundamental. J Neurosci. 1995;15:4693–4711. doi: 10.1523/JNEUROSCI.15-06-04693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethoff S, Wildgruber D, Grodd W, Ethofer T. Response and habituation of the amygdala during processing of emotional prosody. Neuroreport. 2009;20:1356–1360. doi: 10.1097/WNR.0b013e328330eb83. [DOI] [PubMed] [Google Scholar]
- Yukie M. Connections between the amygdala and auditory cortical areas in the macaque monkey. Neurosci Res. 2002;42:219–229. doi: 10.1016/s0168-0102(01)00325-x. [DOI] [PubMed] [Google Scholar]