Summary
Recent studies have revealed that microRNAs (miRNAs) regulate plant adaptive responses to nutrient deprivation. However, the functional significance of miRNAs in adaptive responses to nitrogen (N) limitation remains to be explored.
The Arabidopsis miR169 was strongly down-regulated, whereas its targets, NFYA (Nuclear Factor Y, subunit A) family members, were strongly induced by nitrogen N starvation. Analysis of the expression of miR169 precursors showed that MIR169a was substantially down-regulated in both roots and shoots by N starvation. Accumulation of the NFYA family members was suppressed in transgenic Arabidopsis with constitutive expression of MIR169a.
Transgenic Arabidopsis plants overexpressing MIR169a accumulated less N and were more sensitive to N stress than the wild type. N sensitivity of 35S::MIR169a might be attributable to impaired uptake systems.
These results provide evidence that miRNAs have functional roles in helping plants to cope with fluctuations in N availability in the soil.
Keywords: Arabidopsis, miR169, NFYA, nitrate transporter, nitrogen starvation
Introduction
Nitrogen (N) is required for the normal growth and development of plants, and is the key environmental factor limiting crop productivity worldwide. The application of N fertilizer has increased greatly in recent years as farmers strive to obtain high yields. These large inputs of external N combined with decreasing N use efficiency have contributed to severe environmental degradation since the 1990s (Ju et al., 2009), and substantial research has been directed at improving the N use efficiency of plants. To respond to variations in N availability in the soil, plants have evolved multifaceted strategies, including morphological, physiological and biochemical adaptations (Stitt et al., 2002; Vidal & Gutiérrez, 2008). These adaptations in response to variable N availability are at least partially dependent on changes in gene expression. Many stress-induced genes are known or presumed to have roles in resistance to N stress.
A number of stress-regulated genes encode regulatory proteins, such as transcription factors, that are important in regulating the expression of still other downstream genes (Singh et al., 2002). Arabidopsis ANR1 (Arabidopsis Nitrate Regulated 1) was the first transcription factor identified as a component involved in the nitrate regulation of root architecture (Zhang & Forde, 1998). Overexpression of ZmDOF1 (DNA binding with one finger) in Arabidopsis and potato (Solanum tuberosum) improved N assimilation and growth under low-N conditions (Yanagisawa et al., 2004). Recently, nodule inception-like protein 7 (NLP7) was reported to be a positive regulator of the primary nitrate response (Castaings et al., 2009); Chlorina 1 (CHL1) (nitrate transporter AtNRT1.1) was assumed to utilize dual-affinity binding and a phosphorylation switch to sense a wide range of nitrate concentrations in the soil (Ho et al., 2009); NRT2.1, a high-affinity nitrate transporter, may be the dominant nitrate sensor under N-limited conditions (Ho & Tsay, 2010). However, a detailed understanding of the molecular basis underlying N sensing/signaling is still lacking.
With the discovery of small regulatory 21- to 24-nt RNAs, researchers recognized the important roles of small RNAs in posttranscriptional gene regulation (Hamilton & Baulcombe, 1999; Carrington & Ambros, 2003; Bartel, 2004). These small RNAs are mainly composed of microRNAs (miRNAs) and small interfering RNAs (siRNAs). miRNAs are processed from single-stranded RNA precursors capable of forming hairpin structures by the ribonuclease III-like enzyme Dicerlike 1 (DCL1) or DCL4. Unlike miRNAs, siRNAs are generated from long double-stranded RNAs by RNA polymerase 6 (RDR6)/RDR2 or from overlapping antisense mRNAs.
Plant miRNAs are involved in various developmental processes, including flowering, leaf and root development, embryo development, and auxin signaling (Carrington & Ambros, 2003; Bartel, 2004; Jones-Rhoades et al., 2006). Recent studies have also revealed that miRNAs regulate plant adaptive responses to nutrient deprivation (Jones-Rhoades & Bartel, 2004; Fujii et al., 2005; Hsieh et al., 2009; Pant et al., 2009). For example, miR395 plays important roles in coordinating sulfur assimilation and translocation by adjusting the mRNA levels of ATP sulfurylase (APS1, APS3 and APS4) and a low-affinity sulfur transporter (Jones-Rhoades & Bartel, 2004). miR398 mediates copper homeostasis by directing the degradation of copper/zinc superoxide dismutase mRNA when copper is limited (Yamasaki et al., 2007). miR399 regulates phosphate homeostasis in Arabidopsis through the suppression of a ubiquitin-conjugating E2 enzyme, Phosphate 2 (PHO2) (Fujii et al., 2005; Chiou et al., 2006). Recently, researchers determined that miR167 mediates lateral root initiation and growth in response to N (Gifford et al., 2008). By using quantitative RT-PCR of the primary transcripts of miRNAs, Pant et al. (2009) also found several N-responsive miRNAs in Arabidopsis, and the abundances of several miRNAs were strongly dependent on phosphorus (P) or N status in rapeseed (Brassica napus) phloem sap, flagging them as candidate systemic signals. However, the functional significance of these miRNAs in adaptive responses to N limitation remains to be explored.
Here, we show that the expression of different gene members of the miR169 family in both the roots and shoots of Arabidopsis is significantly down-regulated by N starvation. Based on analysis of miR169 precursors, only MIR169a appears to be down-regulated in both the roots and shoots of Arabidopsis by N starvation. Furthermore, transgenic plants with constitutive expression of MIR169a decrease the expression of nitrate transporter genes (AtNRT1.1 and AtNRT2.1) and display the early senescence phenotype induced by low inorganic N under different growth conditions (i.e. on agar medium, and in hydroponic culture). The results suggest that miRNA169 has functional roles in helping plants to cope with fluctuations in Navailability in the soil.
Materials and Methods
Plant material and growth conditions
Arabidopsis thaliana (L.) Heynh of the Columbia (Col) ecotype was used for all experiments. hua enhancer 1 (hen1-1; mutations in RNA methyltransferase) and hyponastic leaves 1 (hyl1; mutations in the dsRNA-binding protein) were in the Landsberg (Ler) or Nosssen-0 genetic backgrounds, as indicated in the text and figures. For the hydroponic culture, the seeds were germinated directly on the tops of modified Eppendorf tubes. The basic nutrients in hydroponic solutions consisted of 0.5 mM Ca(NO3)2, 2 mM K2SO4, 2 mM KH2PO4, 1 mM MgSO4, 100 µM Fe-EDTA, 1 µM H3BO3, 0.2 µM ZnSO4, 0.2 µM MnSO4, 5 × 10−2 µM CuSO4, and 5 × 10−2 µM Na2MoO4. The nutrient solution was replaced with fresh solution every 2 d. After they had grown for 5 wk, Arabidopsis plants were supplied with N-free nutrient solutions to simulate N-deficiency conditions, as indicated in the figure legends. Nutrient solutions were renewed daily to ensure pH stability.
The basic components of the agar medium were half-strength Murashige and Skoog (MS) medium, except for N, as indicated in the legend to Fig. 5(d).
Fig. 5.
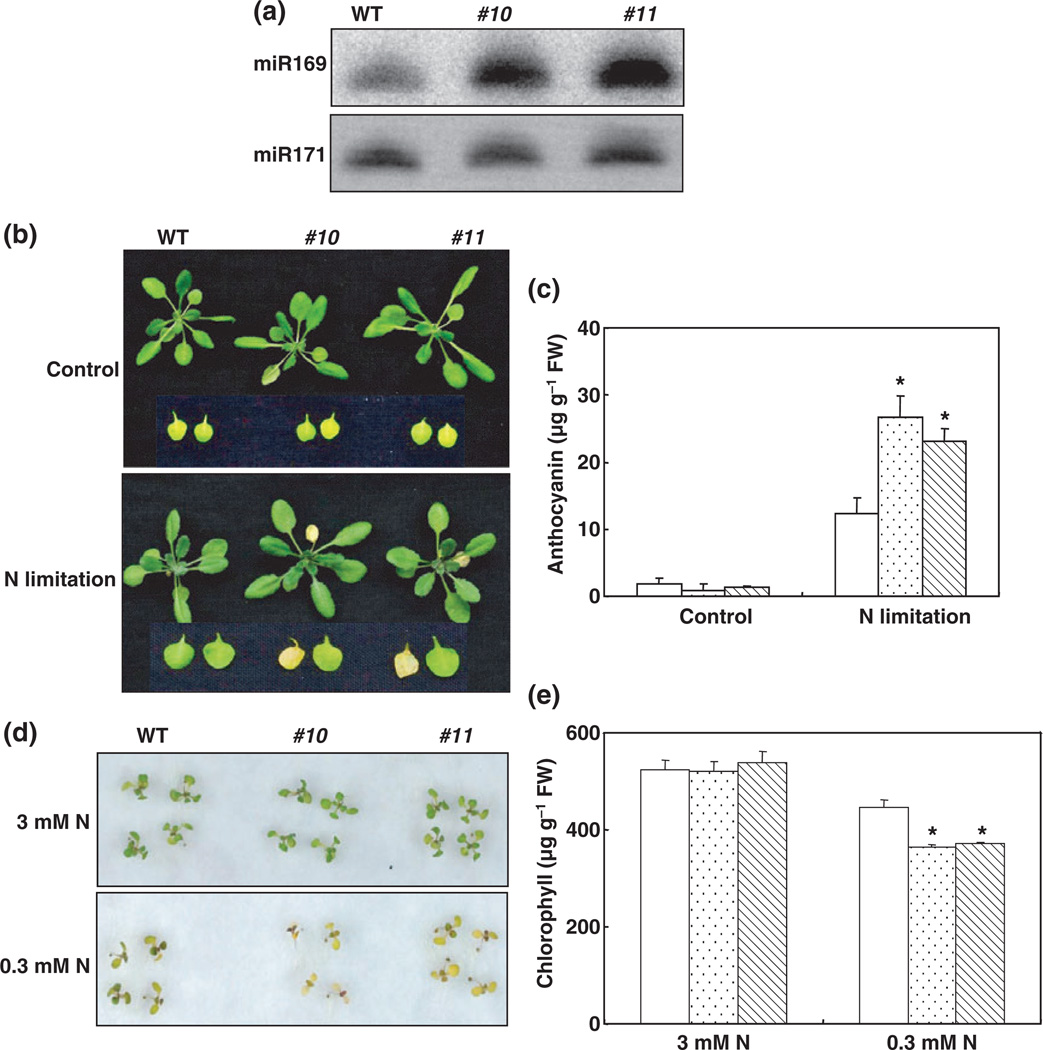
35S::MIR169a plants are more sensitive to nitrogen (N)-deficiency stress. (a) Overexpression of MIR169a in transgenic Arabidopsis. Small RNA northern blot analysis of miR169a levels in the wild type (WT) and representative transgenic lines was carried out. miR171 is shown as a loading control. (b) N-deficient phenotype of 35S::MIiR169a transgenic plants under hydroponic conditions. Plants were grown hydroponically in a nutrient solution containing 0.5 mM Ca(NO3)2 for 5 wk and then transferred to N-free medium for 3 d. Representative pictures are shown. (c) Anthocyanin content in leaves of Col and 35S::miR169a transgenic plants (Col, open bars; #10, stippled bars; #11, hatched bars) with or without N-starvation treatment for 5 d. Error bars represent SE for four independent experiments. *, P < 0.05 (t-test); significant difference from WT. FW, fresh weight. (d) Effects of N deficiency on the growth of transgenic seedlings on agar medium containing 3 mM N (1 mM NH4NO3 and 1 mM KNO3) or 0.3 mM N (0.1 mM NH4NO3 and 0.1 mM KNO3). Seeds were germinated and grown for 10 d. Representative pictures are shown. (e) Chlorophyll content in Col and 35S::miR169a transgenic seedlings (WT, open bars; #10, stippled bars; #11, hatched bars). Error bars represent SE for four independent experiments. *, P < 0.05 (t-test); significant difference from the WT.
For the N-starvation experiment in soil, plants were grown as described by Fan et al. (2009). In brief, Arabidopsis seedlings were grown in a mixture of perlite and vermiculite (1 : 2) and were irrigated with nutrient solution containing 0.5 mM Ca(NO3)2 for 4 wk. The plants were then watered with N-free nutrient solution. The wild-type (WT) and mutant plants were grown in the same pot.
Plants were kept in a growth chamber with a light intensity of 150 µmol m−2 s−1, 60–70% relative humidity, and a day : night temperature regime of 22 : 18°C.
Constructs and generation of transgenic plants
To generate pMDC32:MIR169a constructs, a 200-bp fragment surrounding the miRNA sequence including the fold-back structure was amplified from genomic DNA with the following primers: miR169a, forward 5′-CAC CTG GGT ATA GCT AGT GAA ACG CG-3′ and reverse 5′-CCT TAG CTT GAG TTC TTG CGA-3′. The amplified fragments were introduced into the pENTR™/D-TOPO vector (Invitrogen) and cloned into pMDC32 (Curtis & Grossniklaus, 2003) using LR reactions (Invitrogen).
The plasmid was electroporated into Agrobacterium tumefaciens GV3101 and Arabidopsis plants were transformed using the floral dip method (Clough & Bent, 1998). Transgenic plants were selected using 35 µg ml−1 hygromycin. T3 or T4 homozygous lines were used for all experiments.
RNA analysis
Total RNA was extracted from WT and transgenic plants with Trizol reagent (Invitrogen). For enrichment of small RNAs, high-molecular-weight RNA was selectively precipitated by the addition of one volume of 20% PEG-1M NaCl (Llave et al., 2002). Low-molecular-weight RNA was fractioned on 17% denaturing polyacrylamide gels. The blots were probed and washed as described previously (Borsani et al., 2005; Li et al., 2008).
For real-time RT-PCR, 5 µg of total RNA isolated with the RNeasy plant mini kit (Qiagen) was used for first-strand cDNA synthesis using SuperScript III first-strand synthesis supermix (Invitrogen). Primers specific for the precursor of miR169 were used to detect expression levels of miR169 (Li et al., 2008). Primers were also designed to detect the transcription level of NFYA (Supporting Information Table S1). Quantitative real-time PCR was carried out in an ABI 7500 system (Applied Biosystems, Foster City, CA, USA) using the SYBR Premix Ex Taq™ (perfect real time) kit (TaKaRa Biomedicals, Kyoto, Japan). PCR included a preincubation at 95°C for 3 min followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 15 s and extension at 72°C for 45 s. The PCR products were loaded on 1.5% agarose gels and photographed after staining with ethidium bromide. Primer efficiencies were measured and calculated (Ramakers et al., 2003). The relative expression level was calculated using the comparative Ct method. Results were normalized to the expression of Tubulin Beta Chain 4 (Tub4). Each experiment was replicated at least three times.
5′-RACE analysis
To obtain the cleavage transcripts, 5′-RACE (Rapid Amplification of cDNA Ends) analysis was performed using the GeneRacer Kit (Invitrogen). Total RNA from 4-wk-old leaves was directly ligated to the GeneRacer RNA Oligo adaptor without calf intestinal phosphatase treatment. The GeneRacer oligo dT primer was then used for cDNA synthesis. Initial PCR was carried out using the GeneRacer 5′-primer and gene-specific outer primers (NFYA1: 5′-AACACCTAACATAACTCGCTCT-3′; NFYA5: 5′-TTG TACTCTCAGAGAATCGG -3′). Nested PCR was carried out using 1 µl of the initial PCR reaction, GeneRacer 5′-primer and gene-specific inner primers (NFYA1: 5′-CTC GCTCTTTGTACATTCATCA-3′; NFYA5: 5′-AGAGA ATCGGAAGTTAACAA-3′). RACE fragments were cloned and sequenced after gel purification.
Germination and green seedling assay
Approximately 50 seeds from WT plants and transformants were plated in Petri dishes containing agar medium as described in the section ‘Plant material and growth conditions’ (0.8% agar, 0.6% sucrose). After the seeds had been stratified at 4°C in the dark for 96 h, they were allowed to germinate at 22°C in 16-h light : 8-h dark conditions with 70 µmol m−2 s−1 light intensity. A seed was considered to have germinated when the radicle had visibly protruded from the seed coat. Ten days later, the percentage of green seedlings was recorded, based on the presence of obvious green cotyledons. For hydroponic culture, the percentage of green old leaves (first pair of rosettes) was scored after Arabidopsis plants had been supplied with N-free nutrient solutions for 3 d.
Chlorophyll content measurement
Chlorophyll contents were measured as described by Woodward & Bennett (2005). The pigments were extracted with 5 ml of dimethylformamide for 24 h in the dark, and the optical densities OD664 and OD647 for each sample were measured. The shoot chlorophyll content was calculated as: ((OD664 × 7.04) + (OD647 × 20.27)) × 5/sample weight (g) = µg chlorophyll g−1 FW.
Measurement of anthocyanin content
Anthocyanin contents were measured as described previously (Rabino & Mancinelli, 1986). The pigments were extracted with 99 : 1 methanol : HCl (v/v) at 4°C, the OD530 and OD657 for each sample were measured, and OD530 – 0.25 × OD657 was used to compensate for the contribution of chlorophyll and its products to the absorption at 530 nm.
Elemental assay
Arabidopsis plants were separated into shoot and root. After drying at 120°C for 30 min and 65°C for 72 h, the samples were milled to a fine powder for N analysis. N analysis was performed using a carbon and nitrogen analyzer (Elemental Analyzer EA1108; Carlo Erba Strumentazione, Rodano, Italy).
Results
miR169 is down-regulated by N starvation
We previously reported that NFYA5 is important for drought resistance, and its induction by drought stress occurs at both the transcriptional and posttranscriptional levels (Li et al., 2008). The posttranscriptional regulation of NFYA5 was determined to be dependent on miR169, mainly miR169a (Li et al., 2008). In an analysis of the genomic response to low concentrations of nitrate, the steady-state mRNA levels of NFYA5 and other members of the NFYA family were significantly increased (Wang et al., 2003), which prompted us to investigate the function of miR169 in the response to N stress.
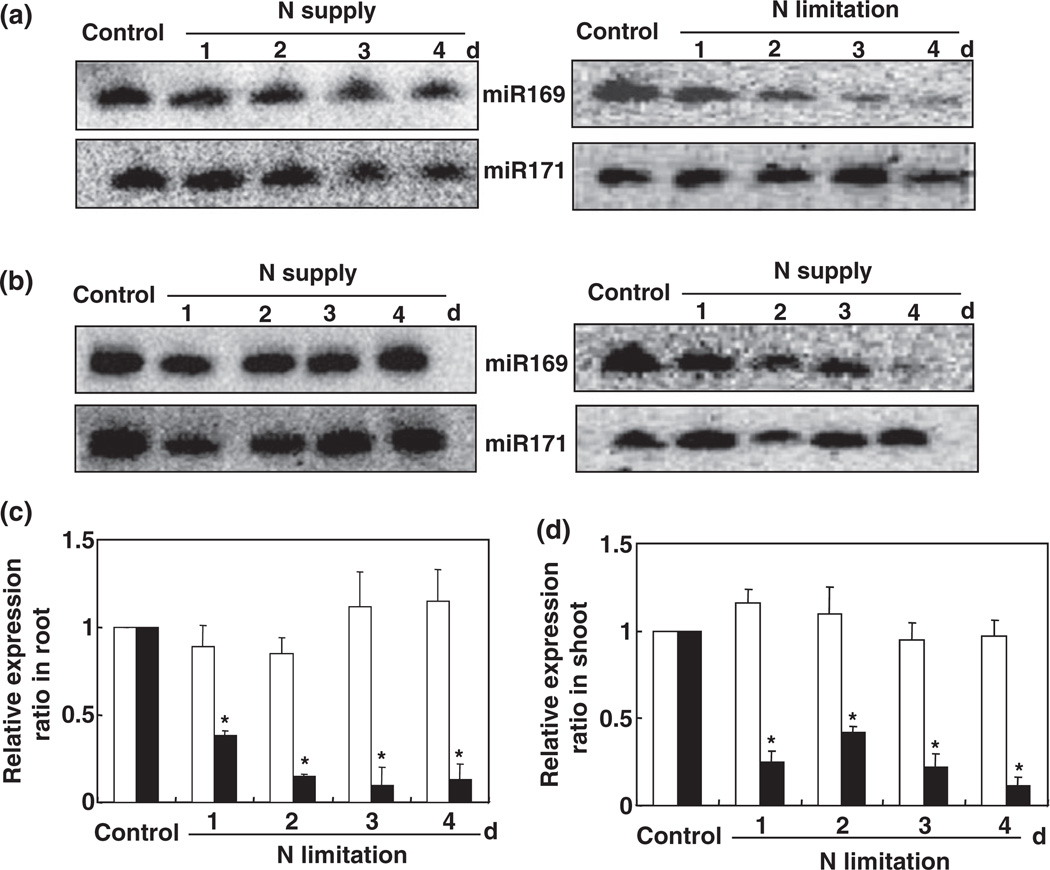
To confirm the physiological status of the Arabidopsis plants (i.e. to confirm that the plants were N-stressed), the expression of the marker gene AMT1:5 (At3g24290; an ammonium transporter) was analyzed and determined to be significantly induced by N starvation, as reported previously (Pant et al., 2009; Fig. S1). To test the regulation of miR169 by N stress, a time-course experiment was carried out in which hydroponically grown 5-wk-old Arabidopsis plants were subjected to N starvation. Small RNA northern blot analysis using an oligonucleotide probe complementary to ASRP1815 (http://asrp.cgrb.oregonstate.edu; 5′-TAG CCA AGG ATG ACT TCC C-3′) clearly showed that the expression of miR169 was substantially suppressed byNstarvation in both the roots and shoots of the Arabidopsis plants (Fig. 1a,b). Compared with the control, the relative miR169 expression ratios in the roots and shoots after 4 d of N starvation were 87% and 89% lower, respectively (Fig. 1c,d).
Fig. 1.
miR169 is down-regulated by nitrogen (N) deficiency in roots (a) and shoots (b). Arabidopsis plants were grown hydroponically in a nutrient solution containing 0.5 mM Ca(NO3)2 for 5 wk and then transferred to N-free or N-replete medium for the indicated times before small RNA was isolated from leaves and roots. Five micrograms of small RNA from each sample was loaded per lane and hybridized with a 32P-labeled probe of ASRP1815. The blot reprobed with miR171 is shown as a loading control. The relative abundances in Arabidopsis plants grown in the N-free (closed bars) or N-replete (open bars) medium are shown in (c) (root) and (d) (shoot). *, P < 0.05 (t-test); significant difference from wild type (WT).
NFYA3, NFYA5, NFYA8 and NFYA2 expression is induced by N starvation
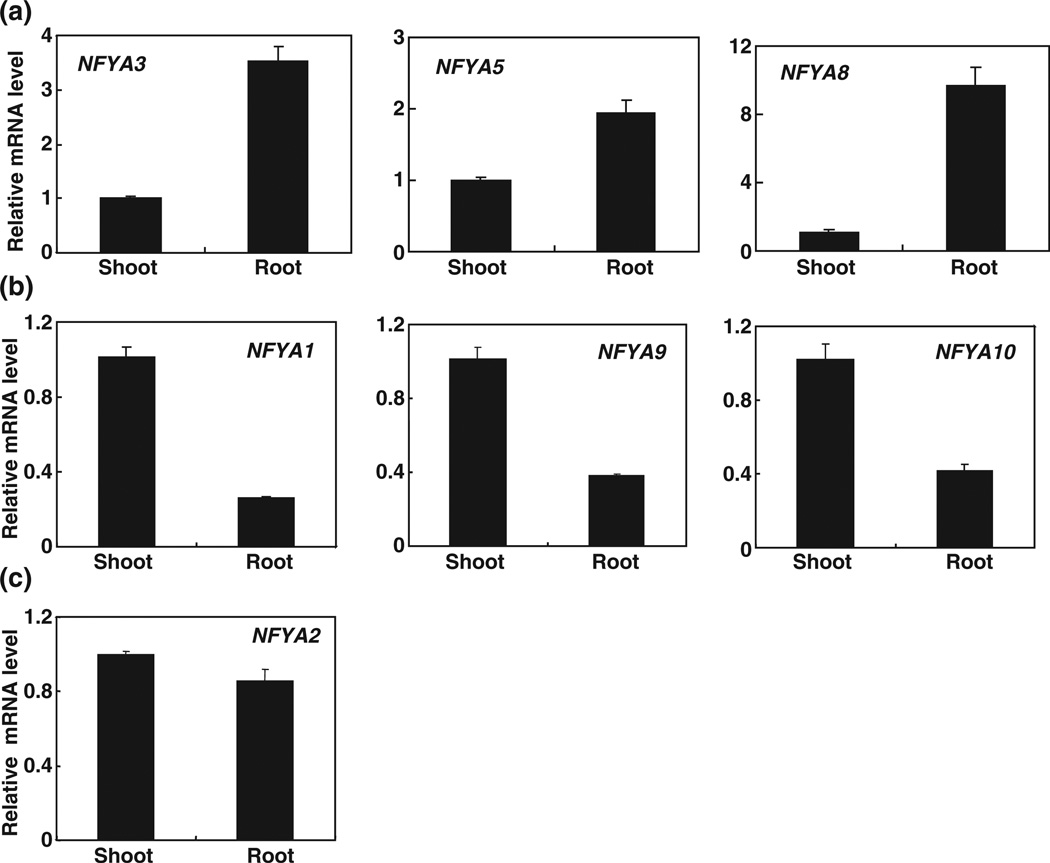
In addition to NFYA5, other members of NFYA family, including NFYA1, NFYA2, NFYA3, NFYA8, NFYA9 and NFYA10, are also potential targets of miR169 (Jones-Rhoades & Bartel, 2004; Table S2). Although NF-Y transcription factors occur in almost all eukaryotes, the biological roles of most of the NF-Y family members in plants are poorly understood. Thus, we first examined the expression pattern of NFYA transcription factors by quantitative RT-PCR. In contrast to the tissue-specific expression patterns described by Siefers et al. (2009), we found that all the members of the NFYA family were expressed in both roots and shoots of 5-wk-old Arabidopsis plants (Fig. 2). According to the different transcripts that accumulated in the roots vs shoots, we divided the members of the NFYA family into three subgroups. Group I contained NFYA3, NFYA5 and NFYA8, whose transcripts mainly accumulated in the roots (Fig. 2a). Group II contained NFYA1, NFYA9 and NFYA10, whose transcripts mainly accumulated in the shoots (Fig. 2b). Group III contained NFYA2, whose transcripts accumulated equally in the roots and shoots (Fig. 2c).
Fig. 2.
Tissue expression patterns of NFYA (Nuclear Factor Y, subunit A). Total RNA was isolated from leaves and roots of 5-wk-old Arabidopsis wild-type plants grown under hydroponic conditions. NFYA3, NFYA5 and NFYA8 were mainly accumulated in roots (a); NFYA1, NFYA9 and NFYA10 were strongly expressed in shoots (b); and NFYA2 was equally distributed in shoots and roots (c). The expression levels were normalized to that of Tub4 (Tubulin Beta Chain 4). Results are the mean ± SE for three biological replicates.
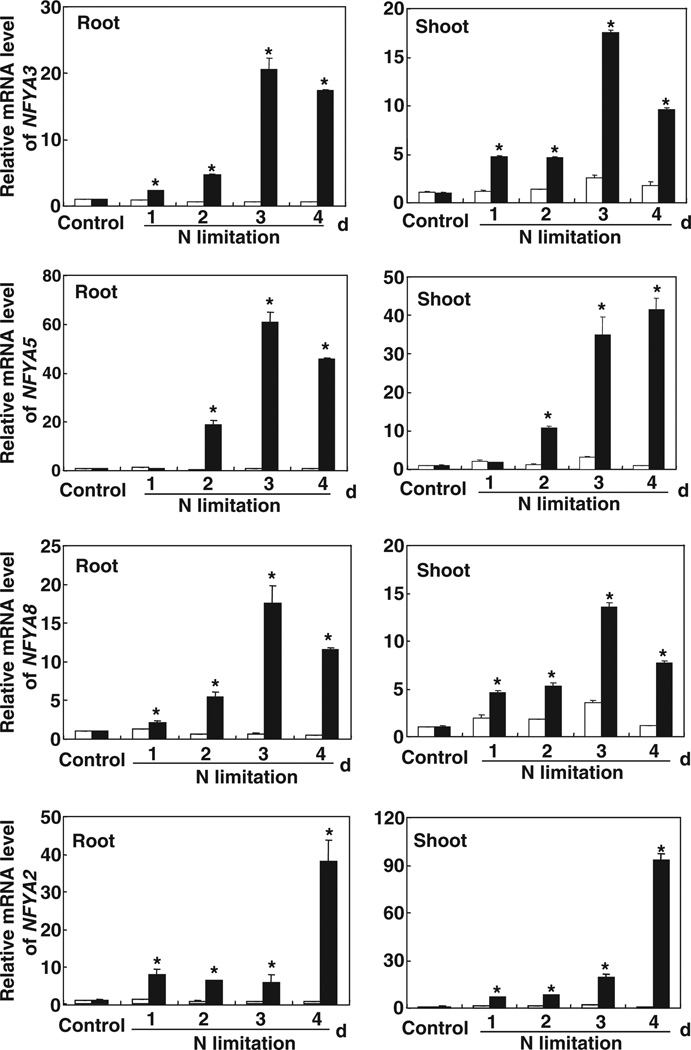
In both roots and shoots, NFYA3, NFYA5, NFYA8 and NFYA2 were strongly induced by N starvation (Fig. 3). In contrast to other members of the NFYA family in group II, The NFYA5 mRNA level in roots increased only after 24 h of N starvation, and it increased c. 60-fold 3 d after the Arabidopsis plants had been transferred to the N-free medium (Fig. 3). With the exception of NFYA1 in the roots, the expression of members of the NFYA family in group II also increased in N-starved roots and shoots (Fig. S2), which was in agreement with the reduction of miR169 expression in N-starved roots and shoots of Arabidopsis (Fig. 1). These results suggested that NFYA mRNA was cleaved by the targeting of miR169 and that this cleavage was directly related to the N status of the Arabidopsis plants.
Fig. 3.
Regulation of NFYA (Nuclear Factor Y, subunit A) expression by nitrogen (N). Relative quantification of NFYA3, NFYA5, NFYA8 and NFYA2 expression in the roots and shoots of 5-wk-old hydroponically grown Arabidopsis plants, either supplied with 0.5 mM Ca(NO3)2 (open bars) or transferred from the N solution to an N-free medium (closed bars) for 1, 2, 3 or 4 d, was carried out by real-time RT-PCR analysis. The expression levels were normalized to that of Tub4 (Tubulin Beta Chain 4). Results are the mean ± SE for three biological replicates. *, P < 0.05 (t-test); significant difference from the wild type (WT).
Expression profiling of MIR169 loci in response to N deprivation
The miR169 family in Arabidopsis contains 14 members and can be divided into four subgroups based on the sequence of the miRNA produced. MIR169a represents the first subgroup, MIR169b and MIR169c form the second group, MIR169d–g constitute the third group and the fourth group is made up of MIR169h–n. According to the Arabidopsis Small RNA Project (http://asrp.cgrb.oregonstate.edu/db/microRNA.html?fid=12/), miR169a is the main contributor to the total miR169 level, with c. 90% of miR169 sequences from leaves of soil-grown Col plants being miR169a.
Because their sequence similarity resulted in cross-hybridization, the miR169 family members were difficult to differentiate in small RNA blots. To determine which of the MIR169 loci contributed to the decrease in miR169 in response to N deprivation, we carried out real-time RT-PCR using miR169 locus-specific primers with RNA extracted from N-stressed Arabidopsis plants. RNA was isolated from hydroponically grown 5-wk-old plants that had been subjected to N stress for 2 d. We did not detect the expression of MIR169g, which was in agreement with the results of Pant et al. (2009). For primer pairs, the average amplification efficiency was > 86%, indicating that the primers could be used to detect the expression changes reliably.
In roots, with the exception of MIR169e and MIR169f, the expression of MIR169 loci was significantly down-regulated by N starvation (Fig. 4a). For example, the expression level of MIR169a was only c. 22% of that in N-sufficient conditions (Fig. 4a). In shoots, however, only MIR169a expression was substantially reduced in response to N starvation (Fig. 4b), which was consistent with the down-regulation of the mature miRNA by N deprivation (Fig. 1). In previous research, we found that MIR169a was the major miRNA locus important for the regulation of NFYA expression (Li et al., 2008). Thus, we undertook further experiments to determine the potential role of MIR169a in the adaptation to N stress in Arabidopsis.
Fig. 4.
Differential expression of miR169 genes in response to nitrogen (N) deficiency in roots (a) and shoots (b). Total RNA was isolated from 5-wk-old hydroponically grown Arabidopsis plants that were grown with 0.5 mM Ca(NO3)2 or without Ca(NO3)2 for 2 d. Quantifications were normalized to the expression of Tub4 (Tubulin Beta Chain 4). Open bars, control; closed bars, N limitation. Results are the mean ± SE for three biological replicates. *, P < 0.05 (t-test); significant difference from the wild type (WT).
35S::MIR169a transgenic plants are hypersensitive to N starvation
We searched the publicly available T-DNA collections and obtained a T-DNA insertion mutant (SALK_113174 in the Col background) from the Arabidopsis Biological Resource Center to further investigate the function of miR169a. Plants homozygous for the T-DNA insertion were identified by PCR, and sequencing of the T-DNA flanking region confirmed that the insertion site was located in the promoter region of miR169a (Fig. S3). Small RNA northern blot analysis showed that the miR169 transcript was not affected (Fig. S3). Thus, we generated transgenic Arabidopsis plants overexpressing the precursor of miR169a under the constitutive CaMV 35S promoter (Fig. 5a).
First, we quantified the effects of miR169a overexpression on its potential targets using the whole plant (NFYA1, NFYA3, NFYA5, NFYA8 and NFYA9; http://asrp.cgrb.oregonstate.edu). With the exception of NFYA1, the mRNA levels of potential targets (NFYA3, NFYA5, NFYA8 and NFYA9) were significantly decreased (Fig. S4), and similar results were obtained in other lines. To further elucidate the relationship between NFYA1 and miR169, we determined the expression level of NFYA1 in hen1 and hyl1, in which the biogenesis of miR169 is blocked (Li et al., 2008). The level of NFYA1 mRNA did not show significant variation in hen1, hyl1 and the corresponding WT (Fig. S5), suggesting that NFYA1 was not the target of miR169 as predicted by the ASRP database (http://asrp.cgrb.oregonstate.edu). We further carried out cleavage site mapping of NFYA1 using RNA obtained from 35S::MIR169a transgenic plants by 5′-RACE PCR, and used NFYA5, for which the cleavage site has been reported by Combier et al. (2006), as a positive control. The cleavage site of NFYA5 was easily identified (data not shown), but we could not identify that of NFYA1, further suggesting that NFYA1 was not the true target of miR169. Interestingly, overexpression of MIR169a also caused a large decrease in the transcripts of NFYA2 and NFYA10 (Fig. S4). These results indicated that MIR169a was the major miRNA locus regulating NFYA expression.
We then investigated the responses of 35S::MIR169a transgenic plants to N starvation in hydroponic solution. Under nutrient-sufficient conditions, growth was similar for transgenic and WT plants (Fig. 5b). However, when plants were transferred to the N-free medium for 3 d, c. 80% of 35S::MIR169a rosettes (75% for #10 and 82% for #11) showed the typical N-deficient phenotype, with discoloration of leaves, in contrast to 25% of WT (Fig. 5b). Another indicator of N-starvation sensitivity is the accumulation of the purple flavonoid pigment anthocyanin in leaves (Peng et al., 2008). The anthocyanin concentration in 35S::MIR169a plants after N starvation for 5 d was c. 25 µg g−1 fresh weight (Fig. 5c), which was 2 times that of the WT.
We also grew 35S::MIR169a plants on agar medium containing 0.3 mM N (0.1 mM NH4NO3 and 0.1 mM KNO3) or in soil. Although the germination rates were similar for WT and 35S::MIR169a transgenic plants grown on agar medium containing different concentrations of N (data not shown), the plants differed greatly at postgerminative growth stages. Unlike WT plants, 35S::MIR169a transgenic plants growing on the medium containing 0.3 mM N showed a typical N-deficient phenotype: leaves were discolored (Fig. 5d), and the chlorophyll content was significantly lower in the MIR169a transgenic plants (Fig. 5e), which may be attributable to variation in the capacity for N acquisition. A similar phenomenon was observed when 35S::MIR169a transgenic plants were grown in soil (data not shown), again supporting the conclusion that 35S:MIR169a transgenic plants were more sensitive to N stress.
Overexpression of MIR169a alters the N-starvation response in plants
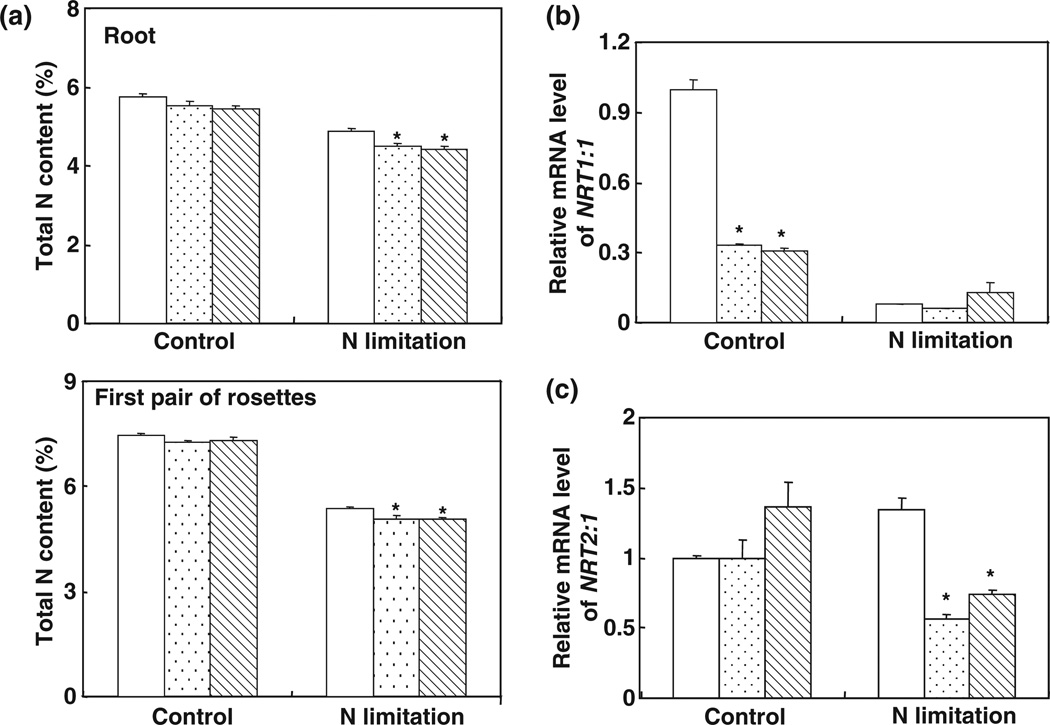
To test whether the hypersensitivity to N starvation of 35S::MIR169a plants was attributable to variation in the capacity for N acquisition, we first analyzed the total N contents in the roots and shoots of hydroponically grown 35S::MIR169a transgenic plants under N-replete and N-limiting conditions. Under N-limiting conditions, the total N contents in roots of 35S::MIR169a transgenic plants were c. 4.5%, which was 7.6% lower than that of the WT (Fig. 6a). Similar results were obtained for old leaves (the first pair of the rosette; Fig. 6a).
Fig. 6.
Decreased nitrogen (N) content in 35S::MIR169a plants. (a) N concentration in the roots and the first pair of rosettes of wild-type (WT) or 35S::MIR169a transgenic plants (WT, open bars; #10, stippled bars; #11, hatched bars) grown hydroponically with full nutrients for 5 wk and then N-starved for 3 d. Error bars indicate SD (n = 4). *, P < 0.05 (t-test); significant difference from the WT. (b) NRT1.1 (nitrate transporter 1.1) mRNA levels in 35S::MIR169a transgenic plants. Total RNA from roots described in (a) was used for reverse transcription followed by quantitative PCR. The expression levels were normalized to that of Tub4 (Tubulin Beta Chain 4). (Col, open bars; #10, stippled bars; #11, hatched bars.) Results are the mean ± SE for three biological replicates. *, P < 0.05 (t-test); significant difference from the WT. (c) NRT2.1 mRNA levels in 35S::MIR169a transgenic plants (Col, open bars; #10, stippled bars; #11, hatched bars). Total RNA from roots described in (a) was used for reverse transcription followed by quantitative PCR. The expression levels were normalized to that of Tub4. Error bars represent SEs (n = 3). Results are the mean ± SE for three biological replicates. *, P < 0.05 (t-test); significant difference from the WT.
In Arabidopsis, the molecular mechanisms of nitrate uptake have been well characterized. Three types of nitrate transporter have been identified in Arabidopsis: the AtNRT1 family, with 53 members; the AtNRT2 family, with seven members; and the AtCLC (Arabidopsis thaliana chloride channels) family, with seven members (Miller et al., 2007; Tsay et al., 2007; De Angeli et al., 2009). NRT1.1 and NRT2.1 played a central role in nitrate uptake. NRT1.1 mRNA accumulation was decreased in transgenic plants overexpressing MIR169a relative to the WT; this was especially true under N-sufficient conditions, when NRT1.1 mRNA accumulation was c. 65% less in plants overexpressing MIR169a than in the WT (Fig. 6b). Expression of NRT2:1 was induced by N starvation in the WT but was inhibited by N starvation in 35S::MIR169a plants, and the level of NRT2:1 mRNA in 35S::MIR169a plants under N starvation was only 56–74% of that in the WT (Fig. 6c). These results suggest that the N-uptake system in 35S::MIR169a transgenic plants may be impaired.
Discussion
We previously reported that NFYA5 defined one of the drought stress-responsive transcriptional cascades and that the novel feature of NFYA5 regulation under drought stress was the involvement of miR169 (Li et al., 2008). NFYA5 and other members of the NFYA family were also induced by N starvation in 9- or 10-d-old Arabidopsis seedlings (Wang et al., 2003; Pant et al., 2009). In roots and shoots of mature Arabidopsis, members of the NFYA family, especially NFYA3, NFYA5 and NFYA8, were strongly induced, while miR169 was suppressed by N starvation (Figs 3, S2). Of the putative targets, NFYA5 and NFYA2 had previously been experimentally demonstrated to be the targets of mir169 (Jones-Rhoades & Bartel, 2004; Li et al., 2008). Overexpression of MIR169a suppressed the accumulation of NFYA mRNA, with the exception of NFYA1 mRNA. In hen1and hyl1, the level of NFYA1 mRNA was similar to that of the corresponding WT. There were four mismatches between NFYA1 and miR169a and one mismatch located in the miR169a 5′ region, which was important for target RNA cleavage (Mallory et al., 2004). Thus, we inferred that NFYA1 was not the target as predicted by the ASRP database. We also noted that the expression of NFYA1, NFYA9 and NFYA10 was not consistent with that of miRNA169, indicating that NFYA1, NFYA9 and NFYA10 may be regulated by miRNA169 at the translational level, which has been demonstrated to be a widespread mechanisms of plant miRNA-guided silencing (Brodersen et al., 2008).
Deficiencies in nutrients such as N, phosphorus (Pi), potassium (K) and sulfur (S) can induce oxidative stresses (Shin et al., 2005). NFYA5 was reported to regulate downstream genes involved in oxidative stress responses, such as those encoding a subunit of the cytochrome b6-f complex, glutathione S-transferases (GST), peroxidases and an oxidoreductase family protein (Li et al., 2008). In a recent study on Medicago truncatula, symbiotic nodule development was found to be regulated by miR169, and overexpression of MIR169a led to a developmental block of nodule formation (Combier et al., 2006). These findings indicate a potential link between low N status and miR169. Here, we clearly showed that miR169 was critical for the N-starvation response in Arabidopsis. N starvation down-regulated miR169 expression, thus relieving repression of NFYA by miR169. miR169 is encoded by many loci. In roots, all of these loci, but especially MIR169a loci, were substantially down-regulated by N stress. In shoots, however, only MIR169a loci were substantially down-regulated, which demonstrated that closely related miRNAs that were predicted to target the same genes had in fact different functions. The results differed from those of Pant et al. (2009), perhaps because of differences in experimental conditions. Pant et al. (2009) used 9-d-old Arabidopsis seedlings, while we largely used mature Arabidopsis plants. The sequence read frequency data in the ASRP database (http://asrp.cgrb.oregonstate.edu/db/microRNA.html?fid=12/) suggest that miR169a represents nearly 90% of the miR169 population in leaves of soil-grown Col plants. Thus, a substantial down-regulation of miR169a would result in a reduction in the overall miR169 level.
35S::MIR169a plants were hypersensitive to N starvation under all of the growth conditions used in the current study, and yellowing of the leaves was the main symptom (Fig. 5). The association between the yellowing phenotype and decreased total N concentration (Fig. 6a) suggested that the phenotype was a symptom of N deficiency, which could be at least partly attributable to the reduced capacity of N-uptake systems in 35S::MIR169a plants (Fig. 6b,c). In Arabidopsis, the molecular mechanisms of nitrate uptake are well characterized, and both low-affinity and high-affinity nitrate uptake systems are involved in nitrate uptake. AtNRT2.1 belongs to the high-affinity uptake system, while AtNRT1.1 (CHL1) functions as a dual-affinity transporter involved in both high- and low-affinity uptake (Liu et al., 1999; Little et al., 2005). CHL1 was reported to function in stomatal opening and to contribute to drought susceptibility in Arabidopsis (Guo et al., 2003). The features of the chl1 phenotypes were similar to the drought-stress hypersensitivity described in 35S::MIR169a transgenic plants (Li et al., 2008), which suggested an association between AtNRT1.1 and miR169. Using the PlantCARE program (http://bioinformatics.psb.ugent.be/webtools/plantcare), we found four CCAAT sequences in the 2-kb promoter region of AtNRT1.1 and one CCAAT sequence in the 2-kb promoter region of AtNRT2.1, indicating direct regulation of AtNRT1.1 and AtNRT2.1 by members of the NFYA family. Overexpression of MIR169a significantly decreased the expression of AtNRT1.1 under N-sufficient conditions (Fig. 6b). Recently, AtNRT1.1 was reported to function as a nitrate sensor in plants (Ho et al., 2009), and Pant et al. (2009) reported a high abundance of miR169 in phloem sap during N-sufficient growth and a sharp decrease under N-limiting conditions, indicating that miR169 may function as a potential long-distance signal to regulate N-starvation responses in plants.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Science Foundation of China (NSFC) (grant numbers 30970221 and 31071139) and the Program for New Century Excellent Talents in University (grant number NCET-09-0732) to WX and an Innovative Group Grant from the NSFC (grant number 30821003) to FS.
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article.
Fig. S1 Marker gene expression is significantly induced by nitrogen starvation in Arabidopsis.
Fig. S2 Analysis of the responses of members of the NFYA (Nuclear Factor Y, subunit A) family in group II to nitrogen (N) starvation.
Fig. S3 Detection of miR169 transcript by small RNA northern blot analysis. miR171 is shown as a loading control.
Fig. S4 Detection of corresponding NFYA (Nuclear Factor Y, subunit A) gene transcripts in 35S::MIR169a transgenic plant lines by real-time RT-PCR.
Fig. S5 Detection of NFYA 1 (Nuclear Factor Y, subunit A1) gene transcripts in hua enhancer 1 (hen1), hyponastic leaves 1 (hyl1) and their corresponding wild types by real-time RT-PCR.
Table S1 List of primers used in this study
Table S2 The potential targets of miR169
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu JK. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell. 2005;123:1279–1291. doi: 10.1016/j.cell.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320:1185–1190. doi: 10.1126/science.1159151. [DOI] [PubMed] [Google Scholar]
- Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301:336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- Castaings L, Camargo A, Pocholle D, Gaudon V, Texier Y, Boutet-Mercey S, Taconnat L, Renou JP, Daniel-Vedele F, Fernandez E, et al. The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant Journal. 2009;57:426–435. doi: 10.1111/j.1365-313X.2008.03695.x. [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL. Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell. 2006;18:412–421. doi: 10.1105/tpc.105.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Combier JP, Frugier F, Billy F, Boualem A, El-Yahyaoui F, Moreau S, Vernié T, Ott T, Gamas P, Crespi M, et al. MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Genes & Development. 2006;20:3084–3088. doi: 10.1101/gad.402806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier-Brygoo H. CLC-mediated anion transport in plant cells. Philosophical Transactions of the Royal Society B Biological Science. 2009;364:195–201. doi: 10.1098/rstb.2008.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SC, Lin CS, Hsu PK, Lin SH, Tsay YF. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell. 2009;21:2750–2761. doi: 10.1105/tpc.109.067603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK. A miRNA involved in phosphate-starvation response in Arabidopsis. Current Biology. 2005;15:2038–2043. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. Cell-specific nitrogen responses mediate developmental plasticity. Proceedings of the National Academy of Sciences, USA. 2008;105:803–808. doi: 10.1073/pnas.0709559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FQ, Young J, Crawford NM. The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell. 2003;15:107–117. doi: 10.1105/tpc.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF. CHL1 function as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Ho CH, Tsay YF. Nitrate, ammonium, and potassium sensing and signaling. Current Opinion in Plant Biology. 2010;13:604–610. doi: 10.1016/j.pbi.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Hsieh LC, Lin SI, Shih ACC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiology. 2009;151:2120–2132. doi: 10.1104/pp.109.147280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress induced miRNA. Molecular Cell. 2004;14:787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annual Review of Plant Biology. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- Ju XT, Xing GX, Chen XP, Zhang SL, Zhang LJ, Liu XJ, Cui ZL, Yin B, Christie P, Zhu ZL, et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proceedings of the National Academy of Sciences, USA. 2009;106:3041–3046. doi: 10.1073/pnas.0813417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Lu XY, Cui X, Jin H, Zhu JK. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell. 2008;20:2238–2251. doi: 10.1105/tpc.108.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE. The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proceedings of the National Academy of Sciences, USA. 2005;102:13693–13698. doi: 10.1073/pnas.0504219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Huang CY, Tsay YF. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell. 1999;11:865–874. doi: 10.1105/tpc.11.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave C, Xie Z, Kasschau KD, Carrington JC. Cleavage of scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, Barton MK, Bartel DP. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5′ region. EMBO Journal. 2004;23:3356–3364. doi: 10.1038/sj.emboj.7600340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. Journal of Experimental Botany. 2007;58:2297–2306. doi: 10.1093/jxb/erm066. [DOI] [PubMed] [Google Scholar]
- Pant BD, Musialak-Lange M, Nuc P, May P, Buhtz A, Kehr J, Walther D, Scheible WR. Identification of nutrient-responsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiology. 2009;150:1541–1555. doi: 10.1104/pp.109.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng MS, Hudson D, Schofield A, Tsao R, Yang R, Gu HL, Bi YM, Rothstein SJ. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. Journal of Experimental Botany. 2008;59:2933–2944. doi: 10.1093/jxb/ern148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabino R, Mancinelli AL. Light, temperature, and anthocyanin production. Plant Physiology. 1986;81:922–924. doi: 10.1104/pp.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Shin R, Berg RH, Schachtman DP. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiology. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- Siefers N, Dang KK, Kumimoto RW, Bynum WE, Tayrose G, Holt BF. Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiology. 2009;149:625–641. doi: 10.1104/pp.108.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KB, Foley RC, Oñate-Sánchez L. Transcription factors in plant defense and stress responses. Current Opinion of Plant Biology. 2002;5:430–436. doi: 10.1016/s1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- Stitt M, Muller C, Matt P, Gibon Y, Carillo P, Morcuende R, Scheible WR, Krapp A. Steps towards an integrated view of nitrogen metabolism. Journal of Experiment Botany. 2002;53:959–970. doi: 10.1093/jexbot/53.370.959. [DOI] [PubMed] [Google Scholar]
- Tsay YF, Chiu CC, Tsai CB, Ho CH, Hsu PK. Nitrate transporters and peptide transporters. FEBS Letter. 2007;581:2290–2300. doi: 10.1016/j.febslet.2007.04.047. [DOI] [PubMed] [Google Scholar]
- Vidal E, Gutiérrez RA. A systems view of nitrogen nutrient and metabolite responses in Arabidopsis. Current Opinion of Plant Biology. 2008;11:521–529. doi: 10.1016/j.pbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Wang R, Okamoto M, Xing X, Crawford NM. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiology. 2003;132:556–567. doi: 10.1104/pp.103.021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AJ, Bennett IJ. The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulenis. Plant Cell, Tissue and Organ Culture. 2005;82:189–200. [Google Scholar]
- Yamasaki H, Abdel-Ghany SE, Cohu CM, Kobayashi Y, Shikanai T, Pilon M. Regulation of copper homeostasis by micro-RNA in Arabidopsis. Journal of Biology Chemistry. 2007;282:16369–16378. doi: 10.1074/jbc.M700138200. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T. Metabolic engineering with Dof1 transcription factor in plants: improved nitrogen assimilation and growth under low-nitrogen conditions. Proceedings of the National Academy of Sciences, USA. 2004;101:7833–7838. doi: 10.1073/pnas.0402267101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.