Abstract
Spinal cord injury normally results in life-long disabilities and a broad range of secondary complications. Advances in therapeutic delivery during the past few decades offer hope for such victims. However, the limited functional improvement shown in in vivo studies hinders effective therapeutic application in clinical practice. Recent studies showed that gene vectors can transfect cells present in the lesion of an injured spinal cord (endogenous cells) and thereby produce therapeutic molecules with long-lasting biological effects that promote neural tissue regeneration. In this article we review recent advances in non-viral gene delivery into neural cells and their use for gene therapy in spinal cord injury.
Keywords: Spinal cord, gene therapy, delivery, biomaterials, regeneration
Introduction
Spinal cord injury (SCI) can cause life-long disabilities and a broad range of secondary complications. Primary trauma to the spinal cord causes secondary injuries due to a complex cascade of molecular and cellular events which lead to loss of neurons, demyelination, and formation of a glial scar [1]. Advances in therapeutic methods during the past a few decades offer hope for SCI victims. However, the limited evidence so far of functional improvement in in vivo studies has hindered the advancement of therapeutic applications in clinical practice.
Cell therapy involves transplantation of a variety of cell types, such as Schwann cells [2], olfactory ensheathing glial cells [3] and neural stem cells [4]. Implantation of genetically engineered stem cells that secrete neurotrophic factors is a promising cell therapy approach. However, the behaviour of implanted cells is not well understood and not well controlled in a hostile microenvironment. Biomaterial scaffolds designed to reconstruct the architecture of an injured spinal cord can provide structural guidance for axonal regeneration and prevent scar tissue infiltration [5–9]. Despite recent advances, functional improvement is restricted when the regenerated axons reach the limits of their regrowth capacity in the channels of such engineered conduits. Also of limited effectiveness were molecular therapies designed to promote axonal regeneration, such as application of neurotrophins [10] and the targeting of inhibiting factors (such as chondroitinase ABC) [11,12]. Such molecules delivered directly into cerebrospinal fluid or into the intraparenchymal space functioned only transiently due to a short half-life and rapid elimination by a combination of cerebrospinal fluid flow and cell metabolism. In addition, repeated injection or continuous infusion of the therapeutic into the spinal cord often causes additional trauma and infection.
Gene vectors that transfect cells present in the lesion of an injured spinal cord (endogenous cells) and thereby produce therapeutic molecules may create long-lasting biological effects.
Although recombinant viral vectors are efficient for cell transduction and show promise for gene delivery into neurons, their therapeutic potential is limited by their immunogenicity [13,14], toxicity, and a high risk of insertional mutagenesis that may induce neoplasia [15,16]. Non-viral gene vectors provide a safer alternative approach for gene therapy. Therapeutic gene delivery by non-viral vectors generates functional proteins and the vectors remain episomal in the host cell. As non-viral gene vectors are not restricted to the size of delivered DNA [17], they have higher DNA-carrying capacity than viral vectors. Non-viral vectors can also be modified to target gene delivery to specific neuronal subpopulations [18,19].
In the past two decades, nanocarriers were developed to improve gene transfection efficiency with lower cytotoxicity. Gene carriers based on the cationic lipid, polyethylenimine (PEI), and dendritic and hyperbranched polymer were investigated for their neural cell transfection ability in vitro. Cell transfection efficacy was enhanced by improving nuclear import of the plasmid DNA and by targeting the transfection at specific cell populations [18,19]. In vivo gene transfection of local reactive cells with non-viral vectors was assessed in a SCI model [6,7]. Vectors delivering therapeutic genes, such as B-cell lymphoma-2 (Bcl-2) and glial cell line-derived neurotrophic factor (GDNF) in vivo yielded neuroprotective effects and enhanced neurogenesis [20,21]. In addition, biomaterial scaffolds such as poly(lactide-co-glycolide) (PLG) bridges that provide structural guidance for spinal neural tissue regeneration and a reservoir for the sustained gene delivery have been investigated for spinal cord repair in recent years [6,7,22]. In this review we will focus on the recent advances of neural cell transfection using non-viral gene vectors and their use for gene delivery in spinal cord regeneration after injury.
Pathology of SCI and cell targeting for gene therapy
In primary SCI, mechanical trauma causes direct neurological damage that induces neural tissue necrosis at the point of injury. Then a secondary injury occurs by a cascade of cellular and molecular reactions that may progress over several days or weeks [23–25]. SCI quickly induces a series of cellular events that occurs at an early stage after injury that may persist for months. Several different cell types migrate to the site of SCI and contribute to the inflammatory response and scar tissue formation. Within a few minutes post-SCI, myeloid cells are activated and create a heterogeneous multifunctional network that can either cause damage or promote repair of spinal cord neural tissue [26]. Microglia are among the first such cells to migrate into the lesion from the surrounding tissue [27,28]. Neutrophils arrive between 3 and 24 hours [29–31] and monocytes are the next to arrive 2–3 days post-injury [31]. These cells damage the neural tissue by releasing proinflammatory cytokines and proteases. However, they also help to remove injured tissue debris and they release protective cytokines that promote neuronal regeneration and tissue repair. In response to demyelination, oligodendrocyte progenitor cells that produce the proteoglycan neuroglycan 2 (NG2) and express platelet-derived growth factor-α receptor on the cell membrane are recruited from the grey and white matter for remyelination of regenerated spinal cord axons at the site of injury [32,33]. Damage to the spinal cord meningeal surface also initiates invasion of fibroblastic meningeal cells that restore the continuous layer of cells around neural tissue. Multipotential progenitor cells line the central canal of the adult rodent spinal cord [34]. In response to SCI, these multipotential progenitor cells (also known as ependymal cells) proliferate dramatically and differentiate into astrocytes and oligodendrocytes [35]. Astrocytes become the main cellular component of the glial scar because they proliferate and migrate into the injured area to eventually fill the vacant space.
Following SCI, therapeutic approaches strive to improve axonal regeneration by enhancing guided axonal growth and counter the influence of inhibitory factors. An axon growth-promoting molecule may directly promote axonal regrowth or indirectly enhance axonal regeneration by blocking inhibitory signals. The regenerated axons may subsequently establish new synapses to restore physiological function [36]. The strategy of non-viral gene therapy for regeneration of injured spinal cord is to transfect the endogenous cells with gene vectors that lead to the expression of functional molecules, such as growth factors, in a sustained fashion and thereby generate long-lasting biological effects (Figure 1). In a recent study, multichannelled neural conduits delivering plasmids encoding either firefly luciferase or EGFP[RE1] were implanted into the rat spinal cord after lateral hemisection surgery. Three days after implantation, the gene was expressed at the injury site. The endogenous cells in the lesion of an injured spinal cord, such as Schwann cells, oligodendrocytes, macrophages, reactive astrocytes, fibroblasts, and endothelial cells were seen in the pores and channels of the neural conduits and they did contain the plasmid-delivered genes [7]. This study demonstrated successful transfection of reactive and proliferative cells with non-viral vectors and that such delivery of a therapeutic gene may regulate cell and tissue organization and promote axonal growth.
Figure 1.
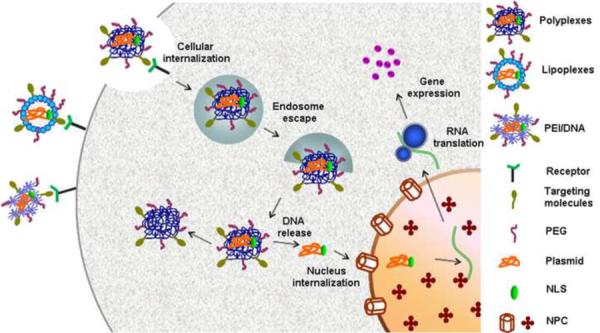
Plasmid delivery using nanocarriers.
Polyplexes, lipoplexes or PEI complexed plasmids can bind to specific receptors on the cell surface and then be internalized through endocytosis. The unbound DNA enters the nucleus through NPC, undergoes transcription in the nucleus, and eventual RNA translation and gene expression in the plasma.
Abbreviations: NLS: xxx[re4]; NPC: nuclear pore complex; PEG: polyethylene glycol; PEI: polyethylenimine.
Non-viral vector gene transfection of glial cells
Glial cells are the major endogenous cells involved in the host healing response associated with SCI. In vitro studies of gene transfection into glial cells provide important insights into how to accomplish the in vivo delivery of therapeutic genes to treat spinal cord injuries. Studies showed that nanocarriers promote the efficiency of gene transfection into cultured primary glial cells (Table 1) [37–40]. An early study showed that liposomes would enable transfection of mature astrocyte monolayers with efficiencies of 3.3% [37]. When the same astrocyte cultures were lipofected repeatedly, transfection efficiency of the β-galactosidase gene was improved from 2.6 to 17% [38]. The disulfide-containing cationic lipid, 1′,2′ dioleoyl-sn-glycero-3′-succinyl-2-hydroxyethyl disulfide ornithine conjugate (DOGSDSO), improved gene transfection efficiency in astroglial and microglial cultures from newborn rat cerebral cortex. The reversible disulfide bond in DOGSDSO was cleaved by the relatively high intracellular concentrations of reductive substances in the cytoplasm. The rapid intracellular degradation of the lipid increased the content of free DNA and decreased the toxicity of the reagent [39]. A recent study reported a 65% transfection efficiency into a rat spinal cord-derived glial cell line on lipoplex [DOTAP:PC (10:1)-pGFP]-precoated coverslips [40].
Table 1.
Transfection efficiency of cationic lipid, PEI and dendrimeric systems for glial cell and neuron transfection in vitro.
| Vector | Cell type | Source of cells | Transfection efficiency (%) | Refs |
|---|---|---|---|---|
| Cationic lipid | Astrocyte | Neonatal rat cortex | 3.3 | [37] |
| Astrocyte | Newborn mouse cerebral cortex | 17 | [38] | |
| Astrocyte, microglial | Newborn rat cerebral cortex | N/A | [39] | |
| Glial cell | Embryonic rat spinal cord | 65 | [40] | |
| Neuron | Embryonic rat hippocampus | 3 | [44] | |
| Neuron | Embryonic rat ventral mesencephalon | 2.4 | [45] | |
| Neuron | Embryonic rat cortex and hippocampus | 20–30 | [48] | |
| SH-SY5Y cells | Human neuroblastoma cell line | 16–25 | [49] | |
| PEI | Rat C6 glial cells, astrocytes | Adult rat hippocampus | 14 | [42] |
| Dendrimer | Neuron, astrocytes, microglia, and oligodendr ocytes | Embryonic mouse cortices | 40 | [43] |
PEI-complexed DNA can transfect rat brain-derived glial and neuronal cell lines. A PEI with a molecular weight of 25 kDa is more effective than a PEI with a molecular weight of 50–100 kDa for glial cell transfection. For neuronal cell transfection, PEI of 50–100 kDa is more effective than PEI of 25 kDa [41]. When transfected with Lipofectamine or PEI-complexed plasmid DNA, rat C6 glial cells and primary astrocytes generated the same total level of transgene product. However, Lipofectamine transfected significantly more cells than PEI while the cells transfected with PEI produced more transgene products per cell [42].
The negatively charged phosphate groups of the DNA backbone interact electrostatically with the positively charged amino groups of the dendritic or hyperbranched polymers and the DNA. This DNA–polymer interaction reorganized the DNA into small packets for efficient cellular delivery. A polyamidoamine (PAMAM) dendrimer grafted with arginine (Arg) for DNA delivery provided high transfection efficiency and low cytotoxicity in primary cortical cells, such as astrocytes, microglia, and oligodendrocytes compared with other gene carriers such as native PAMAM, PEI, and Lipofectamine. Efficient gene knock-down also resulted following transfection of cultured primary cortical cells with PAMAM-Arg polyplexed plasmid encoding HMGB1 shRNA [43]. These studies show that the transfection efficiency of glial cells was improved using synthetic gene vectors. Transfection of these cells with therapeutic genes and the expression of growth factors will provide neuroprotective effects and enhance axonal regeneration in the spinal cord repair.
Non-viral vector gene transfection of neurons
The transfection efficiency of primary neurons is normally low. Improvement of transfection efficiencies for neurons may significantly improve spinal cord axonal regeneration. Cationic lipids have been used extensively to transfect cultured neurons. In the earlier studies, neuron transfection efficiency was low (1–3%) for cultured primary neuronal cells [44–46]. Several experimental variables such as cell density, liposome-DNA concentrations, and liposome-DNA complexing time may affect the transfection efficiency and cell viability. The presence or absence of antibiotics and serum in the cell culture media can also affect cell transfection efficiency [47]. It was reported that Lipofectamine 2000 significantly improved transfection efficiency in primary neurons (20–25%) [48]. A recent study found low cytotoxicity and high transfection efficiency (16%) for the Arg-Glu2C(16) lipoplexes in neuronal SH-SY5Y cells. Because of the low cytotoxicity of Arg-Glu2C(16) lipoplexes, the proportion of transfected cells could be increased to 25% by increasing the concentration of lipoplexes applied to the cells [49]. Delivery of therapeutic genes by biomaterial scaffolds was also tested in vitro. In one study, PC12 cells were transfected with nerve growth factor (NGF) lipoplexes released from PLG scaffolds [50] and showed that biomaterials can act effectively as a carrier for non-viral gene vector delivery.
Plasmid DNA polyplexed with PEI also yielded significant gene expression in cultured chick embryonic neurons and in newborn mouse brains. It is likely that PEI generates higher gene delivery efficiency than poly-l-lysine (PLL) because of its enhanced endosomal release capability [51,52]. Real-time confocal particle tracking showed that the intracellular transport properties of PEI/DNA polyplexes and adenoviral vectors were similar. After transfection into primary neurons, both PEI/DNA polyplexes and adenoviruses quickly accumulated near the cell nucleus. The PEI/DNA polyplexes moved slower in neurites than in the cell bodies, whereas adenoviruses moved at equal rates in both cell compartments. However, PEI/DNA polyplexes and adenoviruses followed different intracellular trafficking pathways. The majority of PEI/DNA polyplexes trafficked through the endolysosomal pathway and ended up in late endosomes or lysosomes, whereas adenoviruses efficiently escaped from endosomes [53]. When PEI-based polyplexes were grafted with the hydrophilic polymer poly(ethylene glycol) (PEG) and administered to the central nervous system (CNS), particle aggregation and cellular toxicity of the polymer was reduced [54,55]. Both viral and non-viral vectors actively traffic toward the nucleus through the microtubule network and its associated motor proteins rather than by passive diffusion through the cytoplasm [56,57]. Microtubule-based vector transport is a critical function for neuron transfection because it provides communication between the cell body and axon regions. Alternatively, retrograde transport of non-viral vectors following uptake into neuronal cells does occur [53,58] and PEI/DNA complexes undergo retrograde transport from axon terminals to the neuronal soma in primary neurons [53]. Although these studies demonstrate development of nanocarriers for the gene delivery into neurons, the transfection efficiency is still low and thus prevents its clinical application to the treatment of neurological disorders. The nuclear membrane is recognized as a major barrier to the transfection of non-dividing cells. Thus, improved designs of gene vectors targeting the mechanism of nuclear importation may significantly enhance neuron transfection.
Neuron-specific gene transfection by cell receptor targeting
Internalization of vectors into a cell requires that the vector first associates with the cell plasma membrane. By binding to cell membrane lipids, non-viral vectors can establish non-specific associations with most cell types. However, a strategy based on ligand binding to specific membrane receptor proteins would prevent widespread delivery to off-target cells. Vector expression in specific cell types can accurately regulate cellular functions and limit unnecessary host inflammatory responses. Neurons express various classes of receptors including those for neuropeptides, neurotrophins and neurotoxins. Targeting such receptors would likely facilitate specific non-viral gene delivery.
Plasmid DNA polyplexed with PLL and conjugated with neurotensin (NT) underwent targeted uptake by cells expressing the high-affinity neurotensin receptor [19]. Neurotrophin receptors are expressed by specific neuron subtypes such as dorsal root ganglia (DRG) neurons, sympathetic neurons, and basal forebrain cholinergic neurons. When plasmid DNA and a targeting peptide containing the NGF loop 4 hairpin motif were complexed with PEI, they efficiently and specifically transfected DRG neurons expressing the NGF receptor TrkA [18]. Invasion of neurotoxins into the targeted neurons is an efficient process. After internalization of tetanus toxin into motor neuron presynaptic terminals, the molecules undergo efficient retrograde transport. After self-assembled vectors composed of PEI and tetanus toxin fragment c (TTC) were specifically transfected into cultured DRG neurons, they did express neurotrophic factor [59]. PEI was conjugated with Tet1 which mimics the receptor binding properties of TTC for specific cell transfection. The Tet1-modified PEI/DNA polyplexes were specifically taken up by cells expressing receptors for TTC [60,61]. When plasmid DNA complexed with Tet1-PEG-PEI was injected into the lateral ventricle, only adult neural progenitor cells were specifically transfected whereas untargeted PEG-PEI complexes transfected a heterogeneous population of cells [62]. These works suggest that neuron receptor-targeted gene transfection can provide specific cellular internalization and avoid undesired gene expression in neighbouring tissues.
Nuclear targeting of gene transfection
Microinjection of plasmid DNA into the cell nucleus resulted in significantly higher transgene expression than a cytoplasmic injection [63]. The nuclear envelope is a major barrier to effective gene transfer and the efficacy of nuclear delivery determines the level of gene expression [63]. The processes of DNA replication and RNA transcription in the nucleus are separated from the translation of mRNA and protein production in the cytoplasm by the nuclear membrane. Breakdown of the nuclear membrane would enhance delivery of plasmids into a cell nucleus.
Most non-viral gene transfection strategies depend on cell division. However, most tissue cells are non-dividing which presents a major barrier to therapeutic efficacy. It is recognized that the nuclear pore complex (NPC) regulates access of plasmid DNA to the nucleus [63,64]. Proteins up to 40 kDa, particles of approximately 9 nm, or DNA molecules of 210–350 bp can passively diffuse into the nucleus through the NPC which is composed of 30 different nucleoporins [65,66]. Thus the access of DNA complexes (100–500 nm) and plasmids to the nucleus is severely limited. However, the nuclear membrane is selectively permeable to molecules [i.e. heterogeneous nuclear ribonucleoprotein A1 (hnRNP), transcription factors, polymerase] containing nuclear localization signals (NLSs). Consequently, the incorporation of NLS peptides into non-viral gene carriers was investigated as a means to promote the nuclear uptake of DNA vectors [56].
The first NLS peptide sequence discovered was the sequence PKKKRKV (a monopartite NLS) in the SV40 large T-antigen [67]. This sequence used for non-viral gene delivery was named DNA nuclear targeting sequence (DTS). Non-viral gene vectors conjugated with NLS peptide improved the efficiency of gene delivery into neurons and increased gene expression. The non-classical NLS of heterogeneous nuclear ribonucleoprotein (hnRNP) A1 was termed M9 and M9-assisted plasmid delivery resulted in 20–100-fold higher transfection efficiency than lipofection alone. The enhanced gene transfection occurred after in vitro transfection into embryonic-derived retinal ganglion cells, rat pheochromocytoma (PC12) cells, embryonic rat ventral mesencephalon neurons, and the clinically relevant human NT2 cells or retinoic acid-differentiated NT2 neurons [68]. Those results suggest that the non-classical NLS is more potent than the classical NLS (SV40) for nuclear targeting in post-mitotic neurons.
Although nuclear localization sequence [re2]peptides can promote the nuclear uptake of DNA, the extent of uptake is highly variable and, to date, is generally disappointing. Although luciferase reporter activity increased significantly in one study [69], others [70,71] found no effect or only modest increases in gene expression following linkage of nuclear localization sequence peptides to the DNA vector. Further studies sought to optimize the DTS so as to improve non-viral gene delivery. For instance, consensus sequences for nuclear factor kappa B (NFκB) [72,73] and glucocorticoid responsive elements (GREs) [74] were introduced into the polyplexed plasmids to enhance nuclear uptake. Farrow [75] reported a hybrid mRNA/DNA gene delivery system that could bypass the nuclear barrier for neuron transfection and thus facilitate cytoplasmic gene expression. This study demonstrated that the co-delivery of mRNA encoding the T7 RNA polymerase (T7 RNAP) along with a T7-driven plasmid produced a 10–2,200 fold higher gene expression in primary dorsal root ganglion neuronal (DRGN) cultures compared to a cytomegalovirus (CMV)-driven plasmid.
Improvement of neuronal regeneration in SCI with in vivo gene transfection
Gene delivery into the spinal cord is a promising approach for treatment of spinal cord traumatic injury. SCI in adult mammals causes atrophy or loss of axotomized neurons. Injection of plasmids encoding a therapeutic gene had neuroprotective effects. In an adult Sprague-Dawley rat spinal cord hemisection study, axons from the right side of Clarke's Nucleus (CN) were cut unilaterally at the T8 level. A lipofectamine-complexed plasmid encoding Bcl-2 was injected into the right side of segment T8 in the normal spinal cord, or just caudal to the hemisection site. The atrophy and loss of axotomized Clarke's nucleus neurons in adult rats were reduced after intraspinal injection of the complexes [20]. Further studies assessed whether the same treatment protects axotomized red nucleus (RN) neurons. Plasmids encoding the human Bcl-2 gene were complexed with cationic lipids and then the complexes were injected just rostral to the hemisection site and 87% of RN neurons survived. These results indicate that intraspinal administration of complexed plasmids encoding the Bcl-2[re3] gene can prevent retrograde cell loss and reduce atrophy of axotomized RN and Clarke's nucleus neurons [76]. Another study showed that in vivo transfer of lipoplexed plasmids encoding GDNF promoted axonal regeneration and enhanced locomotion function recovery [16]. After lipoplexed plasmid encoding GDNF was injected into the injured spinal cord, GDNF expression increased in the injected areas 7 days post-injection. Four weeks later, anterograde tract tracing confirmed regeneration of the corticospinal tracts. Inclined plane test and Basso, Beattie, and Bresnahan (BBB) locomotor scores showed improved locomotion function of rat hind limbs. Insulin-like growth factor–I (IGF-I) is a neurotrophic factor that promotes the growth of projection neurons. Plasmids encoding IGF-I that were injected into the rat's tail vein 30 minutes after spinal cord hemisection induced a series of molecular events including improved activation of Akt and attenuated activation of GSK-3b, p35, and tau hyperphosphorylation [77]. The IGF-I gene transfer promoted motor recovery, anti-inflammatory responses, and antiapoptotic effects after SCI. Ultrasound microbubble-mediated transfection was applied to the spinal cord in adult rats to improve gene transfection efficiency. The naked plasmid DNA and microbubble mixture was injected into cerebrospinal fluid by lumbar puncture and then the spinal cord was exposed to ultrasound. The ultrasound treatment significantly increased luciferase expression compared with naked plasmid DNA alone [78].
The pathological disorder of SCI progresses over long periods of time and it may need the stable expression of functional genes at therapeutic levels for months. To achieve prolonged transgene expression in the rat spinal cord, repeated intrathecal administration of PEI-complexed plasmid DNA was performed [55]. The polyplexes were injected into the lumbar subarachnoid space. Single injections of polyplexes generated transgene expression in the spinal cord at 40-fold higher levels than did naked plasmid DNA. Repeated injection reduced gene expression because of apoptotic cell death. However, polyethylene glycol (PEG)-grafted PEI polyplexed plasmids did not reduce gene expression after repeated intrathecal injections. Although lumbar puncture is a routine clinical procedure and repeated injection of plasmids complexed with PEG-grafted PEI into the spinal cord may prolong the time span of in vivo transgene expression, it increases the chance of infection and extra trauma to the spinal cord.
Biomaterial scaffolds with optimized geometries provide guidance architecture to direct tissue formation after SCI and provide a reservoir for prolonged gene delivery (Figure 2). Lipoplexed plasmid DNA was immobilized onto the multichannel poly[lactide-co-glycolide] (PLG) neural conduits and the neural conduits were implanted in the hemisectioned rat spinal cord [6]. Transgene expression levels in the conduits delivering lipoplexes were twofold higher than that of naked plasmid and the expression persisted for three weeks. In a further study, multichannel neural conduits with encapsulated plasmids implanted into the hemisectioned spinal cord resulted in axon growth across the channels and high gene expression levels at the implant site. The synergy of gene delivery with scaffold architecture may induce improved tissue regeneration with complex architectures [7]. Autologous tissue derived from preligated peripheral nerves was fabricated as neural conduit to carry non-viral vector expressing brain-derived neurotrophic factor and the fabricated conduit was grafted into wounded rat spinal cord. The implanted conduit in spinal cord generated less immune-response and improved axonal regeneration and motor function recovery [79].
Figure 2.
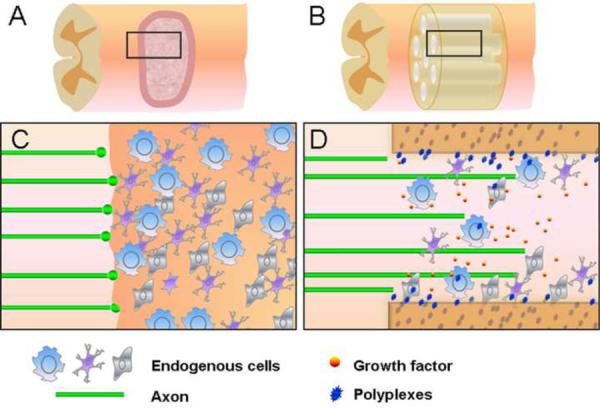
Neural conduit delivering plasmid polyplexes improves spinal cord axonal regeneration. (a,c) Inhibition of axonal regeneration by glial scar after spinal cord injury. (b) Neural conduit delivering plasmid polyplexes is implanted into the injured spinal cord. (d) Neural conduits simultaneously provide structural guidance for axonal regeneration and act as a reservoir for sustained gene delivery. Plasmid polyplexes released from neural conduit transfect the endogenous cells and secret growth factors which enhance axonal growth.
In a recent study, we fabricated multichannel collagen conduits delivering plasmids encoding neurotrophin-3 polyplexed with PEGylated hyperbranched 2-(dimethylamino)ethyl methacrylate (DMAEMA) for implantation into the transected spinal cord. We observed aligned axon growth through the conduit channels one month post-surgery. The conduits delivering pNT-3 polyplexes induced significantly more axonal growth compared with control conduits (Figure 3). The increased NT-3 levels in the neural tissue implanted with conduits carrying pNT-3 polyplexes was measured using ELISA. Our results indicate that functionalized biomaterial scaffolds designed to improve axonal regeneration by enhancing guided axonal growth and by countering the influence of inhibitory factors provide a promising approach for the repair of SCI. In addition to gene delivery by biomaterial scaffolds, the transplantation of gene-modified cells is another promising approach for gene therapy. Olfactory-ensheathing glia (OEG) transfected with cationic lipoplexed plasmids encoding NT3 was implanted into the rat spinal cord after contusion. Three months later, regeneration of axons was observed and hind limb function of rats was improved [80]. Because of the complex nature of injured spinal cord, an efficient therapeutic effect may be achieved by a combinatorial application of gene therapy with biomaterials and cell transplantation.
Figure 3.
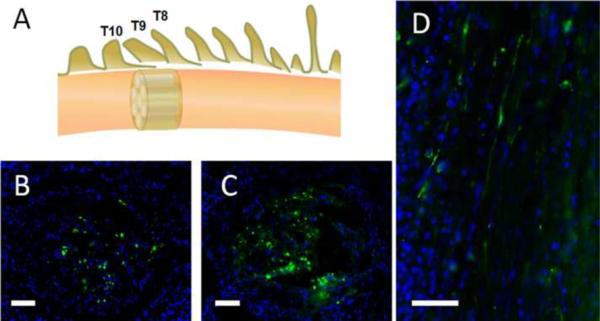
Longitudinal sections and cross-sections show axonal growth in the channels of multichannel collagen conduits. (a) At the thoracic 8– 10 (T8–T10) vertebral levels, the spinal cord was completely transected and a 2 mm gap was created. A 2mm-long multichannel collagen conduit was implanted in the gap and aligned with the rostral and caudal spinal cord stumps. (b,c) Comparison of regenerated axons in a typical channel on the cross-sections of control conduit (b) or conduit treated with pNT-3 polyplexes (c). (d) The aligned regenerated axons in a channel of conduits. The histological sections were stained with antineurofilament monoclonal antibody (green) and DAPI (blue). Scale bar: 50mm.
Concluding remarks
The target of spinal cord regeneration and functional recovery is to enhance axonal re-growth and control the inhibitory environment. Non-viral gene vectors that transfect the endogenous cells and produce therapeutic molecules may generate long-lasting biological effects. Recent advances in nanocarriers for gene delivery show promising results in both glial cell and neuronal transfection. However, to maximize the therapeutic potential of non-viral gene therapy, improved gene transfection efficiency is critical. Studies have focused on the major physical barriers encountered in non-viral gene delivery such as the cell and nuclear membrane and the latter seems to be the greater challenge in neural gene therapy. Implantation of engineered neural conduits in the injured spinal cord simultaneously provides structural guidance for neural tissue regeneration and acts as a reservoir for sustained gene delivery. The function of combinatorial application of biomaterial scaffolds and gene vectors may alleviate the low transfection efficiency of non-viral vectors. Delivered by the scaffolds, gene vectors may sustainably transfect endogenous cells and generate a long-lasting biological effect.
Highlights
The development of nano-carriers improved neural cell transfection efficiency in vitro.
Gene vectors can transfect endogenous cells and create long-lasting biological effects.
Non-viral gene therapy enhanced neurogenesis after spinal cord injury.
Biomaterials provide structural guidance for regeneration and a reservoir for gene delivery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Teaser: Non-viral gene vectors can transfect cells present in the lesion of an injured spinal cord and thereby produce therapeutic molecules with long-lasting biological effects that promote neural tissue regeneration.
References
- 1.Profyris C, et al. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol. Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Raisman G. Schwann cells induce sprouting in motor and sensory axons in the adult rat spinal cord. J. Neurosci. 1994;14:4050–4063. doi: 10.1523/JNEUROSCI.14-07-04050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, et al. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa Y, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 5.Moore MJ, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–429. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 6.De Laporte L, et al. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009;30:2361–2368. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Laporte L, et al. Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol. Ther. 2009;17:318–326. doi: 10.1038/mt.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson HE, et al. Neural stem cell- and Schwann cell-loaded biodegradable polymer scaffolds support axonal regeneration in the transected spinal cord. Tissue Eng. Part A. 2009;15:1797–1805. doi: 10.1089/ten.tea.2008.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen BK, et al. Comparison of polymer scaffolds in rat spinal cord: a step toward quantitative assessment of combinatorial approaches to spinal cord repair. Biomaterials. 2011;32:8077–8086. doi: 10.1016/j.biomaterials.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnell L, et al. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 11.Jefferson SC, et al. Chondroitinase ABC promotes recovery of adaptive limb movements and enhances axonal growth caudal to a spinal hemisection. J. Neurosci. 2011;31:5710–5720. doi: 10.1523/JNEUROSCI.4459-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang WC, et al. Gait analysis of spinal cord injured rats after delivery of chondroitinase ABC and adult olfactory mucosa progenitor cell transplantation. Neurosci. Lett. 2010;472:79–84. doi: 10.1016/j.neulet.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Dewey RA, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat. Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 14.Peden CS, et al. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall E. Clinical research. Gene therapy a suspect in leukemia-like disease. Science. 2002;298:34–35. doi: 10.1126/science.298.5591.34. [DOI] [PubMed] [Google Scholar]
- 16.Marshall E. Gene therapy. Second child in French trial is found to have leukemia. Science. 2003;299:320. doi: 10.1126/science.299.5605.320. [DOI] [PubMed] [Google Scholar]
- 17.de Jong G, et al. Efficient in-vitro transfer of a 60-Mb mammalian artificial chromosome into murine and hamster cells using cationic lipids and dendrimers. Chromosome Res. 2001;9:475–485. doi: 10.1023/a:1011680529073. [DOI] [PubMed] [Google Scholar]
- 18.Zeng J, et al. Self-assembled ternary complexes of plasmid DNA, low molecular weight polyethylenimine and targeting peptide for nonviral gene delivery into neurons. Biomaterials. 2007;28:1443–1451. doi: 10.1016/j.biomaterials.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Arango-Rodriguez ML, et al. Biophysical characteristics of neurotensin polyplex for in vitro and in vivo gene transfection. Biochim. Biophys. Acta. 2006;1760:1009–1020. doi: 10.1016/j.bbagen.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, et al. DNA plasmid that codes for human Bcl-2 gene preserves axotomized Clarke's nucleus neurons and reduces atrophy after spinal cord hemisection in adult rats. J. Comp. Neurol. 1999;404:159–171. doi: 10.1002/(sici)1096-9861(19990208)404:2<159::aid-cne2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Lu KW, et al. Cationic liposome-mediated GDNF gene transfer after spinal cord injury. J. Neurotrauma. 2002;19:1081–1090. doi: 10.1089/089771502760341983. [DOI] [PubMed] [Google Scholar]
- 22.De Laporte L, et al. Patterned transgene expression in multiple-channel bridges after spinal cord injury. Acta Biomater. 2010;6:2889–2897. doi: 10.1016/j.actbio.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Privat A. Pathophysiology and treatment of spinal cord injury. Bull. Acad. Natl Med. 2005;189:1109–1117. [PubMed] [Google Scholar]
- 24.Schwab JM, et al. Experimental strategies to promote spinal cord regeneration–an integrative perspective. Prog. Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Park E, et al. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J. Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 26.Hawthorne AL, Popovich PG. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics. 2011;8:252–261. doi: 10.1007/s13311-011-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 28.Dibaj P, et al. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia. 2010;58:1133–1144. doi: 10.1002/glia.20993. [DOI] [PubMed] [Google Scholar]
- 29.Beck KD, et al. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiwai H, et al. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am. J. Pathol. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stirling DP, Yong VW. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J. Neurosci. Res. 2008;86:1944–1958. doi: 10.1002/jnr.21659. [DOI] [PubMed] [Google Scholar]
- 32.Almad A, et al. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8:262–273. doi: 10.1007/s13311-011-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, et al. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J. Neurosci. 2006;26:2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulbatski I, et al. Oligodendrocytes and radial glia derived from adult rat spinal cord progenitors: morphological and immunocytochemical characterization. J. Histochem. Cytochem. 2007;55:209–222. doi: 10.1369/jhc.6A7020.2006. [DOI] [PubMed] [Google Scholar]
- 35.Meletis K, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:E182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwab ME. Repairing the injured spinal cord. Science. 2000;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 37.McKalip D, et al. Liposomal mediated transfection of mature rat astrocytes in vitro: a comparison of ten reagents. Neurosci. Lett. 2000;295:64–66. doi: 10.1016/s0304-3940(00)01587-1. [DOI] [PubMed] [Google Scholar]
- 38.Wu BY, et al. Multi-lipofection efficiently transfected genes into astrocytes in primary culture. J. Neurosci. Methods. 2000;102:133–141. doi: 10.1016/s0165-0270(00)00285-5. [DOI] [PubMed] [Google Scholar]
- 39.Ajmani PS, et al. Enhanced transgene expression in rat brain cell cultures with a disulfide-containing cationic lipid. Neurosci. Lett. 1999;277:141–144. doi: 10.1016/s0304-3940(99)00856-3. [DOI] [PubMed] [Google Scholar]
- 40.Rakotoarivelo C, et al. Mild surfection of neural cells, especially motoneurons, in primary culture and cell lines. Exp. Neurol. 2007;204:118–130. doi: 10.1016/j.expneurol.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, et al. Polyethylenimine strategies for plasmid delivery to brain-derived cells. Methods. 2004;33:144–150. doi: 10.1016/j.ymeth.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Tinsley RB, et al. Improved non-viral transfection of glial and adult neural stem cell lines and of primary astrocytes by combining agents with complementary modes of action. J. Gene Med. 2004;6:1023–1032. doi: 10.1002/jgm.584. [DOI] [PubMed] [Google Scholar]
- 43.Kim JB, et al. Enhanced transfection of primary cortical cultures using arginine-grafted PAMAM dendrimer, PAMAM-Arg. J. Control. Release. 2006;114:110–117. doi: 10.1016/j.jconrel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Kaech S, et al. Improved lipid-mediated gene transfer into primary cultures of hippocampal neurons. Mol. Brain Res. 1996;35:344–348. doi: 10.1016/0169-328x(95)00238-n. [DOI] [PubMed] [Google Scholar]
- 45.Wiesenhofer B, Humpel C. Lipid-mediated gene transfer into primary neurons using FuGene: comparison to C6 glioma cells and primary glia. Exp. Neurol. 2000;164:38–44. doi: 10.1006/exnr.2000.7414. [DOI] [PubMed] [Google Scholar]
- 46.da Cruz MT, et al. Improving lipoplex-mediated gene transfer into C6 glioma cells and primary neurons. Exp. Neurol. 2004;187:65–75. doi: 10.1016/j.expneurol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Dalby B, et al. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Ohki EC, et al. Improving the transfection efficiency of post-mitotic neurons. J. Neurosci. Methods. 2001;112:95–99. doi: 10.1016/s0165-0270(01)00441-1. [DOI] [PubMed] [Google Scholar]
- 49.Obata Y, et al. Evaluation of cationic liposomes composed of an amino acid-based lipid for neuronal transfection. Nanomedicine. 2010;6:70–77. doi: 10.1016/j.nano.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Whittlesey KJ, Shea LD. Nerve growth factor expression by PLG-mediated lipofection. Biomaterials. 27:2477–2486. doi: 10.1016/j.biomaterials.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boussif O, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonawane ND, et al. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 53.Suk JS, et al. Quantifying the intracellular transport of viral and nonviral gene vectors in primary neurons. Exp. Biol. Med. (Maywood) 2007;232:461–469. [PubMed] [Google Scholar]
- 54.Tang GP, et al. Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials. 2003;24:2351–2362. doi: 10.1016/s0142-9612(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 55.Shi L, et al. Repeated intrathecal administration of plasmid DNA complexed with polyethylene glycol-grafted polyethylenimine led to prolonged transgene expression in the spinal cord. Gene Ther. 2003;10:1179–1188. doi: 10.1038/sj.gt.3301970. [DOI] [PubMed] [Google Scholar]
- 56.van der Aa MA, et al. The nuclear pore complex: the gateway to successful nonviral gene delivery. Pharm. Res. 2006;23:447–459. doi: 10.1007/s11095-005-9445-4. [DOI] [PubMed] [Google Scholar]
- 57.Dohner K, et al. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell. 2002;13:2795–2809. doi: 10.1091/mbc.01-07-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suh J, et al. Efficient active transport of gene nanocarriers to the cell nucleus. Proc. Natl Acad. Sci. USA. 2003;100:3878–3882. doi: 10.1073/pnas.0636277100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliveira H, et al. Targeted gene delivery into peripheral sensorial neurons mediated by self-assembled vectors composed of poly(ethylene imine) and tetanus toxin fragment c. J. Control. Release. 2010;143:350–358. doi: 10.1016/j.jconrel.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Liu JK, et al. A novel peptide defined through phage display for therapeutic protein and vector neuronal targeting. Neurobiol. Dis. 2005;19:407–418. doi: 10.1016/j.nbd.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 61.Park IK, et al. Neuron-specific delivery of nucleic acids mediated by Tet1-modified poly(ethylenimine) J. Gene Med. 2007;9:691–702. doi: 10.1002/jgm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwon EJ, et al. Targeted nonviral delivery vehicles to neural progenitor cells in the mouse subventricular zone. Biomaterials. 2010;31:2417–2424. doi: 10.1016/j.biomaterials.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Capecchi MR. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980;22:479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- 64.Ludtke JJ, et al. The effect of cell division on the cellular dynamics of microinjected DNA and dextran. Mol. Ther. 2002;5:579–588. doi: 10.1006/mthe.2002.0581. [DOI] [PubMed] [Google Scholar]
- 65.Luby-Phelps K. Cytoarchitecture and physical properties of cytoplasm: volume, viscosity, diffusion, intracellular surface area. Int. Rev. Cytol. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. [DOI] [PubMed] [Google Scholar]
- 66.Schaffer DV, et al. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol. Bioeng. 2000;67:598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 67.Kalderon D, et al. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 68.Ma H, et al. Non-classical nuclear localization signal peptides for high efficiency lipofection of primary neurons and neuronal cell lines. Neuroscience. 2002;112:1–5. doi: 10.1016/s0306-4522(02)00044-1. [DOI] [PubMed] [Google Scholar]
- 69.Zanta MA, et al. Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc. Natl Acad. Sci. USA. 1999;96:91–96. doi: 10.1073/pnas.96.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bremner KH, et al. Factors influencing the ability of nuclear localization sequence peptides to enhance nonviral gene delivery. Bioconjug. Chem. 2004;15:152–161. doi: 10.1021/bc034140k. [DOI] [PubMed] [Google Scholar]
- 71.van der Aa M, et al. Covalent attachment of an NLS-peptide to linear DNA does not enhance transfection efficiency of cationic polymer based gene delivery systems. J. Control. Release. 2005;101:395–397. [PubMed] [Google Scholar]
- 72.Breuzard G, et al. Nuclear delivery of NFkappaB-assisted DNA/ polymer complexes: plasmid DNA quantitation by confocal laser scanning microscopy and evidence of nuclear polyplexes by FRET imaging. Nucleic Acids Res. 2008;36:E71. doi: 10.1093/nar/gkn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Gaal EV, et al. DNA nuclear targeting sequences for non-viral gene delivery. Pharm. Res. 2011;28:1707–1722. doi: 10.1007/s11095-011-0407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dames P, et al. Targeting of the glucocorticoid hormone receptor with plasmid DNA comprising glucocorticoid response elements improves nonviral gene transfer efficiency in the lungs of mice. J. Gene Med. 2007;9:820–829. doi: 10.1002/jgm.1082. [DOI] [PubMed] [Google Scholar]
- 75.Farrow PJ, et al. Cytoplasmic expression systems triggered by mRNA yield increased gene expression in post-mitotic neurons. Nucleic Acids Res. 2006;34:E80. doi: 10.1093/nar/gkl442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shibata M, et al. Single injections of a DNA plasmid that contains the human Bcl-2 gene prevent loss and atrophy of distinct neuronal populations after spinal cord injury in adult rats. Neurorehabil. Neural Repair. 2000;14:319–330. doi: 10.1177/154596830001400408. [DOI] [PubMed] [Google Scholar]
- 77.Hung KS, et al. Gene transfer of insulin-like growth factor-I providing neuroprotection after spinal cord injury in rats. J. Neurosurg. Spine. 2007;6:35–46. doi: 10.3171/spi.2007.6.1.35. [DOI] [PubMed] [Google Scholar]
- 78.Shimamura M, et al. Gene transfer into adult rat spinal cord using naked plasmid DNA and ultrasound microbubbles. J. Gene Med. 2005;7:1468–1474. doi: 10.1002/jgm.793. [DOI] [PubMed] [Google Scholar]
- 79.Tinsley RB, et al. Use of engineered peripheral nerve autografts for spinal cord repair. Neuroreport. 2006;27:261–265. doi: 10.1097/01.wnr.0000199462.09165.12. [DOI] [PubMed] [Google Scholar]
- 80.Wu J, et al. Ex vivo non-viral vector-mediated neurotrophin-3 gene transfer to olfactory ensheathing glia: effects on axonal regeneration and functional recovery after implantation in rats with spinal cord injury. Neurosci. Bull. 2008;24:57–65. doi: 10.1007/s12264-008-0057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


