Abstract
Obesity contributes to a multitude of serious health problems. Given the demonstrated role of the endogenous cannabinoid system in appetite regulation, the purpose of the present study was to evaluate structural analogs of two cannabinoids, rimonabant (cannabinoid CB1 receptor antagonist) and O-2050 (sulfonamide analog of Δ8-tetrahydrocannabinol), that showed appetite suppressant effects in previous studies. Structure–activity relationships of these two lead compounds were examined in several assays, including cannabinoid CB1 and CB2 receptor binding, food intake, and an in vivo test battery (locomotor activity, antinociception, ring immobility, and body temperature) in mice. Rimonabant and O-2050 reliably decreased feeding in mice; however, their analogs decreased feeding only at higher doses, even though some compounds had quite good cannabinoid CB1 binding affinity. Results of the in vivo test battery were inconsistent, with some of the compounds producing effects characteristic of cannabinoid agonists while other compounds were inactive or were antagonists against an active dose of Δ9-tetrahydrocannabinol. These results demonstrate that reduction of food intake is not a characteristic effect of pyrazole and sulfonamide cannabinoid analogs with favorable cannabinoid CB1 binding affinity, suggesting that development of these classes of cannabinoids for the treatment of obesity will require evaluation of their effects in a broad spectrum of pharmacological assays.
Keywords: Antagonist, Cannabinoid, Feeding, Hemopressin, Pyrazole, Rimonabant, Sulfonamide, (Mouse)
1. Introduction
Obesity contributes to a multitude of serious health problems, including diabetes, stroke, and cardiovascular disease (Brown et al., 2009). Despite this substantial health impact, pharmaco-therapy for treatment of obesity is largely limited to two drugs, phentermine (a sympathomimetic) and orlistat (oral lipase inhibitor) (for a review, see Powell et al., 2011). Current medication development efforts are focused on several avenues through which appetite may be modulated, including appetite-related peptides such as neuropeptide Y and AgRP (agouti-related protein), as well as combination drugs that modulate more than one system (Powell et al., 2011). The results of past studies strongly suggest that the endogenous cannabinoid system plays a role in appetite regulation (for a review, see Cota et al., 2003), and represents an additional target for medication development. Exogenous administration of cannabinoids also has been shown to affect feeding behavior. Agonists of cannabinoid CB1 receptors, such as Δ9-tetrahydrocannabinol, the primary psychoactive substituent of the marijuana plant, increase food intake in humans and rodents (Hart et al., 2002; Koch, 2001; Williams et al., 1998), whereas antagonists, such as the prototypic cannabinoid CB1 receptor antagonist rimonbant, decrease food intake (McLaughlin et al., 2003; Rowland et al., 2001; Wiley et al., 2005). Interestingly, O-2050, a side chain analog of Δ8-tetrahydrocannabinol, which has been described as a neutral cannabinoid CB1 receptor antagonist (Gardner and Mallet, 2006, but see Wiley et al., 2011), also has been reported to decrease feeding behavior (Gardner and Mallet, 2006; Wiley et al., 2011).
In humans, rimonabant was originally marketed as an anti-obesity agent and as an aid to smoking cessation until its adverse psychiatric effects were revealed during advanced clinical trials after its release in Europe (Christensen et al., 2007); however, the extent to which the latter effects are related to rimonabant interaction with the cannabinoid CB1 receptor is unknown. Although several of rimonabant’s in vivo effects in rodents have been shown to be cannabinoid CB1 receptor-mediated, including decreased feeding behavior (Wiley et al., 2005), not all of its effects are mediated by this mechanism, e.g., stimulation of locomotor activity (Bass et al., 2002). Similarly, the results of one study have suggested that rimonabant’s effects on mood may be attenuated through blockade of kappa opioid receptors (Lockie et al., 2011). Given that conclusive evidence is lacking with regard to whether rimonabant’s negative affective effects are mediated via its actions at central cannabinoid CB1 receptors, investigation of cannabinoid-based medications for appetite regulation is still considered a viable alternative, particularly for peripherally restricted compounds (Alonso et al., 2012; Christopoulou and Kiortsis, 2011; Sasmal et al., 2011). One of the first steps in this type of investigation is evaluation of the activity and efficacy of promising compounds. To this end, the present study examined the effects of structural analogs of rimonabant and of the sulfonamide cannabinoid, O-2050, on food intake using a model previously used to show the appetite suppressant effects of these two lead compounds (Wiley et al., 2011; Wiley et al., 2005).
2. Materials and methods
2.1. Subjects
Some tests described herein were performed at Virginia Commonwealth University (VCU) and some were completed at Research Triangle Institute (RTI), as described in the drug section. Adult male ICR mice were obtained from Harlan (Dublin, VA) for testing at VCU or from Harlan (Frederick, MD) for testing at RTI. All animals at both institutions were individually housed in clear plastic cages in a temperature-controlled (20–24 °C) environment with a 12-h light–dark cycle. For the feeding experiments, each ICR mouse was tested with each dose of a single drug, presented in randomized Latin square order. Separate groups of mice were tested with each dose of each compound in the mouse test battery. All tests were conducted during the light part of the light–dark cycle. The studies reported in this manuscript were carried out in accordance with guidelines published in the guide for the care and use of laboratory animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at each institution.
2.2. Apparatus
Weight of food pellets was measured with a Mettler AT261 Delta Range scale (Toledo, OH) at 0.01 mg accuracy at VCU. At RTI, food weight was measured with an ACCULAB Model EC-211 scale (Bradford, MA) at 0.1 g accuracy. At VCU, assessment of spontaneous activity in mice occurred in standard activity chambers interfaced with a Digiscan Animal Activity Monitor (Omnitech Electronics, Inc., Columbus, OH). A standard tail-flick apparatus and a digital thermometer (Fisher Scientific, Pittsburgh, PA) were used to measure antinociception and rectal temperature, respectively. The ring immobility device consisted of an elevated metal ring (diameter=5.5 cm, height=16 cm) attached to a wooden stand. At RTI, locomotor sessions with heterodimer 6b were conducted in standard activity chambers interfaced with San Diego Instruments Photobeam Activity System software (San Diego, CA). Antinociception was measured with the use of a standard tail-flick apparatus (Stoelting, Wood Dale, IL). Rectal temperature was measured using a standard rodent rectal thermometer (Physitemp, Clifton, NJ).
2.3. Procedure
2.3.1. Membrane preparations
Chinese Hamster Ovary (CHO) cells stably expressing the human cannabinoid CB1 or CB2 receptor were cultured in a 50:50 mixture of DMEM and Ham F-12 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 mg/ml G418, and 5% fetal calf serum. Cells were harvested by replacement of the media with cold PBS containing 0.4% EDTA followed by agitation. Membranes were prepared by homogenization of cells in 50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4, centrifugation at 50,000 ×g for 10 min at 4 °C, and resuspension in the same buffer at 1.5 mg/ml. Membranes were stored at −80 °C until use.
2.3.2. Radioligand binding
Membranes were diluted with assay buffer B (50 mM Tris-HCl; pH 7.4, 3 mM MgCl2, 0.2 mM EGTA). Reactions containing membrane (10 μg protein) were incubated with 1 nM [3H](−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxy-propyl)cyclohexanol [CP55,940] and varying concentrations of test compounds in assay buffer B containing 0.5% BSA. Non-specific binding was measured in the presence of 1 μM unlabeled CP55,940. The assay was incubated for 60 min at 30 °C, and terminated by rapid filtration under vacuum through Whatman GF/B glass fiber filters that were pre-soaked in Tris buffer containing 5 g/L BSA (Tris-BSA), followed by five washes with cold Tris-BSA. Bound radioactivity was determined by liquid scintillation spectrophotometry at 45% efficiency for 3H.
2.3.3. Feeding behavior
Twenty-four hours before the start of a feeding trial, all food was removed from the home cages of mice to be tested. The next day mice were transported to the laboratory at least one hour before the beginning of the feeding trial. They were injected with the test compound at the specified pre-session injection interval. Subsequently, they were placed in a clear plastic cage with paper towels lining the bottom and allowed access to a pre-measured amount of their regular lab chow. At the end of one hour, mice were removed from the test cage and placed back into their home cage. The amount of food left in the test cage, including crumbs, was measured, and amount consumed was calculated. Mice received up to two feeding trials per week, separated by at least 72 h.
2.3.4. In vivo pharmacology
The pyrazole and sulfonamide analogs were also evaluated in separate groups of mice in a battery of four tests, in which cannabinoid agonists produce a characteristic profile of in vivo effects (Martin et al., 1991): suppression of locomotor activity, antinociception in the tail flick assay, decreased rectal temperature and ring immobility. Prior to injection, rectal temperature and baseline latency in the tail flick test were measured in the mice. The latter procedure involved exposing the mouse’s tail to an ambient heat source (i.e., bright light) and recording latency (in s) for tail removal. Typical control latencies were 2–4 s. A 10 s maximal latency was used in order to avoid damage to the mouse’s tail. After measurement of temperature and baseline tail flick latency, mice were injected i.v. with vehicle or drug. Five minutes later they were placed into individual activity chambers for 10 min. Spontaneous activity was measured as the total number of beam interruptions during the entire session, which was expressed as percent inhibition of the control (vehicle) group’s activity. Tail-flick latency was measured at 20 min post-injection. Antinociception was expressed as the percent maximum possible effect using a 10-s maximum test latency. Rectal temperature was measured at 30 min after injection and was expressed as the difference between pre- and post-injection rectal temperatures. At 40 min post-injection, the mice were placed on a 5.5 cm ring attached at a height of 16 cm to a ring stand, and the amount of time the animals remained motionless during a 5 min period was recorded. The time that each animal remained motionless on the ring was divided by 300 s and multiplied by 100 to obtain a percent immobility rating. Hetero-dimer 6b was also tested in separate groups of mice; however, the procedure at RTI differed from that described for VCU above in the following ways: (1) i.p. (vs. i.v.) administration, (2) mice were placed into the locomotor chambers 40 min after injection for 5 min and were re-evaluated in the tail flick and temperature assays immediately upon removal, and (3) mice were not tested in the ring immobility procedure.
2.4. Drugs
Rimonabant, obtained from the National Institute on Drug Abuse (Rockville, MD), was suspended in a vehicle of absolute ethanol, Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ), and saline in a ratio of 1:1:18. The pyrazole and sulfonamide analogs (Fig. 1) were synthesized at Organix, Inc. (Woburn, MA) and were prepared in the 1:1:18 vehicle. The following pyrazole compounds were assessed: 6-bromo-1-(2,4-dichlorophenyl)–N-(piperidin-1-yl)-1H-indazole-3-carboxamide [O-1654], 1-(2,4-dichlorophenyl)-5-[4-(hexan-2-yl)phenyl]-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide [O-1690], 4-(4-chlorophenyl)-3-(2,4-dichlorophenyl)–N-(piperidin-1-yl)-5-propylbenzamide [O-1788], 3-bromopropyl carboxamide analog [O-2154], 3-cyanopropyl carboxamide analog [O-2155], 5-[4-(5-bromopentyl)phenyl]-pyrazole analog [O-3258], and N-allyl carboxamide analog [O-4373]. The following sulfonamide analogs were assessed: 6-[(6aR,10aR)-1-hydroxy-6,6,9-trimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-3-yl]-6-methyl-N-(4-sulfamoyl-phenyethyl)heptanamide [O-606], 7-[(6aR,10aR)-1-hydroxy-6, 6,9-trimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-3-yl]-7-methyl-N-(4-sulfamoylphenethyl]octanamide [O-1689], 7-[(6aR,10aR)-6,6,9-trimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]-chromen-3-yl]–N-(4-sulfamoylphenethyl)]]hept-5-ynamide [O-1720], N-{6-[(6aR,10aR)-1-hydroxy-6,6,9-trimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-3-yl]hex-4-yn-1-yl}ethane-sulfonamide [O-1991], N-{5-[(6aR,10aR)-1-hydroxy-6,6,9-tri-methyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-3-yl]-5-methylhexyl}ethanesulfonamide [O-2113], and 3-(6′-methane-sulfamidohex-2-yne)-Δ8-tetrahydrocannabinol [O-2050]. All pyrazole and sulfonamide analogs were evaluated at Virginia Commonwealth University. N,N′-[(methylimino)diheptane-7, 1-diyl]bis[5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide] [heterodimer 6b] and 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)–N-{[4-(methanesulfonamido-methyl)cyclohexyl]methyl}-4-methyl-1H-pyrazole-3-carboxamide [13226–70] were synthesized at RTI (Research Triangle Park, NC) and were mixed in a solution of 95% sesame oil and 5% dimethylacetamide. Hemopressin (NIDA) was dissolved in saline and the pH was adjusted to 7±0.5 with sodium phosphate. These three compounds were evaluated at RTI. For feeding studies at both locations, all drugs were administered to the mice intraperitoneally (i.p.) at a volume of 0.1 ml/10 g body weight. Pre-session injection time was 60 min for all feeding studies. Since all in vivo effects were believed to be cannabinoid CB1 receptor mediated, choice of test doses for each compound was based upon cannabinoid CB1 binding affinity, although limited quantities of some of the compounds prevented testing of higher doses. In addition, our maximum dose for the feeding experiments was 100 mg/kg due to limits of solubility for many of the compounds.
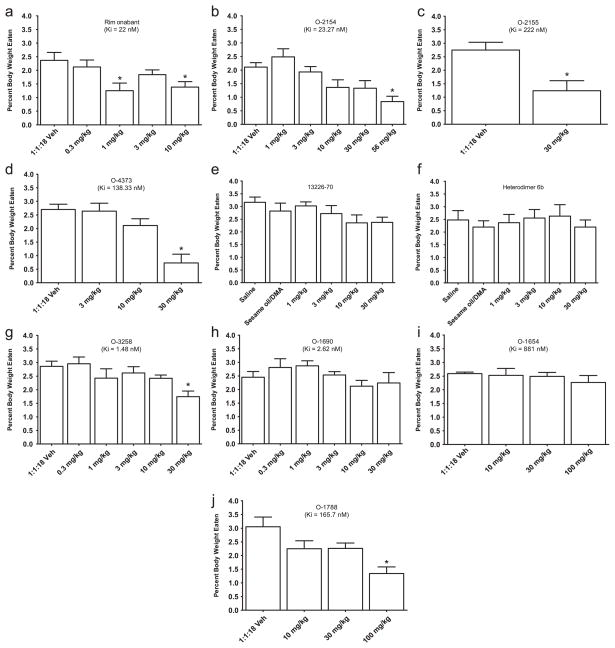
Fig. 1.
Effects of rimonabant and pyrazole-derived cannabinoids on food intake expressed as % of body weight. For each compound, bars represent the mean (± S.E.M.) of data from the same 10 mice at each dose. * Indicates significant difference from vehicle control (p<0.05).
2.5. Statistical analysis
Amount of food eaten (in g) was divided by body weight (in g) and converted to a percentage to obtain % body weight eaten, the dependent measure for the feeding experiments. For each dose-effect curve in the feeding study, a repeated measures analysis of variance (ANOVA) was used to analyze this dependent measure. Tukey post-hoc tests (α=0.05) were used to specify differences revealed by significant ANOVAs.
For the tetrad tests, rectal temperature values were expressed as the difference between control temperature (before injection) and temperature following drug administration (Δ °C). Spontaneous activity was measured as total number of photocell beam interruptions during the entire session and was expressed percent inhibition of the control (vehicle) group’s activity. Antinociception was calculated as percent of maximum possible effect {% maximal possible effect=[(test−control latency)/(10−control)] × 100}. Ring immobility (or catalepsy) was expressed as percent of time spent immobile during a 300-s session. Based on data obtained from numerous previous studies with cannabinoids, maximal cannabinoid effects in each procedure were estimated as follows: 90% inhibition of spontaneous activity, 100% maximum possible effect in the tail flick procedure, −6 °C change in rectal temperature, and 60% ring immobility. ED50s were defined as the dose at which half maximal effect occurred. For drugs that produced one or more cannabinoid effects, ED50s were calculated separately using least-squares linear regression on the linear part of the dose-effect curve for each in vivo measure, plotted against log10 transformation of the dose.
3. Results
3.1. Rimonabant/pyrazole analogs
Fig. 1 shows the effects of rimonabant/pyrazole analogs with a range of cannabinoid CB1 receptor binding affinities on food intake in mice. As expected, the prototypic pyrzole cannabinoid CB1 receptor antagonist rimonabant significantly decreased feeding at doses of 1 and 10 mg/kg by 47% and 41% compared to vehicle, respectively [panel A; F(4,40)=6.3, P<0.05]. Significant decreases in feeding were also observed with the 3-substituted pyrazole analogs, including 56 mg/kg O-2154 [panel B; F(5,45)=8.37, P<0.05], 30 mg/kg O-2155 [panel C; F(1,9)=21.49, P<0.05], and 30 mg/kg O-4373 [panel D; F(3,27)=14.47, P<0.05], producing 60%, 55%, and 73% decreases compared to vehicle, respectively. In contrast, neither 133226-70 (the other 3-substituted analog) nor heterodimer 6b (with dimer attached at the 3 position) affected food intake at doses up to 30 mg/kg [13226–70: panel E, F(5,45)=1.43, P>0.05; heterodimer 6b: panel F, F(5,45)=0.42, P>0.05]. 5-Substituent pyrazole analogs showed similar diversity. Whereas 30 mg/kg O-3258 significantly decreased feeding by 39% compared to vehicle [panel G; F(5,50)=6.45, P<0.05], O-1690 did not alter food intake at doses up to 30 mg/kg [panel H; F(5,45)=1.38, P>0.05]. Finally, conversion of the pyrazole core to an indazole (O-1654) also did not affect food intake at doses up to 100 mg/kg [panel I; F(3,24)=0.5, P>0.05] whereas its conversion to a phenyl (O-1788) significantly decreased feeding at a dose of 56 mg/kg by 56% compared to vehicle [panel J; F(3,27)=6.21, P<0.05].
As described above, manipulation of the substituents of the rimonabant/pyrazole template resulted in varying effects on food intake that were not necessarily predicted by the substituent’s position. The effects of the set of rimonabant analogs on food intake also were not predicted by their cannabinoid CB1 receptor binding affinities or by their effects in a battery of in vivo tests in mice (Table 1). For example, the cannabinoid CB1 receptor binding affinities of compounds that decreased feeding at a dose of 30 mg/kg (O-2155, O-3258, and O-4373) ranged from 1.5 to 222 nM whereas another compound (O-1690) with a cannabinoid CB1 receptor affinity of 2.6 nM did not affect feeding at doses up to 30 mg/kg. Further, effects of the compounds differed in the test battery in mice. Some of the compounds produced effects that are characteristic of cannabinoid agonists (O-2154, O-2155, and O-4373), including suppression of locomotor activity, antinociception, hypothermia, and catalepsy. O-4373 was moderately potent at producing these effects, with ED50s ranging from 2.3 to 7 mg/kg. While limited quantities of O-2154 and O-2155 precluded testing a full dose-effect curve for these compounds, doses of 3 and 10 mg/kg, respectively, produced at least 50% of maximal cannabinoid agonist-like activity (see maximums in statistical analysis section) in each of the four tests. Each of these compounds also decreased feeding at higher doses (30 or 56 mg/kg). Other compounds were inactive when tested alone in mice (O-1690, O-1788, and heterodimer 6b) and/or blocked the characteristic agonist effects of an active dose of Δ9-tetrahydrocannabinol (O-3258 and O-1690) (Wiley et al., 2001). Notably, O-3258, one of the compounds that acted as an antagonist in vivo, increased motor activity when administered alone and suppressed feeding, effects that are shared by rimonabant (Wiley et al., 2005).
Table 1.
Effects of rimonabant analogs in cannabinoid receptor binding and in a battery of pharmacological tests in mice.
| Compound | Structure | CB1 | CB2 | SA | %MPE | RT | RI |
|---|---|---|---|---|---|---|---|
| Rimonabanta,b |
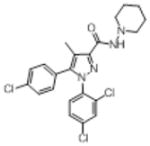
|
6 (0.2) | 702 (62) | stim (10) | inactive (10) | inactive (10) | inactive (10) |
| O-2154c |
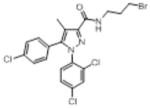
|
38 (9) | 731 (106) | 70% (3) | 50% (3) | 34.2 (3) | 35% (3) |
| O-2155c |
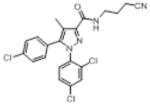
|
222 (78) | 1848 (569) | 86% (10) | 89% (10) | 34.6 (10) | 47% (10) |
| O-4373c |
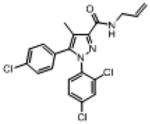
|
147 (11) | 1703 (124) | 2.3 (1.0–5.2) | 7 (1.9–5.0) | 2.9 (2.1–5.0) | 4.2 (2.5–7.0) |
| 13226–70d |
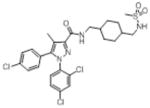
|
54.8 | 897 | nd | nd | nd | nd |
| Heterodimer 6be |
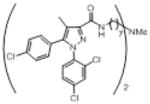
|
17.3 (0.45) | 683 | inactive (30) | inactive (30) | inactive (30) | inactive (30) |
| O-3258 |
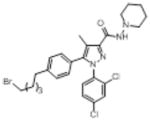
|
1.5 (0.08) | 203 (35) | stim (10) | inactive (10) | inactive (10) | inactive (10) |
| O-1690f |
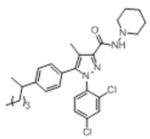
|
2.6 (0.13) | nd | inactive (30) | inactive (30) | inactive (30) | inactive (30) |
| O-1654 |
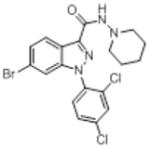
|
881 (44) | 1185 (446) | nd | nd | nd | nd |
| O-1788 |
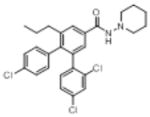
|
166 (30) | nd | inactive (30) | inactive (30) | inactive (30) | inactive (30) |
Cannabinoid CB1 and CB2 receptor binding values reflect displacement of [3H]CP55,940 and are expressed as Kis (± SEM) in nM. SA =spontaneous activity (expressed as % inhibition of activity of vehicle control group; stim=stimulation of activity compared to vehicle control group), MPE=% maximum possible antinociceptive effect in tail flick assay, RT=rectal temperature (expressed as change in temperature compared to pre-drug assessment), RI=ring immobility (expressed as % time immobile on an elevated ring), nd=not determined. For the in vivo test results, values are expressed as ED50s (mg/kg with 95% confidence limits) where appropriate. If compound was inactive or if quantities allowed testing of a single dose only, maximum effect is listed with dose (mg/kg) at which it occurred in parentheses. Previously published data are indicated by italicized text, with citations provided below.
Cannabinoid CB1 receptor binding and in vivo results from Wiley et al. (2012).
Cannabinoid CB2 receptor binding from Showalter et al. (1996).
All binding and in vivo data from Wiley et al. (2012).
Binding data from Fulp et al. (2011).
Binding data from Zhang et al. (2010).
All binding and in vivo data from Wiley et al. (2001).
3.2. Sulfonamide analogs of Δ8-tetrahydrocannabinol
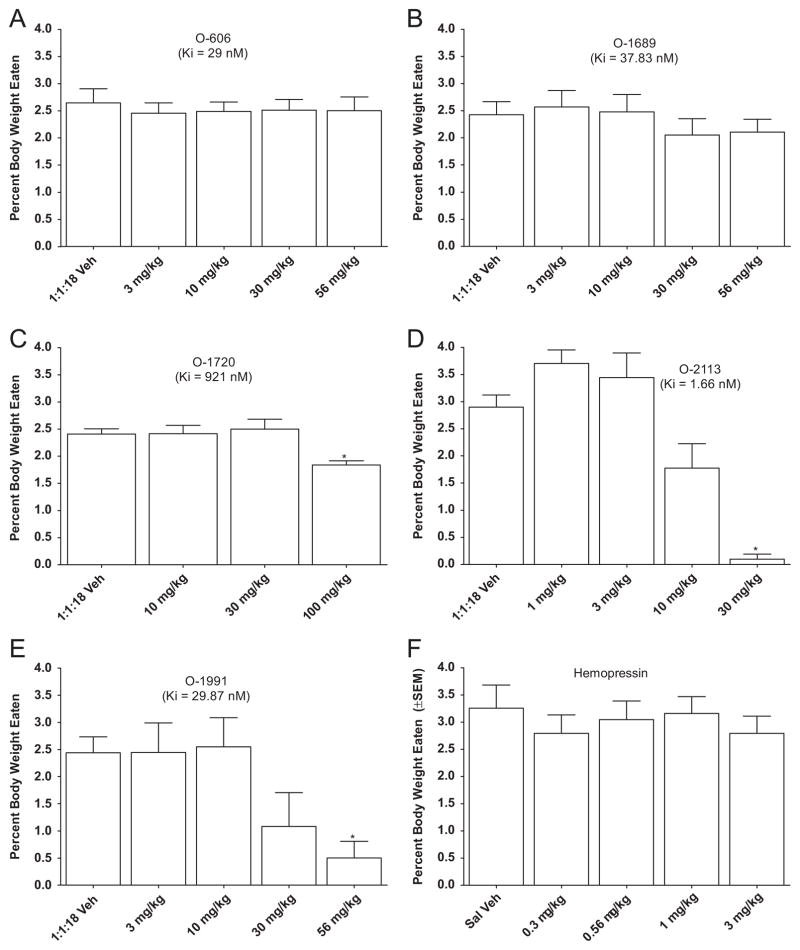
Fig. 2 shows the effects of sulfonamide analogs of Δ8-tetra-hydrocannabinol on food intake in mice. In addition, the effects of the neuropeptide hemopressin are presented. The sulfonamide analogs, O-606 and O-1689, failed to affect food intake, despite their reasonable cannabinoid CB1 receptor affinity of 29 nM and 38 nM, respectively [O-606: panel A, F(4,36)=0.23, P>0.05; O-1689: panel B, F(4,40)=1.18, P>0.05]. In contrast, O-1720 has poor cannabinoid CB1 receptor affinity (921 nM), yet it significantly decreased feeding by 24% at a dose of 100 mg/kg [panel C, F(3,30)=5.25, P<0.05], as did 30 mg/kg O-2113 and 56 mg/kg O-1991, which resulted in 97% and 79% decrease, respectively [O-2113: panel D, F(4,36)=21.34, P<0.05; O-1991: panel E, F(4,32)=5.67, P<0.05]. The neuropeptide hemopressin did not affect food intake at doses up to 3 mg/kg [panel F, F(4,36)=0.39, P>0.05].
Fig. 2.
Effects of sulfonamide cannabinoids and hemopressin on food intake expressed as % of body weight. For each compound, bars represent the mean (± S.E.M.) of data from the same 10 mice at each dose. * Indicates significant difference from vehicle control (p<0.05).
As with the rimonabant analogs, the cannabinoid CB1 receptor binding affinities of the sulfonamide cannabinoids (Table 2), did not reflect their efficacy in suppressing food intake. For example, although four sulfonamide cannabinoids showed reasonable cannabinoid CB1 receptor binding affinities, only two compounds (O-1991 and O-2113) affected food intake. These two compounds were also the only two to exhibit agonist-like in vivo effects in mice, including suppression of locomotor activity, antinociception and hypothermia. Interestingly, O-2050, a sulfonamide analog previously shown to decrease food intake in this procedure (Wiley et al., 2011), did not affect responses in the in vivo test battery in mice.
Table 2.
Effects of hemopressin and of sulfonamide side chain analogs of Δ8-tetrahydrocannabinol on cannabinoid receptor binding and in a battery of pharmacological tests in mice.
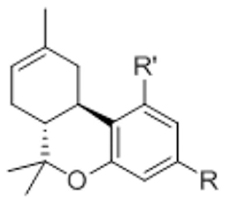
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R | R’ | CB1 | CB2 | SA | %MPE | RT |
| O-2050a |
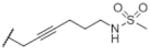
|
OH | 2.5 (0.4) | 0.2 (0.1) | 69% (30) | inactive (30) | inactive (30) |
| O-606 |
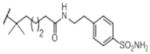
|
OH | 29 (6) | nd | 60% (100) | inactive (100) | inactive (100) |
| O-1689 |
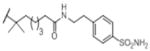
|
OH | 38 (0.47) | nd | 46% (30) | inactive (30) | 32.6 (30) |
| O-1720 |
|
H | 921 (118) | 787 (21) | nd | nd | nd |
| O-2113a |
|
OH | 1.7 (0.3) | 0.08 (0.02) | 0.4 (0.1–1.8) | 0.3 (0.1–1) | 1.4 (0.3–4.6) |
| O-1991a |
|
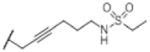
OH | 30 (13) | 1.4 (0.2) | 2 (1–3) | 0.9 (0.6–1.4) | 0.8 (0.4–1.6) |
| Hemopressin | – | – | 410,000 | 410,000 | nd | nd | nd |
See Table 1 for key and list of abbreviations.
Previously published data are indicated by italicized text, with citation provided below.
All binding and tetrad data of indicated compounds from Wiley et al. (2011).
4. Discussion
The primary purpose of this study was to examine the effects of analogs of rimonabant, the prototypic cannabinoid CB1 receptor antagonist, and O-2050, a sulfonamide analog of Δ8-tetrahy-drocannabinol, on feeding behavior in mice. As expected, rimonabant decreased food intake in moderately deprived mice in the present study and in a previous study using the same procedure (Wiley et al., 2005). These results are consistent with previous studies in which rimonabant decreased food intake over a range of experimental conditions, including in deprived and non-deprived rats (De Vry et al., 2004; McLaughlin et al., 2003; Rowland et al., 2001; Verty et al., 2004), in wildtype mice (Di Marzo et al., 2001; Poncelet et al., 2003), in lean and obese (fa/fa) Zucker rats (Vickers et al., 2003), in rats fed diets high in fat or carbohydrates (Verty et al., 2004), and in a diet-induced obesity model in mice (Ravinet Trillou et al., 2004).
In the present study, substitutions at the 3, 4 and 5 positions on the pyrazole core of the rimonabant template affected cannabinoid CB1 receptor affinity, nature of and potency of activity in a tetrad of in vivo tests in mice and food intake, as did conversions of the pyrazole to phenyl (O-1788) or indazole O-1654. O-3258, a pyrazole analog with substitution of a 4-(5-bromopentyl)phenyl group for the 4-phenylchloro at the 5-position of the pyrazole core, had excellent affinity for the cannabinoid CB1 receptor and showed a profile that was most like that of rimonabant, including reduction of food intake, stimulation of motor activity, and antagonism of the effects of an active dose of Δ9-tetrahydrocannabinol in the tetrad tests in mice (Bass et al., 2002; Compton et al., 1996; Wiley et al., 2005). While these effects were also shared by O-3257 [5-(4-(5-azidopentyl)phenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide and O-3259 1-(2,4-dichlor-ophenyl)-4-methyl-5-(4-(5-nitropentyl)phenyl)–N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide], as shown in a previous study with the same protocol for measuring food intake (Wiley et al., 2005), O-3258 was less potent at decreasing food intake than would have been predicted by its cannabinoid CB1 receptor affinity. Whereas doses as low as 1 mg/kg of O-3257 or O-3259 suppressed eating previously, a dose of 30 mg/kg O-3258 was required here. Each of the former compounds is structurally similar to O-3258, with the exception that the terminal moeity on the pentyl group contains nitrogen (N3 and NO2 for O-3257 and O-3259, respectively), and all three have similar cannabinoid CB1 receptor binding affinities (Ki =1.5 nM for O-3258 versus 2.2 nM and 1.2 nM for O-3257 and O-3259, respectively) (Wiley et al., 2005), suggesting that ability to bind to the cannabinoid CB1 receptor may not be the sole factor in determining the propensity of this class of compounds to decrease feeding. In further support of this hypothesis, elimination of the bromo and branching of the pentyl group (as in O-1690) resulted in loss of the effect on feeding without change in cannabinoid CB1 receptor affinity or loss of antagonism of Δ9-tetrahydrocannabinol’s in vivo effects. In contrast, conversion of the pyrazole core to an indazole (O-1654) or phenyl (O-1788) resulted in substantial loss of cannabinoid CB1 receptor affinity, as well as loss of activity in the tetrad tests in mice, albeit O-1788 did decrease feeding at a high (100 mg/kg) dose.
Three of the 3-substituted pyrazoles (O-2154, O-2155, and O-4373) tested here also decreased feeding at higher doses. Although differences in routes of administration (i.p. for feeding and i.v. for tetrad) prevent absolute comparison of dose effects across procedure, it is nonetheless likely that these decreases in food intake were related to suppression of locomotor activity rather than to a specific anorexic effect, as all three analogs produced agonist-like effects in the tetrad tests (including hypomobility) at doses that were 3- to 18-fold lower. Previous work with these and similar 3-substituted pyrazoles demonstated conclusively that the cannabinoid agonist-like effects of this class of analogs were not cannabinoid CB1 receptor-mediated, despite the excellent cannabinoid CB1 receptor binding affinities possessed by some (but not all) of the analogs (Wiley et al., 2012). In contrast, in a series of pyrazole analogs with substitutions at the 3-position, Compound 31 [5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(1-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)-cyclopropyl)-1H-pyrazole-3-carboxamide] showed good cannabinoid CB1 receptor affinity, antagonized the hypothermic effects of WIN55,212-2, and decreased feeding in mice following acute adminstration as well as in a mouse model of diet-induced obesity (Sasmal et al., 2011). These results suggest that substitutions at the 3-position produce compounds with diverse in vivo profiles that range from traditional cannabinoid antagonist effects to pseudo-agonist effects. Some (but not all) of these compounds share rimonabant’s appetite suppressant effects.
Each of the 3-substituted pyrazoles described above undoubtedly reaches the brain, as indicated by full efficacy in each of the tetrad tests following systemic administration. However, previous research has suggested that central action may not be necessary for anorexic action of cannabinoid CB1 receptor antagonists. For example, Gomez et al. (2002) showed that systemic, but not central, administration of rimonabant reduced food intake rats and that destruction of the sensory neurons enervating the gut resulted in loss of rimonabant’s effectiveness in reducing food intake. In addition, peripherally restricted cannabinoids based upon a pyrrole template, have shown promise in reducing food intake in nondeprived mice (LoVerme et al., 2009). In contrast, compound 13226-70, designed as a peripherally restricted 3-substituted rimonabant analog (Fulp et al., 2011), has reasonable cannabinoid CB1 receptor affinity (Ki =54.8 nM), but failed to alter food intake, as did heterodimer 6b, a bivalent ligand composed of two identical cannabinoid CB1 receptor antagonist pharmacophores that is unlikely to cross the blood-brain barrier due to its large topological polar surface area. Further, heterodimer 6b was also not active when tested at doses up to 30 mg/kg (i.p.) in a test battery in mice nor did it antagonize the cannabimimetic effects of Δ9-tetrahydrocannabinol in these tests (present study). These results are consistent with its failure to antagonize the antinociceptive effects of Δ9-tetrahydrocannabinol previously, albeit it partially attenuated those of CP 55,940 (Zhang et al., 2010). Together, these results suggest that these two peripherally restricted analogs would not be effective appetite control agents when administered acutely, but are too limited in nature to be useful in determining whether peripheral restriction of pyrazole analogs is a viable option for this therapeutic indication.
The two sulfonamide substituted compounds, O-606 and O-1689, failed to affect food intake, despite their reasonable cannabinoid CB1 receptor affinity of 29 nM and 38 nM, respectively. In contrast, O-1720 has poor cannabinoid CB1 receptor affinity (921 nM), yet it significantly decreased feeding at a dose of 100 mg/kg, as did 30 mg/kg O-2113 and 56 mg/kg O-1991. The latter two compounds have good affinity at the cannabinoid CB1 receptor (1.7 and 30 nM, respectively) and each produced hypomobility, antinociception and hypothermia at ED50s that were several-fold lower than doses that decreased feeding, suggesting that the feeding effects were nonspecific and may have resulted from overall suppression of activity. In addition, the fact that the sulfonamide cannabinoids did not increase food intake at any dose distinguished this class of compounds from Δ9-tetrahydrocannabinol and other cannabinoid agonists that increased appetite (Wiley et al., 2005; Williams and Kirkham, 2002). Interestingly, O-2050, the only sulfonamide analog that suppressed food intake at doses that did not affect locomotion, did not have agonist or antagonist effects in the mouse pharmacological test battery even when injected centrally (Wiley et al., 2011). While these results suggest that O-2050 may represent a promising lead for development of an appetite suppressant, regulatory issues related to the tetrahydrocannabinol structural template of the compound may be prohibitive in terms of development of this motif.
Finally, in an attempt to replicate previous work showing that low doses of the neuropeptide hemopressin decreased feeding following systemic administration and that this anorexic effect was related to its action as an antagonist of cannabinoid CB1 receptors in the brain (Dodd et al., 2010; Heimann et al., 2007), the effects of hemopressin were evaluated in the present feeding model. In contrast with previous findings, hemopressin did not affect feeding. Further, hemopressin failed to displace [3H]CP55,940 from either cannabinoid CB1 or CB2 receptors (present study and H.A. Navarro, personal communication). Hence, there remains controversy concerning hemopressin’s effects on the endocannabinoid system.
In summary, while the prototypic cannabinoid CB1 receptor antagonist rimonabant and the sulfonamide tetrahydrocannabinol analog O-2050 reliably decrease feeding in moderately food-restricted mice, a series of analogs of these lead compounds were not as effective. Further, this limited efficacy was not reliably associated with poor cannabinoid CB1 receptor binding affinity, as some of the ineffective compounds showed good binding as well as evidence of CNS activity in a test battery in mice. The pharmacological profile of the pyrazoles was especially variable across manipulations of substituents surrounding the pyrazole core, with some compounds producing agonist-like effects and others producing antagonist-like effects. Together, these results emphasize the complex nature of the pharmacological profile of analogs of cannabinoid antagonists and suggest that thorough evaluation of their effects on non-cannabinoid CB1 receptor systems will be necessary if this class of compounds is to be developed for the treatment of obesity.
Acknowledgments
Research supported by NIDA grants DA-03672, DA-09789, DA-005488 and DA-026582 and by NIAAA Grants AA-019740 and AA-017514. The authors would like to thank Ms. Nikita Pulley (RTI) and Ms. Ramona Winckler (VCU) for excellent technical assistance.
References
- Alonso M, Serrano A, Vida M, Crespillo A, Hernandez-Folgado L, Jagerovic N, Goya P, Reyes-Cabello C, Perez-Valero V, Decara J, Macias-Gonzalez M, Bermudez-Silva FJ, Suarez J, de Fonseca FR, Pavon FJ. Antiobesity efficacy of LH-21, a cannabinoid CB(1) receptor antagonist with poor brain penetration, in diet-induced obese rats. B J Pharmacol. 2012;165:2274–2291. doi: 10.1111/j.1476-5381.2011.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Griffin G, Grier M, Mahadevan A, Razdan RK, Martin BR. SR-141716A-induced stimulation of locomotor activity. A structure–activity relationship study. Pharmacol Biochem Behav. 2002;74:31–40. doi: 10.1016/s0091-3057(02)00945-0. [DOI] [PubMed] [Google Scholar]
- Brown WV, Fujioka K, Wilson PW, Woodworth KA. Obesity: why be concerned? Am J Med. 2009;122:S4–S11. doi: 10.1016/j.amjmed.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Christopoulou FD, Kiortsis DN. An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J Clin Pharm Ther. 2011;36:10–18. doi: 10.1111/j.1365-2710.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obes Relat Metab Disord. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Mancini G, Lutz B, Luckman SM. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J Neurosci. 2010;30:7369–7376. doi: 10.1523/JNEUROSCI.5455-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulp A, Bortoff K, Zhang Y, Seltzman H, Snyder R, Maitra R. Towards rational design of cannabinoid receptor 1 (CB1) antagonists for peripheral selectivity. Bioorg Med Chem Lett. 2011;21:5711–5714. doi: 10.1016/j.bmcl.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodriguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Delta(9)-tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, Luchessi AD, Castro LM, Giorgi R, Rioli V, Ferro ES, Devi LA. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci USA. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JE. Delta(9)-THC stimulates food intake in Lewis rats: effects on chow, high-fat and sweet high-fat diets. Pharmacol Biochem Behav. 2001;68:539–543. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- Lockie SH, Czyzyk TA, Chaudhary N, Perez-Tilve D, Woods SC, Oldfield BJ, Statnick MA, Tschop MH. CNS opioid signaling separates cannabinoid receptor 1-mediated effects on body weight and mood-related behavior in mice. Endocrinology. 2011;152:3661–3667. doi: 10.1210/en.2011-1220. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Duranti A, Tontini A, Spadoni G, Mor M, Rivara S, Stella N, Xu C, Tarzia G, Piomelli D. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg Med Chem Lett. 2009;19:639–643. doi: 10.1016/j.bmcl.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, Betz AJ, Ishiwari K, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- Poncelet M, Maruani J, Calassi R, Soubrie P. Overeating, alcohol and sucrose consumption decrease in CB1 receptor deleted mice. Neurosci Lett. 2003;343:216–218. doi: 10.1016/s0304-3940(03)00397-5. [DOI] [PubMed] [Google Scholar]
- Powell AG, Apovian CM, Aronne LJ. New drug targets for the treatment of obesity. Clin Pharmacol Ther. 2011;90:40–51. doi: 10.1038/clpt.2011.82. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology. 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- Sasmal PK, Talwar R, Swetha J, Balasubrahmanyam D, Venkatesham B, Rawoof KA, Neelima Devi B, Jadhav VP, Khan SK, Mohan P, Srinivasa Reddy D, Nyavanandi VK, Nanduri S, Shiva Kumar K, Kannan M, Srinivas P, Nadipalli P, Chaudhury H, Sebastian VJ. Structure–activity relationship studies of novel pyrazole and imidazole carboxamides as cannabinoid-1 (CB1) antagonists. Bioorg Med Chem Lett. 2011;21:4913–4918. doi: 10.1016/j.bmcl.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Verty AN, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett. 2004;354:217–220. doi: 10.1016/j.neulet.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology. 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Breivogel CS, Mahadevan A, Pertwee RG, Cascio MG, Bolognini D, Huffman JW, Walentiny DM, Vann RE, Razdan RK, Martin BR. Structural and pharmacological analysis of O-2050, a putative neutral cannabinoid CB(1) receptor antagonist. Eur J Pharmacol. 2011;651:96–105. doi: 10.1016/j.ejphar.2010.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, Martin BR. CB1 cannabinoid receptor-mediated modulation of food intake in mice. B J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Jefferson RG, Grier MC, Mahadevan A, Razdan RK, Martin BR. Novel pyrazole cannabinoids: insights into CB(1) receptor recognition and activation. J Pharmacol Exp Ther. 2001;296:1013–1022. [PubMed] [Google Scholar]
- Wiley JL, Selley DE, Wang P, Kottani R, Gadthula S, Mahadeven A. 3-Substituted pyrazole analogs of the cannabinoid type 1 (CB1) receptor antagonist rimonabant: cannabinoid agonist-like effects in mice via non-CB1, non-CB2 mechanism. J Pharmacol Exp Ther. 2012;340:433–444. doi: 10.1124/jpet.111.187815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Observational analysis of feeding induced by Delta9-THC and anandamide. Physiol Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gilliam A, Maitra R, Damaj MI, Tajuba JM, Seltzman HH, Thomas BF. Synthesis and biological evaluation of bivalent ligands for the cannabinoid 1 receptor. J Med Chem. 2010;53:7048–7060. doi: 10.1021/jm1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]