Abstract
Objective/Hypothesis
The Matrix Metalloproteinase (MMP), Fibroblast Growth Factor (FGF) and Bone Morphogenetic Protein (BMP) families regulate tissue remodeling in many normal and pathophysiologic processes. We hypothesize that induction of chronic sinonasal inflammation will be associated with changes in regulation of these tissue remodeling cytokines.
Methods
Balb/c mice aged 8–12 weeks were sensitized and treated with intranasal Aspergillus fumigatis (AF) three times per week for 1 week, 3 weeks, 2 months and 3 months (n=8 each time point). Sinonasal tissues were evaluated for changes in MMP, FGF and BMP regulation using standard RT-PCR techniques. Additional snouts were processed for histology and immunohistochemistry. Untreated mouse snouts of identical age were used as controls.
Results
Significant upregulation of MMP8 was observed at 2 months, and MMP1a, MMP7, MMP8 and MMP12 were all significantly upregulated at 3 months. FGF3 was significantly upregulated at 3 weeks and 3 months, and FGF5, FGF6 and FGF8 were all significantly upregulated at 3 months. BMP8b and BMP9 were significantly upregulated at 3 months. Histologic analysis revealed mucosal, stromal and mucin gland hypertrophy, increased mucin production, and metaplasia with loss of cilia. Antibody staining was strongly positive in the AF treated group.
Conclusion
Induction of CRS is associated with time-dependent changes in tissue remodeling cytokine expression occurring in conjuction with inflammatory tissue changes. Antibody staining for upregulated cytokines suggests local production within the sinonasal mucosa. Further study is required to better understand the association between BMP, FGF and MMP regulation and tissue remodeling changes resulting from chronic inflammation.
Keywords: Tissue remodeling, chronic rhinosinusitis, murine model, MMP, BMP, FGF
INTRODUCTION
Current opinion defines chronic rhinosinusitis (CRS) as an array of multifactorial diseases sharing a common factor of chronic sinonasal mucosal inflammation. Research has implicated dysfunction of cytokine expression as a key factor in the underlying molecular pathogenesis of CRS and allergic inflammation.1 Sinonasal osteogenesis, mucosal hypertrophy, basement membrane thickening, fibrosis, subepithelial collagen deposition, angiogenesis and scarring are common forms of tissue remodeling in patients with longstanding CRS.2–4 In addition, significant proliferation of the extracellular matrix is seen in CRS with nasal polyposis.5 These progressive tissue remodeling changes affect normal sinonasal physiology and increase disease symptomatology, as well as surgical difficulty. Few authors have investigated the role of tissue remodeling in CRS, and the underlying molecular mechanisms that ultimately lead to these tissue changes in CRS remain uncharacterized.2,3,6,7 Tissue remodeling processes throughout the body are largely governed by the Matrix Metalloproteinase (MMP) family of enzymes, and the Fibroblast Growth Factor (FGF) and Bone Morphogenetic Factor (BMP) families of cytokines.
MMPs are proteolytic enzymes that regulate remodeling of the extracellular matrix, and regulate other cytokines involved in remodeling of the extracellular matrix.8,9 Studies have demonstrated MMP dysregulation in many pathologic processes, including cancer, atherosclerosis, neurodegenerative diseases and cholesteatoma, as well as in inflammatory diseases such as otitis media, CRS and asthma. Specifically, changes in expression of MMP2 and MMP9 have been associated with human CRS.10–16
Members of the BMP and FGF families of cytokines are responsible for regulation of tissue growth and remodeling in many normal physiologic processes, such as embryological development, bone growth, wound healing, angiogenesis and regulation of inflammation. BMP dysregulation is seen in gastric and colon malignancies, and FGF signaling is known to play a key role in many types of cancer.17,18 Unlike the MMP family, however, little is known regarding the role of BMP and FGF family members in CRS.19
Tissue remodeling is routinely observed in animal models of CRS. The murine model of allergic fungal CRS, initially described by Lindsay, et al., exhibits many of the same histopathologic changes seen in human CRS, including thickening of the lamina propria, mucosal inflammatory cell infiltrate and increase in non-ciliated epithelial cells with increased apocrine secretion.20,21 Bony remodeling of the sinonasal cavity, including osteogenesis, osteitis and periosteal fibrosis has been described in studies using a rabbit model of bacterial CRS.8
The underlying molecular mechanisms governing CRS-associated tissue remodeling, however, remain uncharacterized in animal models as well as in human subjects with CRS. A better understanding of the cellular and molecular processes leading to osteogenesis, fibrosis and angiogenesis in CRS is needed in order to develop novel therapies aimed at halting or reversing CRS-associated tissue remodeling. We have previously demonstrated acute changes in several members of the BMP, FGF and MMP families in a mouse model of acute allergic rhinitis.22 The purpose of this study is to characterize the changes in BMP, FGF and MMP family expression in a mouse model of CRS. We hypothesize that induction of CRS following fungal antigen exposure will result in changes in the level of regulation of several members of the BMP, FGF and MMP families.
METHODS
CRS Mouse Model
The murine model of CRS as described by Lindsay, et al., and later modified by Khalid, et al. was employed.20,21 BALB/C mice age 8–12 weeks were presented with an intraperitoneal challenge of 200 µg of Aspergillus fumigatus (AF) extract (Greer Laboratories, Inc., Lenoir, NC) dissolved in 2 mg of alum and 0.5 ml of phosphate buffered saline. Beginning 7 days following intraperitoneal sensitization, bilateral intranasal challenge with 5 µg of AF extract was administered under anesthesia using an intraperitoneal injection of ketamine (100 mg/ml; 0.067 mg/gram body weight) and xylazine (20 mg/ml; 0.013 mg/gram body weight). Mice were treated with intranasal AF extract 3 times per week for a total of 3 months, at which time they were sacrificed (n=8). Untreated mouse snouts from mice aged 20–24 weeks (n=8) were used as controls. At the time of sacrifice, the sinonasal mucosa, nasal septum, and the underlying vomeronasal and ethmoturbinate bone were dissected from the snouts and placed immediately in RNAlater solution (QIAGEN, Inc., Valencia, CA). Dissections were performed under magnification in order to ensure completeness of the dissection and consistency across all dissections. All harvested sinonasal tissue was included in the RT-PCR analysis.
Quantitative RT-PCR
RNA for RT-PCR was extracted from dissected sinonasal tissue using the RNeasy Mini Kit according to the manufacturer’s instructions (QIAGEN, Inc.). 200 µg of RNA was used to synthesize the cDNA probe using the C-03 RT2 first strand kit (SABiosciences, Frederick, MD). Standardized custom PCR kits optimized for each cytokine, including the primer and probe, were utilized (SABiosciences). Custom arrays were obtained for the following cytokines: BMP1, BMP2, BMP3, BMP4, BMP5, BMP6, BMP7, BMP8a, BMP8b, BMP9, BMP10, FGF1, FGF2, FGF3, FGF4, FGF5, FGF6, FGF7, FGF8, FGF10, MMP1a, MMP2, MMP3, MMP7, MMP8, MMP9, MMP12 and MMP14. The method of semi-quantitative real-time RT-PCR was utilized, and reverse transcription was performed using an ABI Step One Plus system (Applied Biosystems, Inc., Foster City, CA). Using this system, the parameter Ct (threshold cycle) is defined as the fractional cycle number at which the reporter fluorescence generated by cleavage of the probe passes a fixed threshold above baseline. A plot of the log of initial target copy number for a set of standards versus Ct is a straight line. Quantitation of the amount of target in samples is done by measuring Ct and using the standard curve to determine starting copy number. Calculation of Cts, preparation of a standard curve, determination of the starting copy number and calculation of statistical significance was performed using the ΔΔCt method with the aid of the SABiosciences PCR Array Data Analysis Web Portal.22 18S RNA was used as the comparator. In order to determine statistical significance, a two-tailed t-test assuming equal variance was performed using the ΔΔCt values as input.
Histologic Analysis
Experimental mice were treated according to the above protocol using AF extract. Mice were sacrificed after 3 months of nasal challenge. The snouts were harvested, fixed in 3% paraformaldehyde, decalcified, embedded in paraffin, sectioned at 5µm, and stained using standard techniques. Tissues were observed using a Leica DM 2500 microscope and photographs were obtained using Leica application suite v3 software. Serial sections from two treated mice snouts were analyzed for histopathologic changes, and two untreated mouse snouts were used as controls for comparison.
The histopathologic grading scale as described by Khalid, et al. was utilized.21 Every fifth section containing the vomeronasal organ and nasal-associated lymphoid tissue was examined and graded. Inflammation was graded from 0 (no change) to 4 (severe change), and secretory hyperplasia was also graded from 0 (no change) to 4 (severe change). Control sections were used to define the grade of 0. The histologic grades of the treated snouts were then averaged and compared to the untreated controls.
All animal procedures were approved by the OHSU Institutional Animal Care and Use Committee.
Immunohistochemistry
BALB/c mice age 8–12 weeks were sensitized to AF and treated 3 times per week with intranasal challenge of AF for 3 months as described above (n = 2). Snouts were harvested, decalcified, processed and embedded in paraffin using standard techniques. Untreated snouts were used as a control comparison (n = 2). Antibody staining was performed using standard techniques as outlined below.
Antibody staining was performed for MMP1, MMP7, MMP8, FGF8 and BMP9. Serial 5 µm sections were mounted and heated at 50 degrees C for 30 minutes. The slides were deparaffinized using serial ethanol and Citrisolv washes. The antibody was diluted in DaVinci Green Diluent, and DaVinci Green Diluent alone is used as the control. Each section was incubated with antibody at 1:50 and 1:100 concentrations for 1 hour at room temperature. The slides were washed with TBS twice and incubated with 0.15% glycine in TBS for 10 minutes. Blocking was performed using 10% goat serum diluted in TBS for 10 minutes. The secondary antibody (1:50 goat anti-rabbit IgG Alexa Fluor 488) was incubated in the dark for 1 hour. The slides were washed 3 times with 1% goat serum diluted in TBS followed by 3% paraformaldehyde in 0.1 M phosphate buffer. The slides were washed serially with TBS and distilled water, then cover slipped using Fluoromount-G and allowed to dry overnight. Slides were viewed using a Leica DM 2500 microscope and photographs were taken using Leica application suite v3 software. Photographs of representative experimental and control snout sections were obtained using identical settings. Side by side comparison of photographs of the experimental and control snout sections was used to determine the degree and localization of fluorescent staining.
RESULTS
RT-PCR
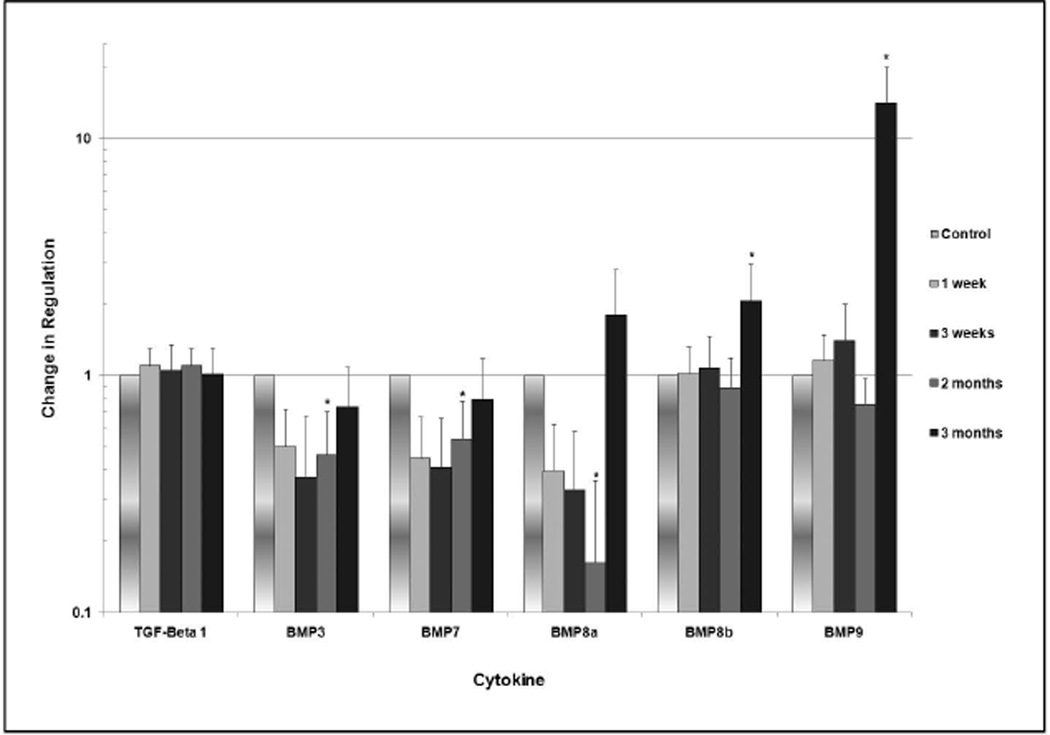
Several members of the BMP family exhibited statistically significant changes in regulation (Figure 1). At two months, significant downregulation of BMP3, BMP7 and BMP8a ranging from 15 to 50% of normal expression was observed (p<0.05). At three months, BMP8b was upregulated by a factor of 2.1, and BMP9 was upregulated by a factor of 14.1 (p<0.05). No significant changes were noted in regulation of BMP1, BMP2, BMP4, BMP5, BMP6, BMP7 and BMP10.
Fig. 1.
Change in degree of regulation of members of the BMP family over 3 months. The vertical axis is formatted in logarithmic scale. Statistically significant values are designated with an asterisk, and 95% confidence intervals are represented by the bars.
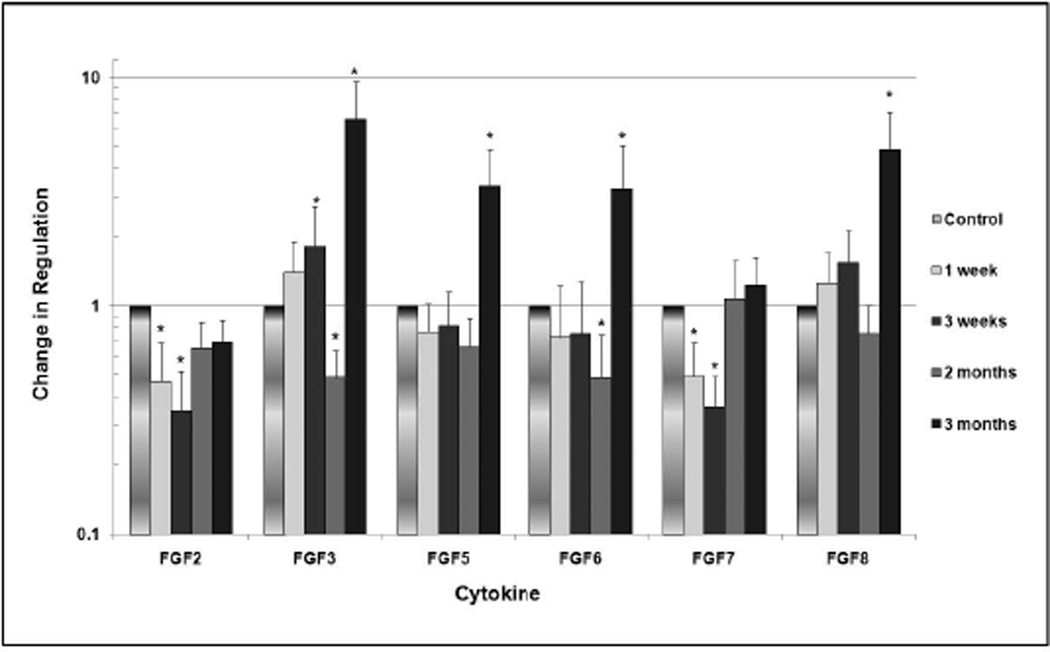
In the FGF family (Figure 2), FGF2 and FGF7 were significantly downregulated to 46–49% of normal expression at 1 week (p<0.05), and downregulation to 35% of normal expression was observed for both FGF2 and FGF7 at three weeks (p<0.05). FGF3 and FGF6 were significantly downregulated to 50% of normal expression at 2 months (p<0.05). Conversely, FGF3 was significantly upregulated by a factor of 1.8 at 3 weeks, and FGF3, FGF5, FGF6 and FGF8 were significantly upregulated by factors ranging from 3.2 to 6.5 at 3 months (p<0.05). No significant changes in regulation were noted for FGF1 and FGF10.
Fig. 2.
Change in degree of regulation of members of the FGF family over 3 months. The vertical axis is formatted in logarithmic scale. Statistically significant values are designated with an asterisk, and 95% confidence intervals are represented by the bars.
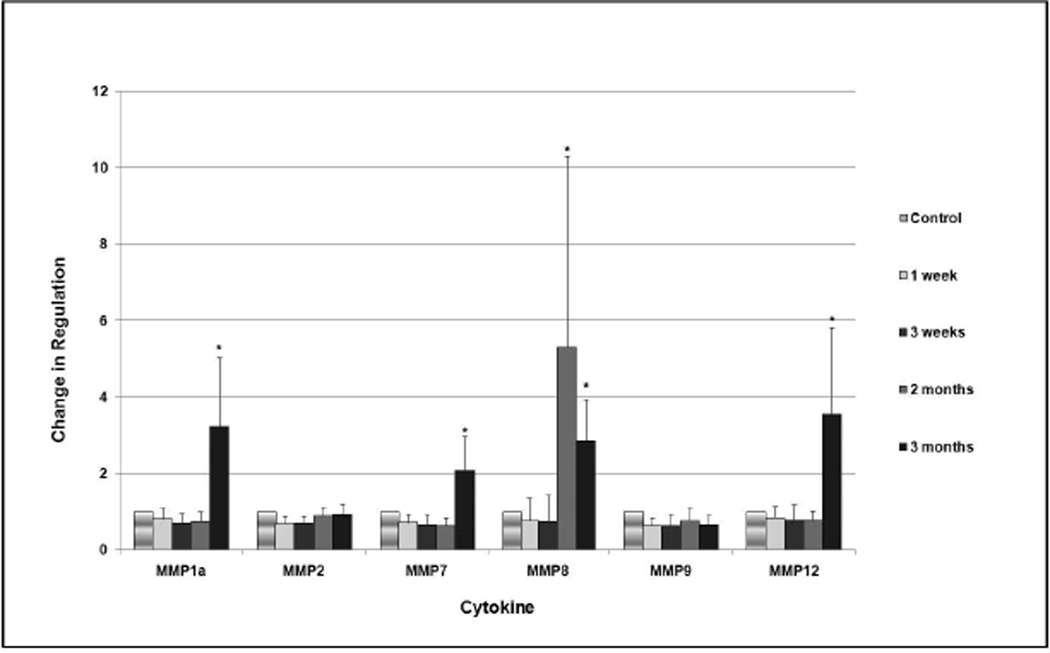
Several members of the MMP family exhibited significant upregulation (Figure 3). At 2 months, MMP8 was upregulated by a factor of 5.3 (p<0.05). MMP1a, MMP7, MMP8 and MMP12 were significantly upregulated at 3 months by factors ranging from 2.1 to 3.5 (p<0.05). No significant changes were noted in expression of MMP2, MMP3, MMP9 and MMP14.
Fig. 3.
Change in degree of regulation of members of the MMP family over 3 months. The vertical axis is formatted in logarithmic scale. Statistically significant values are designated with an asterisk, and 95% confidence intervals are represented by the bars.
Histology
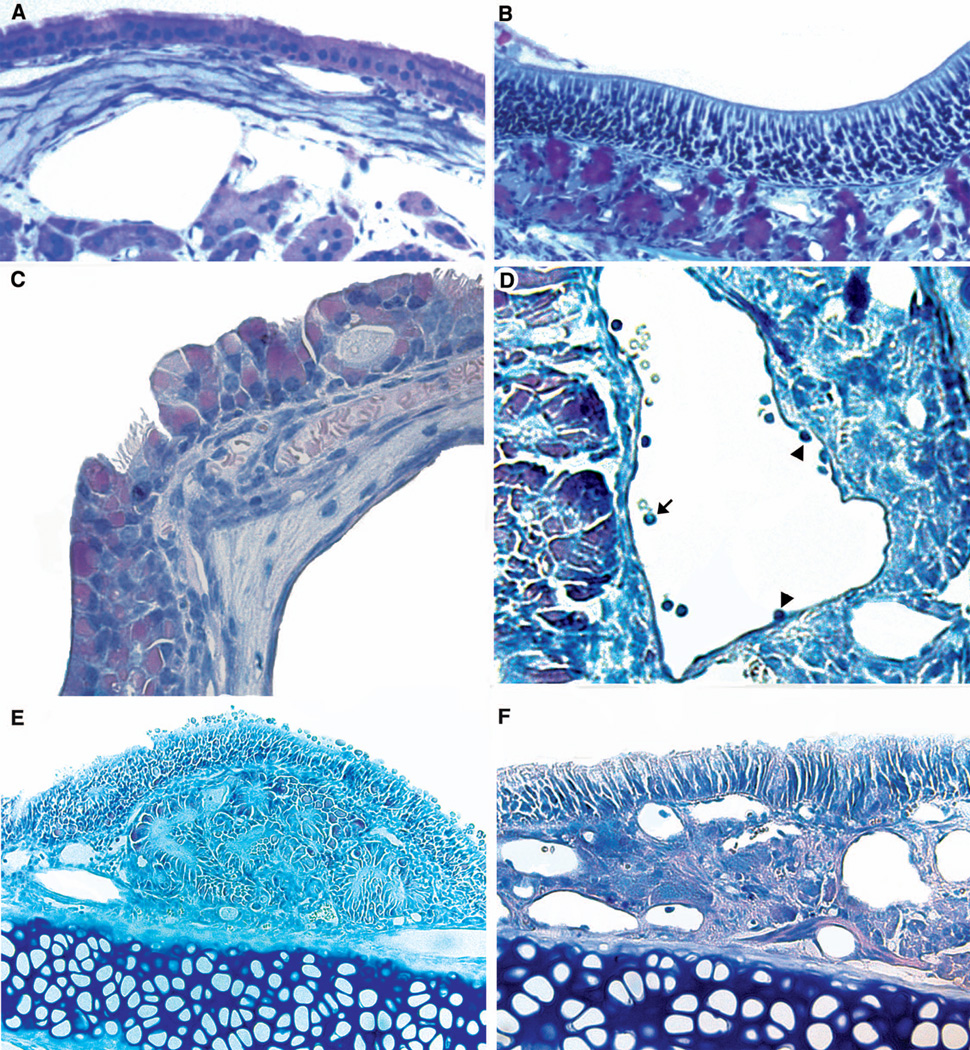
Figure 4 demonstrates the salient histopathologic changes observed in the sinonasal tissue of AF treated mice (Figures 4b, c, d and e) compared to untreated controls (Figure 4a). AF treated mice exhibited mucosal hypertrophy as well as hypertrophy of the underlying stroma and mucous glands. Inflammatory cell infiltration (predominantly eosinophils and lymphocytes) as well as mucosal metaplasia with loss of cilia were also routinely observed.
Fig. 4.
Histologic changes seen in the CRS mouse model as compared to untreated controls (Fig 4a). Rapid mucin staining reveals mucin gland hypertrophy (Fig 4b, arrows indicate mucin glands). Evidence of mucosal metaplasia with loss of cilia (Fig 4c), inflammatory cell infiltrate with prominent intravascular eosinophils (arrow) and inflammatory cells adherent to the vascular endothelium (arrowheads) in the process of diapedesis (Fig 4d), and mucosal and stromal hypertrophy (Figs 4e, 4f) were all observed.
According to the Khalid grading scale, the average inflammatory change was 2.4 and the average change in secretory hyperplasia was 3.3. No obvious bony changes were noted in the AF treated mice as compared to controls.
Immunohistochemistry
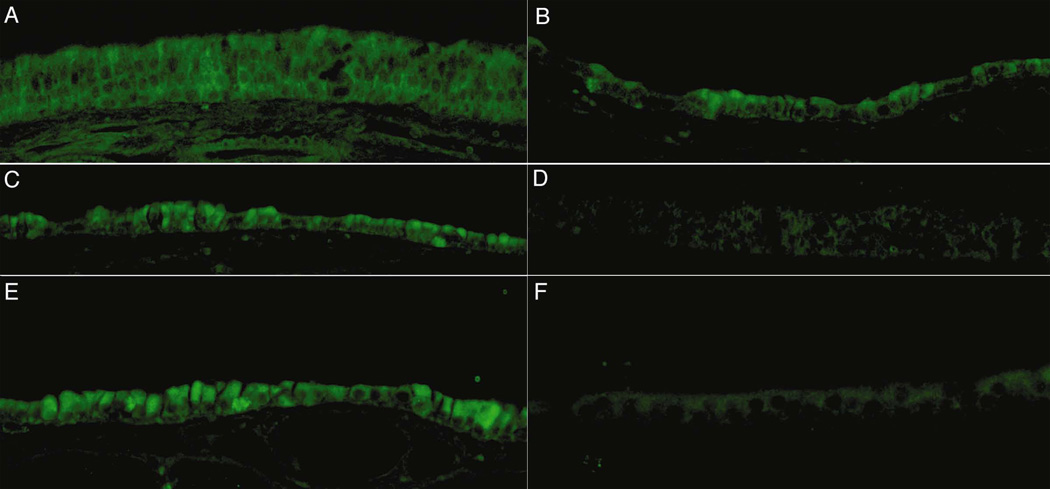
Figure 5 demonstrates patterns of antigen staining in experimental (left column) and control (right column) sinonasal tissue. Strong fluorescent staining localizing primarily to the sinonasal mucosa and the underlying stroma was noted in the AF treated tissue as compared to the untreated controls.
Fig. 5.
Immunohistochemical staining for BMP9 (5a, 5b), FGF8 (5c, 5d), and MMP8 (5e, 5f). Aspergillus treated tissue is shown in the left column, and untreated control tissue is shown on the right. Strong staining was noted with localization to the epithelium.
DISCUSSION
Tissue remodeling is a prominent feature of long-standing CRS. These tissue changes presumably occur as a result of chronic mucosal inflammation, however, outside of clinical experience, there is little evidence to support this. Sinonasal tissue remodeling likely contributes to increased symptomatology and greater challenges in treatment. Some authors have suggested that persistent localized inflammation in patients with sinonasal osteogenesis and osteitis may be resolved only through removal of the underlying diseased bone.6 Additionally, sinonasal angiogenesis and osteogenesis may potentiate surgical difficulty resulting from increased bleeding, poor visualization and greater risk to adjacent structures. Unfavorable wound healing following endoscopic sinus surgery may also be associated with tissue remodeling, as in the commonly encountered scenario involving osteitic stenosis in a previously instrumented frontal recess.
The MMP family is the most thoroughly studied family of tissue remodeling factors, and they have been studied in relation to CRS as well as inflammatory remodeling of the lower airway. Authors have shown that single nucleotide polymorphisms in the MMP9 gene may be a risk factor for development of CRS, and other studies have demonstrated MMP9 plays a significant role in airway remodeling in human asthma. In a mouse model of hypercapnia, MMP8 was demonstrated to be associated with lung matrix remodeling.16,23 Our findings demonstrated significant upregulation of MMP1a, MMP7 and MMP12 at 3 months, as well as significant upregulation of MMP8 at 2 and 3 months. We did not, however, observe any changes in MMP2 and MMP9, both of which have previously been implicated in human CRS. This may be due to differences in molecular physiology between the animal model and human disease.
In the current study, we observed a significant increase in BMP9 expression at 3 months. Very little is known regarding the role BMP members in airway remodeling. BMPs, however, are structurally related to TGF-β, which is known to play a role in CRS and sinonasal fibrosis. Prior studies have demonstrated increased expression of TGF-β in CRS patients with increased fibrosis and no polyposis versus patients with CRS with nasal polyposis.24,25 Additionally, BMPs are known to play a role in embryologic lower airway development. Rosendahl et al. demonstrated upregulation of BMP type 1 receptors with increased expression of BMP2, BMP4, and BMP6, as well as downregulation of BMP5 and BMP7 in inflamed bronchial epithelial cells.26 Due to the structural similarity to TGF-β, it is possible that members of the BMP family are working through activation of the TGF-β receptor; however, it is also possible they are working to induce tissue remodeling through activation of BMP receptors. Further study is needed in order to establish a definite connection between increased BMP9 expression and tissue remodeling.
As with the BMP family, there is little data regarding airway inflammation and remodeling in relation to FGF expression. We observed significant upregulation of FGF3, FGF5, FGF6 and FGF8 following three months of AF treatment. In a prior study of allergic mice infected with respiratory syncytial virus, it was discovered that thickening of the bronchial basement membrane as well as increased lung collagen synthesis occurred in association with increased FGF2 expression.27 To our knowledge, this is the only other study evaluating FGF expression in inflammatory airway remodeling. Further investigation is required before a definite association between FGF3, FGF5, FGF6 and FGF8 and inflammatory sinonasal tissue remodeling can be established.
There are several drawbacks associated with the murine model. Innate immune response to mold and other environmental factors differs between mice and humans. It may also be argued that, from a pathophysiologic standpoint, the AF treated mouse model differs greatly from the diverse collection of multifactorial diseases that fall under the rubric of human CRS. It should be noted, however, that we observed in the mouse model many of the same stigmata of chronic inflammation seen in human CRS, such as mucosal and stromal hypertrophy, metaplasia and ciliary loss. Also, in the absence of a more suitable mouse model of chronic sinonasal inflammation, the AF treated mouse model remains the best option for evaluation of the molecular pathophysiology of CRS given the wide availability of products for study as well as the availability of genetic knockouts.
It should be noted that, despite changes in regulation of many members of the BMP family, we did not observe any obvious bony changes on histologic analysis. This is consistent with prior reports of the AF treated mouse model. CRS is a chronic disease, and three months is relatively early in the disease process. It is likely that further changes in regulation of bone-remodeling cytokines occur over time, and it may be many months before bony changes become apparent. Unfortunately, due to the relatively short average life span of the Balb/c mouse, observation of these changes is not practical, and may not be possible. In our anecdotal experience, human CRS patients often suffer for years before bony changes become apparent on CT scan.
CONCLUSION
Increased expression of several BMP, FGF and MMP family members is seen at 2–3 months in the AF-treated CRS mouse model. Our findings suggest these factors are produced locally within the sinonasal mucosa. These changes in expression are seen in conjunction with tissue changes such as mucosal and stromal hypertrophy, metaplasia and loss of cilia. Further study is required in order to establish a definite association between CRS-related tissue remodeling and changes in expression of BMP, FGF and MMP family members.
Acknowledgments
Grant Support: NIH-NIDCD R01 DC009455 and DC009455-S1 (ARRA) (DRT)
Footnotes
Conflict of interest disclosure: None
WORKS CITED
- 1.Lane AP, Truong-Tran QA, Schleimer RP. Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinol. 2006;20:138–144. [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SH, Shin KS, Lee YS, et al. Impact of chronic rhinosinusitis and endoscopic sinus surgery on bone remodeling of the paranasal sinuses. Am J Rhinol. 2008;22:537–541. doi: 10.2500/ajr.2008.22.3222. [DOI] [PubMed] [Google Scholar]
- 3.Giacchi RJ, Lebowitz RA, Yee HT, Light JP, Jacobs JB. Histopathologic evaluation of the ethmoid bone in chronic sinusitis. Am J Rhinol. 2001;15:193–197. doi: 10.2500/105065801779954148. [DOI] [PubMed] [Google Scholar]
- 4.Sobol SE, Fukaskusa M, Christodoulopoulos P, et al. Inflammation and remodeling of the sinus mucosa in children and adults with chronic sinusitis. Laryngoscope. 2003;113:410–414. doi: 10.1097/00005537-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Rudack C, Prehm P, Stoll W, Maune S. Extracellular matrix components in nasal polyposis. Acta Otolaryngol. 2003;123(5):643–647. doi: 10.1080/0001648021000028133. [DOI] [PubMed] [Google Scholar]
- 6.Khalid AN, Hunt J, Perloff JR, et al. The role of bone in chronic rhinosinusitis. Laryngoscope. 2002;112:1951–1955. doi: 10.1097/00005537-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy DW, Senior BA, Gannon FH. Histology and histomorphometry of ethmoid bone in chronic rhinosinusitis. Laryngoscope. 1999;108(4):502–507. doi: 10.1097/00005537-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature Reviews. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 9.Mannicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Bio. 2008;19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;31(27):5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juhasz A, Sziklai I, Rakosy Z, et al. Elevated levels of tenascin and matrix metalloproteinase 9 correlates with the bone destruction capacity of cholesteatomas. Otol Neurotol. 2009;30:559–565. doi: 10.1097/MAO.0b013e31819fe6ed. [DOI] [PubMed] [Google Scholar]
- 12.Jennings CR, Guo L, Collins HM, Birchall JP. Matrix metalloproteinases 2 and 9 in otitis media with effusion. Clin Otolaryngol. 2001;26:419–494. doi: 10.1046/j.1365-2273.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 13.Can IH, Ceylan K, Caydere M, et al. The expression of MMP-2, MMP-7, MMP-9 and TIMP-1 in chronic rhinosinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2008;139:211–215. doi: 10.1016/j.otohns.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari A, Takeuchi K, Suzuki S, et al. Increased expression of matrix metalloproteinase-2 in nasal polyps. Acta Otolaryngol. 2004;124:1165–1170. doi: 10.1080/00016480410017152. [DOI] [PubMed] [Google Scholar]
- 15.Chen YS, Langhammer T, Westhofen M, Lorenzen J. Relationship between matrix metalloproteinases MMP-2, MMP-9, tissue inhibitor of matrix metalloproteinases 1 and IL-5, IL-8 in nasal polyps. Allergy. 2007;62:66–72. doi: 10.1111/j.1398-9995.2006.01255.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang LF, Chien CY, Tai CF, et al. Matrix metalloproteinase 9 gene polypmorphisms in nasal polyposis. BMC Medical Genetics. 2010;11:85–91. doi: 10.1186/1471-2350-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodach LL, Wiercinska E, de Miranda NF, et al. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134(5):1332–1341. doi: 10.1053/j.gastro.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 18.Turner N, Grose R. Fibroblast growth factor signaling: from development to cancer. Nature Reviews. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 19.Ishibashi T, Tanaka T, Nibu K, Ishimoto S, Kaga K. Keratinocyte growth factor and its receptor messenger RNA expression in nasal mucosa and nasal polyps. Ann Otol Rhinol Laryngol. 1998;107(10):885–890. doi: 10.1177/000348949810701013. [DOI] [PubMed] [Google Scholar]
- 20.Lindsay R, Slaughter T, Britton-Webb J, et al. Development of a murine model of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2006;134:724–730. doi: 10.1016/j.otohns.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 21.Khalid AN, Woodworth BA, Prince A, et al. Physiologic alerations in the murine model after nasal fungal antigenic exposure. Otolaryngol Head Neck Surg. 2008;139:695–701. doi: 10.1016/j.otohns.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Sautter NB, Delaney KL, Trune DR. Altered expression of tissue remodeling genes in a mouse model of acute allergic rhinitis. Int Forum Allergy Rhinol. doi: 10.1002/alr.20059. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryu J, Heldt GP, Nguyen M, Gavrialov O, Haddad GG. Chronic hypercapnia alters lung matrix composition in mouse pups. J Appl Physiol. 2010;109:203–210. doi: 10.1152/japplphysiol.00610.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watelet JB, Claeys C, Perez-Novo C, et al. Transforming growth factor β1 in nasal remodeling: Differences between chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2004;18(5):267–272. [PubMed] [Google Scholar]
- 25.Van Bruaene N, Derycke L, Perez-Novo, et al. TGF-β signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:253–259. doi: 10.1016/j.jaci.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Rosendahl A, Pardali E, Speletas M, et al. Activation of bone morphogenetic protein/smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol. 2002;27:160–169. doi: 10.1165/ajrcmb.27.2.4779. [DOI] [PubMed] [Google Scholar]
- 27.Tourdot S, Mathie S, Hussel T, et al. Respiratory syncytial virus infection provokes airway remodeling in allergy-exposed mice in absence of prior allergen sensitization. Clin Exp Allergy. 2008;38:1016–1024. doi: 10.1111/j.1365-2222.2008.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]