Abstract
OBJECTIVE
Relative adrenal insufficiency in extremely low birth weight infants may contribute to significant morbidity and death. Our objective was to evaluate the relationship between cortisol concentrations and short-term outcomes.
METHODS
Cortisol concentrations were obtained for 350 intubated, extremely low birth weight infants at postnatal age of 12 to 48 hours and at day 5 to 7, as part of a multicenter, randomized trial of hydrocortisone treatment for prophylaxis of relative adrenal insufficiency. Death and short-term morbidity were monitored prospectively. Cortisol levels at each time point were divided into quartiles. The incidence rates of outcomes were determined for each quartile and for infants with cortisol values of <10th percentile or >90th percentile.
RESULTS
Median cortisol values were 16.0 μg/dL at baseline and 13.1 μg/dL on day 5 to 7 in the placebo group. Outcomes did not differ in each quartile between treatment and placebo groups. Low cortisol values at baseline or day 5 to 7 were not associated with increased morbidity or mortality rates and were not predictive of open-label hydrocortisone use. In fact, vasopressor use was lower for infants with lower cortisol values at baseline. Severe intraventricular hemorrhage was more frequent in infants with cortisol levels in the upper quartile at baseline, and values of >90th percentile were significantly associated with higher rates of death, severe intraventricular hemorrhage, periventricular leukomalacia, gastrointestinal perforation, and severe retinopathy of prematurity.
CONCLUSIONS
Low cortisol concentrations were not predictive of adverse short-term outcomes, but high cortisol concentrations were associated with severe intraventricular hemorrhage, and extremely elevated values were associated with morbidity and death. Low cortisol concentrations alone at these 2 time points did not identify the infants at highest risk for adverse outcomes. In contrast, high cortisol values were associated with increased morbidity and mortality rates.
Keywords: bronchopulmonary dysplasia, extremely preterm infants, hydrocortisone, outcomes of high-risk infants
Extremely low birth weight (ELBW) infants are at high risk for death and significant short-term and long-term morbidity. Many factors contribute to the vulnerability of these high-risk infants, including conditions associated with an inflammatory process, such as chorioamnionitis, sepsis, and bronchopulmonary dysplasia (BPD).1,2 The inability of immature infants to respond appropriately to an inflammatory insult likely plays a critical role in this process.1-8 The contribution of relative adrenal insufficiency in modulating the inflammatory response in this high-risk population has been the focus of attention.7-11
Relative adrenal insufficiency occurs during an acute illness when the patient fails to mount an adequate cortisol response. Symptoms in adults and children include hypotension and electrolyte abnormalities, symptoms commonly seen in ELBW infants.12-14 Some studies have reported that low basal cortisol values and/or lower responses to corticotropin are associated with the subsequent development of BPD.1-8,10 Despite this, a large randomized trial of the prophylactic treatment of ELBW infants in the first 2 weeks of life with physiologic replacement doses of hydrocortisone did not reduce mortality or BPD rates, although treatment did reduce those outcomes for infants exposed to chorioamnionitis.9 In that study, treatment with hydrocortisone, in combination with early indomethacin therapy, was associated with an increased incidence of spontaneous gastrointestinal perforation.
The literature is conflicting on the meaning of cortisol concentrations in ELBW infants. Several studies showed that low cortisol values, as a marker of relative adrenal insufficiency, identify infants at highest risk for morbidities such as hypotension15-18 and BPD.1-11 However, when low cortisol values are used as a marker for adequate treatment of pain, lower values are associated with lower mortality and morbidity rates.19,20
Despite conflicting data, the use of hydrocortisone for treatment of symptoms associated with relative adrenal insufficiency, such as hypotension, is now common practice.21 Identifying infants at highest risk for adverse outcomes would allow the focus of therapy to be on infants who might benefit most from treatment. We performed a secondary analysis of data from the randomized trial of hydrocortisone treatment in ELBW infants.9 Our objective was to evaluate the relationship between cortisol concentrations and short-term outcomes. We were specifically interested in establishing clinically useful ranges for cortisol concentrations that could identify infants who were at highest risk for poor outcomes and hence could derive the most benefit from hydrocortisone treatment.
METHODS
Population and Procedures
Cortisol levels were determined for infants enrolled in the multicenter study of low-dose hydrocortisone therapy for prophylaxis of early adrenal insufficiency. Infants were eligible if their birth weight was 500 to 999 g and they required mechanical ventilation at study entry (12–48 hours). The study protocol was approved by institutional review boards at all participating institutions, and parental consent was obtained before enrollment. Infants were assigned randomly to receive normal saline placebo or hydrocortisone sodium succinate (1 mg/kg per day, divided into twice-daily doses, for 12 days, followed by 0.5 mg/kg per day for 3 days). The study population had a mean birth weight of 734 g and a mean gestational age of 25.3 weeks. Limiting enrollment to infants remaining intubated allowed identification of a sicker group of ELBW infants who were at greatest risk for adverse outcomes, particularly BPD, and who might derive the most benefit from hydrocortisone treatment. Full descriptions of the study population and details of the study protocol were reported previously.9
Baseline cortisol concentrations were determined for all infants at study entry, at postnatal age of 12 to 48 hours. Additional cortisol concentrations were determined on day 5 to 7. Values were available for 350 of the 360 ELBW infants enrolled in the randomized trial. To determine physiologic ranges, quartiles were determined at baseline by using 332 values, excluding infants who had received hydrocortisone before study entry. Baseline quartiles included infants in both the placebo and treatment groups, because infants had not yet received the randomly assigned treatment. Similarly, we evaluated outcomes on the basis of cortisol concentrations obtained on day 5 to 7, to determine whether later cortisol values could be used to identify high-risk infants. Cortisol concentrations at day 5 to 7 were available for 311 infants, 153 of whom were in the placebo group. We determined the quartiles for day 5 to 7 on the basis of only the placebo group, to avoid establishing falsely elevated quartiles because of hydrocortisone treatment effects.
Outcomes based on cortisol concentration quartiles were initially examined according to treatment groups, to evaluate the impact of treatment within the different quartiles. Outcomes did not differ according to random assignment of placebo or hydrocortisone treatment, showing the same trends and areas of significance; therefore, data were combined. Outcomes based on day 5 to 7 cortisol levels represent the full cohort, that is, both those who were treated with hydrocortisone and those who received placebo. A subgroup analysis was performed with the infants exposed to chorioamnionitis. The diagnosis of chorioamnionitis was based on review of placental pathologic features by 2 central readers. Interleukin 6 levels, measured at the time of cortisol sampling, were evaluated in relation to the cortisol concentration quartiles.
Statistical Analyses
The χ2 test (or Fisher’s exact test, when appropriate) was used for bivariate analyses of dichotomous outcomes. These analyses are summarized as frequencies and percentages within cortisol quartiles. Logistic regression was used for multivariate analysis of dichotomous outcomes, with adjustment for birth weight, gestational age, prenatal steroid exposure, hydrocortisone exposure before study entry, singleton versus multiple gestation, and vaginal versus cesarean section delivery. These analyses are summarized as adjusted odds ratios and corresponding 95% confidence intervals. Duration of vasopressor use was analyzed by using Poisson regression with bivariate analyses, summarized as means and SDs within cortisol quartiles; multivariate analyses are summarized as risk ratios. All hypothesis testing was 2-sided, with P values of <.05 being considered statistically significant. All analyses were conducted with SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
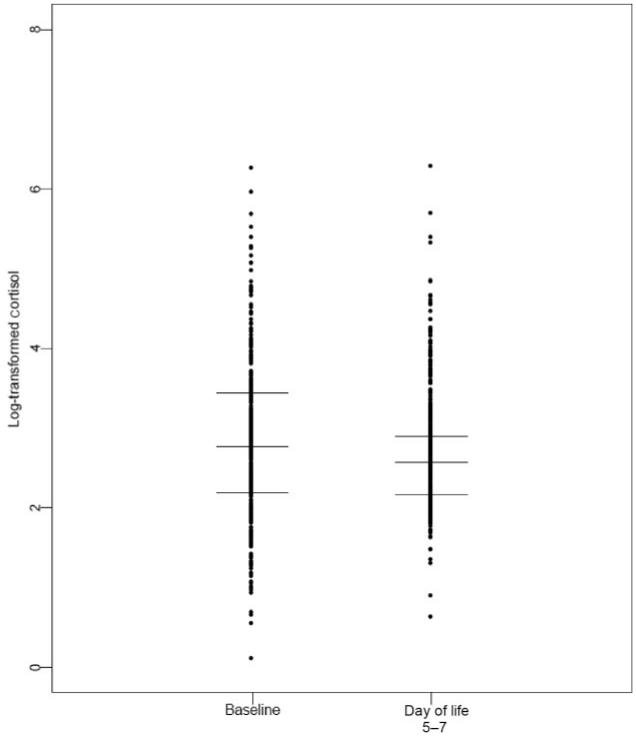
Cortisol concentrations varied widely, both at baseline (postnatal age of 12–48 hours) and at day 5 to 7, with and without treatment (Fig 1). Cortisol values at baseline were significantly higher than those at 5 to 7 days. As expected, infants receiving hydrocortisone had higher cortisol concentrations at 5 to 7 days than did those receiving placebo (Table 1).
FIGURE 1.
Scatter plot of logarithmically transformed cortisol values at baseline and at day 5 to 7, with lines demarcating the median and upper and lower quartiles at each time point.
TABLE 1. Cortisol Concentration Quartiles.
| N | Cortisol Concentration, μg/dL |
|||
|---|---|---|---|---|
| Lower Quartile |
Median | Upper Quartile |
||
| Baseline (12–48 h) | 332 | 8.9 | 16.0 | 31.0 |
| Day 5–7, placebo | 153 | 8.7 | 13.1 | 18.1 |
| Day 5–7, hydrocortisone | 158 | 12.2 | 18.4 | 40.1 |
Baseline cortisol concentrations in the lower quartile were not associated with increased mortality or morbidity rates (Table 2). This held true for both hydrocortisone-treated and placebo-treated infants; therefore, the presented results combine the 2 groups. There was no difference between quartiles in outcomes such as BPD, infection, any intraventricular hemorrhage (IVH), or severe retinopathy of prematurity. There was an increased incidence of severe (grade 3 or 4) IVH among infants whose cortisol concentrations were in the upper quartile at baseline. Mortality rates trended higher with increasing quartiles, although results did not reach statistical significance. Baseline cortisol levels were not predictive of open-label hydrocortisone use. In fact, vasopressor use was lower in the lowest and highest quartiles, compared with the middle quartiles.
TABLE 2. Outcomes According to Cortisol Quartiles at Baseline (12–48 Hours).
| N | Lower Quartile |
Middle Quartiles |
Upper Quartile |
P | Odds Ratio (95% Confidence Interval) |
||
|---|---|---|---|---|---|---|---|
| Lower Quartile vs Middle Quartiles |
Upper Quartile vs Middle Quartiles |
||||||
| Outcomes | |||||||
| Death before postmenstrual age of 36 wk, n/N (%) |
332 | 8/83 (10) | 21/166 (13) | 19/83 (23) | .15 | 1.18 (0.46–3.02) | 2.08 (0.99–4.37) |
| BPD, n/N (%) | 281 | 43/74 (58) | 83/144 (58) | 39/63 (62) | .65 | 0.94 (0.50–1.77) | 1.31 (0.69–2.49) |
| Any IVH, n/N (%) | 320 | 20/82 (24) | 58/159 (36) | 39/79 (49) | .06 | 0.78 (0.41–1.48) | 1.73 (0.98–3.06) |
| Severe IVH, n/N (%) | 320 | 9/82 (11) | 19/159 (12) | 26/79 (33) | .001 | 1.80 (0.71–4.60) | 3.96 (1.91–8.21) |
| PVL, n/N (%) | 214 | 1/60 (2) | 8/110 (7) | 5/44 (11) | .12 | 0.18 (0.02–1.55) | 1.92 (0.55–6.65) |
| Any gastrointestinal perforation, n/N (%) | 332 | 2/83 (2) | 15/166 (9) | 12/83 (14) | .09 | 0.33 (0.07–1.55) | 1.78 (0.76–4.17) |
| Spontaneous gastrointestinal perforation, n/N (%) |
332 | 2/83 (2) | 7/166 (4) | 10/83 (12) | .11 | 0.93 (0.17–4.98) | 2.84 (0.99–8.15) |
| Nosocomial infection, n/N (%) | 332 | 35/83 (42) | 68/166 (41) | 38/83 (46) | .84 | 1.15 (0.65–2.04) | 1.14 (0.66–1.96) |
| Severe ROP, n/N (%) | 282 | 14/74 (19) | 47/145 (32) | 21/63 (33) | .53 | 0.69 (0.32–1.46) | 1.09 (0.55–2.16) |
| Treatments | |||||||
| Open-label hydrocortisone use during treatment, n/N (%) |
332 | 15/83 (18) | 49/166 (30) | 16/83 (19) | .17 | 0.68 (0.34–1.37) | 0.55 (0.28–1.07) |
| Vasopressor use during first 28 d, n/N (%) |
332 | 39/83 (47) | 127/166 (77) | 55/83 (66) | .004 | 0.37 (0.20–0.69) | 0.51 (0.28–0.96) |
| Duration of vasopressor use, median (interquartile range), d |
332 | 0 (0–4) | 4 (1–7) | 2 (0–6) | .21 | NA | NA |
N indicates the number of observations. P values refer to differences between all groups. The denominator for IVH is based on the number of infants with head ultrasound scans before death, the denominator for periventricular leukomalacia is based on the number of infants with head sonograms at 4 to 6 weeks of age, the denominator for BPD is based on survivors at postmenstrual age of 36 weeks, and the denominator for severe (stage 3 and above) retinopathy of prematurity is based on the number of infants diagnosed before death. Severe IVH includes grades 3 and 4. Any gastrointestinal perforation includes all causes. Spontaneous gastrointestinal perforation is not associated with necrotizing enterocolitis. PVL indicates periventricular leukomalacia; ROP, retinopathy of prematurity; NA, not applicable.
Death and short-term morbidity could not be predicted by low cortisol concentrations at day 5 to 7 (Table 3). Again, mortality rates trended higher with increasing quartiles, but results did not reach statistical significance. Rates of both any IVH and severe IVH were higher for infants with cortisol concentrations in the highest quartile on day 5 to 7.
TABLE 3. Outcomes According to Cortisol Quartiles at Day 5 to 7, Using Day 5 to 7 Placebo Cutoff Points.
| N | Lower Quartile |
Middle Quartiles |
Upper Quartile |
P | Odds Ratio (95% Confidence Interval) |
||
|---|---|---|---|---|---|---|---|
| Lower Quartile vs Middle Quartiles |
Upper Quartile vs Middle Quartiles |
||||||
| Outcomes | |||||||
| Death by postmenstrual age of 36 wk, n/N (%) |
311 | 1/56 (2) | 14/134 (10) | 17/121 (14) | .19 | 0.17 (0.02–1.38) | 1.18 (0.54–2.59) |
| BPD, n/N (%) | 278 | 33/55 (60) | 65/119 (55) | 70/104 (67) | .21 | 1.35 (0.68–2.67) | 1.67 (0.94–2.98) |
| Any IVH, n/N (%) | 304 | 19/55 (35) | 35/131 (27) | 54/118 (46) | .02 | 1.56 (0.76–3.20) | 2.23 (1.27–3.90) |
| Severe IVH, n/N (%) | 304 | 9/55 (16) | 10/131 (8) | 28/118 (24) | .007 | 2.49 (0.91–6.82) | 3.63 (1.63–8.12) |
| PVL, n/N (%) | 210 | 2/47 (4) | 5/87 (6) | 10/76 (13) | .21 | 0.65 (0.11–3.66) | 2.18 (0.69–6.96) |
| Any gastrointestinal perforation, n/N (%) | 311 | 3/56 (5) | 10/134 (7) | 16/121 (13) | .37 | 0.78 (0.20–3.03) | 1.64 (0.69–3.88) |
| Spontaneous gastrointestinal perforation, n/N (%) |
311 | 0/56 (0) | 5/134 (4) | 13/121 (11) | .28 | Not estimable | 2.47 (0.82–7.47) |
| Nosocomial infection, n/N (%) | 311 | 21/56 (38) | 63/134 (47) | 57/121 (47) | .60 | 0.72 (0.37–1.40) | 0.86 (0.52–1.43) |
| Severe ROP, n/N (%) | 278 | 15/54 (28) | 38/120 (32) | 30/104 (29) | .59 | 0.88 (0.40–1.94) | 0.72 (0.39–1.34) |
| Treatments | |||||||
| Open-label hydrocortisone use during treatment, n/N (%) |
311 | 18/56 (32) | 32/134 (24) | 23/121 (19) | .02 | 2.01 (0.94–4.31) | 0.62 (0.33–1.18) |
| Vasopressor use during first 28 d, n/N (%) |
311 | 30/56 (54) | 96/134 (72) | 80/121 (66) | .09 | 0.50 (0.25–1.02) | 0.59 (0.33–1.05) |
| Duration of vasopressor use, median (interquartile range), d |
311 | 1 (0–7) | 3 (0–7) | 3 (0–7) | .54 | NA | NA |
N indicates the number of observations. P values refer to differences between all groups. The denominator for IVH is based on the number of infants with head ultrasound scans before death, the denominator for periventricular leukomalacia is based on the number of infants with head sonograms at 4 to 6 weeks of age, the denominator for BPD is based on survivors at postmenstrual age of 36 weeks, and the denominator for severe (stage 3 and above) retinopathy of prematurity is based on the number of infants diagnosed before death. Severe IVH includes grades 3 and 4. Any gastrointestinal perforation includes all causes. Spontaneous gastrointestinal perforation is not associated with necrotizing enterocolitis. PVL indicates periventricular leukomalacia; ROP, retinopathy of prematurity; NA, not applicable.
Logistic regression analyses indicated that only gestational age was independently associated with increased mortality rates, open-label hydrocortisone use, and vasopressor use at both time points. Vasopressor use did not differ between placebo and treatment groups.
To clarify the relationship of true outliers with adverse outcomes, we compared infants with cortisol values of <10th percentile (<5.2 μg/dL) or >90th percentile (>62.8 μg/dL) with infants whose cortisol concentrations were in the middle quartiles (Table 4). Cortisol values of >90th percentile were associated significantly with higher rates of death, severe IVH, periventricular leukomalacia, gastrointestinal perforation, and severe retinopathy of prematurity. Extremely low cortisol concentrations were not predictive of adverse outcomes and were associated with lower rates of vasopressor use.
TABLE 4. Outcomes According to Cortisol Concentrations at Baseline (12–48 Hours) in the 10th and 90th Percentiles.
| N | 10th Percentile (Cortisol Level of <5.2 μg/dL) |
Middle Quartiles | 90th Percentile (Cortisol Level of >62.8 μg/dL) |
P | Odds Ratio (95% Confidence Interval) |
||
|---|---|---|---|---|---|---|---|
| 10th Percentile vs Middle Quartiles |
90th Percentile vs Middle Quartiles |
||||||
| Outcomes | |||||||
| Death by postmenstrual age of 36 wk, n/N (%) |
233 | 4/33 (12) | 21/166 (13) | 10/34 (29) | .04 | 0.95 (0.30–2.98) | 2.88 (1.21–6.85) |
| BPD, n/N (%) | 197 | 14/29 (48) | 83/144 (58) | 16/24 (67) | .40 | 0.69 (0.31–1.53) | 1.47 (0.59–3.65) |
| Any IVH, n/N (%) | 223 | 7/33 (21) | 58/159 (36) | 15/31 (48) | .07 | 0.47 (0.19–1.15) | 1.63 (0.75–3.54) |
| Severe IVH, n/N (%) | 223 | 3/33 (9) | 19/159 (12) | 10/31 (32) | .02 | 0.74 (0.21–2.65) | 3.51 (1.44–8.57) |
| PVL, n/N (%) | 150 | 0/22 (0) | 8/110 (7) | 5/18 (28) | .01 | Not estimable | 4.90 (1.39–17.25) |
| Any gastrointestinal perforation, n/N (%) | 233 | 1/33 (3) | 15/166 (9) | 8/34 (24) | .02 | 0.32 (0.04–2.47) | 3.10 (1.19–8.04) |
| Spontaneous gastrointestinal perforation, n/N (%) |
233 | 1/33 (3) | 7/166 (4) | 6/34 (18) | .02 | 0.71 (0.08–5.97) | 4.87 (1.52–15.56) |
| Nosocomial infection, n/N (%) | 233 | 10/33 (30) | 68/166 (41) | 17/34 (50) | .26 | 0.63 (0.28–1.40) | 1.44 (0.69–3.02) |
| Severe ROP, n/N (%) | 198 | 3/29 (10) | 47/145 (32) | 11/24 (46) | .01 | 0.24 (0.07–0.84) | 1.76 (0.74–4.23) |
| Treatment | |||||||
| Open-label hydrocortisone use during treatment, n/N (%) |
233 | 5/33 (15) | 49/166 (30) | 7/34 (21) | .17 | 0.43 (0.16–1.17) | 0.62 (0.25–1.52) |
| Vasopressor use in first 28 d, n/N (%) | 233 | 15/33 (45) | 127/166 (77) | 24/34 (71) | .002 | 0.26 (0.12–0.56) | 0.74 (0.32–1.67) |
| Duration of vasopressor use, median (interquartile range), d |
233 | 0 (0–3) | 4 (1–7) | 2 (0–6) | .002 | NA | NA |
N indicates the number of observations. P values refer to differences between all groups. The denominator for IVH is based on the number of infants with head ultrasound scans before death, the denominator for periventricular leukomalacia is based on the number of infants with head sonograms at 4 to 6 weeks of age, the denominator for BPD is based on survivors at postmenstrual age of 36 weeks, and the denominator for severe (stage 3 and above) retinopathy of prematurity is based on the number of infants diagnosed before death. Severe IVH includes grades 3 and 4. Any gastrointestinal perforation includes all causes. Spontaneous gastrointestinal perforation is not associated with necrotizing enterocolitis. PVL indicates periventricular leukomalacia; ROP, retinopathy of prematurity; NA, not applicable.
There was significant variation in cortisol levels between baseline and day 5 to 7, with changes not being predictable according to treatment. Only 20 infants who had baseline cortisol concentrations in the lower quartile remained in the lower quartile at day 5 to 7, although 5 received hydrocortisone treatment, and 42 infants had both baseline and day 5 to 7 cortisol concentrations in the upper quartile. All other infants’ values crossed quartiles or remained in the middle quartiles. When the infants who remained in the extreme quartiles (lower or upper quartiles) were examined, there was a trend toward increased mortality, BPD, severe IVH, periventricular leukomalacia, and gastrointestinal perforation rates in the infants who had persistently elevated cortisol levels. There were no differences in vasopressor use or open-label hydrocortisone use.
The analysis described above was repeated with a subgroup of infants diagnosed as having chorioamnionitis. There were no differences in outcomes between quartiles for either baseline or day 5 to 7 cortisol concentrations, with trends similar to those seen for the full cohort. In addition, interleukin 6 levels did not correlate with cortisol concentration quartiles.
DISCUSSION
Despite the evidence in the literature to support the concept of relative adrenal insufficiency in ELBW infants, neither cortisol concentrations in the first 48 hours of postnatal life nor those on day 5 to 7 were helpful in identifying infants, in this large cohort of sick intubated infants, with clinical symptoms of relative adrenal insufficiency who might benefit most from treatment with hydrocortisone. Determining the significance of low cortisol concentrations in ELBW infants is challenging. Cortisol concentrations in utero are low and increase slowly throughout gestation.22 Serial cortisol levels in healthy preterm infants can remain quite low (mean: 4.5 μg/dL) without evidence of adverse consequences or symptoms of relative adrenal insufficiency.23 However, several studies have associated low cortisol levels in sick infants with adverse outcomes, such as the need for surfactant administration,1,3 vasopressor use,16,17 BPD,3-5,24 and death.6 A pilot study of prophylaxis of relative adrenal insufficiency in ELBW infants showed improved rates of survival without BPD after treatment with hydrocortisone.8 Another randomized study looking at early treatment of relative adrenal insufficiency with hydrocortisone was halted after enrollment of only 51 infants because of an increase in gastrointestinal perforations.10 The authors concluded that infants with basal cortisol concentrations below the median had decreased rates of BPD or death when treated with hydrocortisone (2 of 10 infants vs 7 of 10 infants), but this finding should be viewed with caution because of the small sample size.
It has been well established that infants with hypotension that is resistant to vasopressor therapy, a common symptom of relative adrenal insufficiency, respond to treatment with hydrocortisone.15,17 Several investigations have supported a role for decreased cortisol production in the hypotension seen in extremely premature infants. Ng et al16 showed that cortisol concentrations on day 7 of life were correlated inversely with the maximal dose of vasopressor therapy in the first 2 weeks of life and the duration of its use. Arnold et al25 found that cortisol production was correlated significantly with blood pressure. In addition, in an animal model, Yoder et al26 found that extremely premature infant baboons with decreased cortisol production showed cardiac dysfunction, which was responsive to hydrocortisone therapy.
These results are in contrast to our large trial, in which there was no correlation with adverse outcomes for infants with cortisol concentrations in the lowest quartile, either at baseline or at 5 to 7 days. In fact, correlations between cortisol concentrations and the use or duration of vasopressor therapy were the opposite of anticipated, with less vasopressor use for infants in the lowest quartile. Infants in the lower quartile at baseline did not show improvement in outcomes; the incidence of adverse outcomes in the treated group was not different from that in the placebo group for any quartile group. Although relative adrenal insufficiency may contribute to morbidity and death in this vulnerable population, additional factors and variability in cortisol concentrations make it difficult to identify the infants who may benefit most from treatment.
Much of the focus of evaluating cortisol concentrations in ELBW infants has been on establishing inappropriately low values. Many factors, in addition to the acuity of illness, may affect the stress response. For example, cortisol concentrations have been used as a proxy for evaluation of adequate pain treatment, with high concentrations suggesting extreme stress associated with pain. Cortisol values decrease in response to treatment of operative pain, and these decreased values are associated with decreases in morbidity and mortality rates.19,20 Therefore, we chose to evaluate the outcomes of the infants with high concentrations. Cortisol levels in the upper quartile, at both baseline and day 5 to 7, regardless of treatment group, were associated with increased rates of adverse outcomes, particularly severe IVH. A study examining urinary cortisol production rates in preterm infants found a wide variation, with no difference between ill and well preterm infants.27 There was, however, a significant correlation of elevated cortisol production rates with increased incidence of grade 3 to 4 IVH, consistent with our findings. Similarly, Korte et al11 found that 44% of very low birth weight infants with cortisol concentrations of >15 μg/dL had grade 3 to 4 IVH, compared with 14% of infants with cortisol concentrations of <15 μg/dL. It is not clear from our study whether elevated cortisol concentrations precede the infants’ complications, as a marker of a vulnerable population, or whether the elevated value is a result of the complications, particularly severe IVH. The timing of early head ultrasound scans was not coordinated with the timing of cortisol concentration determinations, because this was not part of the original study design.
Increases in adverse outcomes with high cortisol levels were also noted in a trial of prophylactic treatment with thyroid supplementation, in which both death and BPD were more common in infants with either the highest or lowest cortisol concentrations.28 To evaluate whether these increases in adverse outcomes and elevated cortisol concentrations were related to exposure to inflammation, we performed a subgroup analysis of infants exposed to chorioamnionitis. No differences in outcomes according to cortisol levels were found in this group. In addition, an evaluation of interleukin 6 levels determined at the same time as the cortisol levels did not indicate a correlation with cortisol concentrations. To determine whether higher cortisol concentrations acted as a proxy for severity of illness, a subset of sicker infants was defined as infants who continued to require assisted ventilation at day 5 to 7, with the fraction of inspired oxygen times mean airway pressure above the median for the group. These sicker infants showed a similar distribution across the cortisol quartile concentrations and demonstrated similar trends of increased rates of adverse outcomes with increasing cortisol quartile.
High stress values of cortisol may represent a marker for a population that is more vulnerable to adverse out-comes. Having high cortisol concentrations did not decrease the need for or length of vasopressor treatment or the need for open-label hydrocortisone treatment, irrespective of treatment group. Indeed, early gestational age was the only predictor of the need for these therapies. Hypotension is well known to be multifactorial in origin, with relative adrenal insufficiency being only one possible cause. The response to hydrocortisone treatment of hypotension in this population may not represent treatment of relative adrenal insufficiency but may be a reflection of the steroid’s ability to cause an increase in blood pressure.15,17,18 Use of hydrocortisone for treatment of hypotension may be necessary in this population but does not necessarily imply the diagnosis of relative adrenal insufficiency. Corticosteroids affect cardiovascular stability through numerous mechanisms, such as inhibiting catecholamine metabolism and increasing angiotensin type 2 receptors in the myocardium.15 When hydrocortisone was given to neonates requiring high-dose dopamine treatment, the increase in blood pressure was evidenced by an increase in systemic vascular resistance without changes in stroke volume initially, with later improvement in stroke volume.18
Cortisol concentrations obtained in this study represent total serum cortisol, which is the sum of both bound and free cortisol. Free cortisol is the biologically active proportion of the hormone, which can vary from 6% to 20% of total cortisol. Variables such as hypoproteinemia, resulting in lower levels of cortisol-binding protein, can affect the relationship of the total cortisol measured to the biologically active free cortisol. This variability may contribute to the difficulty in establishing clinically relevant cortisol concentrations for sick, intubated, ELBW infants.
CONCLUSIONS
We found that low cortisol concentrations in ELBW infants at 12 to 48 hours and day 5 to 7 of postnatal life did not identify the infants at highest risk for adverse outcomes. Hypotension, a prominent symptom of relative adrenal insufficiency, was most associated with decreasing gestational age, rather than cortisol values. In contrast, high cortisol concentrations were associated with severe IVH. In addition, infants with values of >90th percentile had increased mortality, gastrointestinal perforation, and periventricular leukomalacia rates. Although some infants with adverse outcomes may have low cortisol concentrations, this marker alone is not adequate to identify the population of infants who proceed to develop BPD. Conversely, an elevated cortisol level may serve as a marker for a population of ELBW infants most vulnerable to adverse outcomes. Further evaluation is needed to correlate the timing of the elevated values with the adverse events, particularly severe IVH, to clarify this relationship.
What’s Known on This Subject.
Hydrocortisone is used to treat both hypotension and relative adrenal insufficiency in ELBW infants and has been associated with gastric perforations. Defining normal cortisol concentrations in this population is challenging, with conflicting information in the literature.
What This Study Adds.
We address the clinical usefulness of cortisol concentrations in the first 48 hours of life and on day 5 to 7 in identifying infants at high risk for adverse outcomes, who may benefit most from treatment with hydrocortisone.
ACKNOWLEDGMENTS
The PROPHET (Prophylaxis of Early Adrenal Insufficiency in Tiny Babies) Study Group principal investigators and sites were as follows: Kristi L. Watterberg, MD, University of New Mexico; Jeffrey S. Gerdes, MD, University of Pennsylvania; Cynthia H. Cole, MD, MPH, Tufts University; Susan W. Aucott, MD, Johns Hopkins University; Elizabeth H. Thilo, MD, University of Colorado; Mark C. Mammel, MD, and Robert J. Couser, MD, Children’s Hospital and Clinics of Minnesota, University of Minnesota; Jeffery S. Garland, MD, SM, Wheaton Franciscan Health Care-St Joseph’s, Medical College of Wisconsin; Henry J. Rozycki, MD, Virginia Commonwealth University; Corinne L. Leach, MD, PhD, State University of New York, Buffalo; Conra Backstrom, RN, University of New Mexico; and Michele L. Shaffer, PhD, Pennsylvania State University.
We thank Dr Susan Scott for her critical review of and commentary on the manuscript.
Abbreviations
- ELBW
extremely low birth weight
- IVH
intraventricular hemorrhage
- BPD
bronchopulmonary dysplasia
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in babies who develop bronchopulmonary dysplasia. Pediatrics. 1996;97(2):210–215. [PubMed] [Google Scholar]
- 2.Watterberg KL, Scott SM, Naeye RL. Chorioamnionitis, cortisol and acute lung disease in very low birth weight infants. Pediatrics. 1997;99(2) doi: 10.1542/peds.99.2.e6. Available at: www.pediatrics.org/cgi/content/full/99/2/e6. [DOI] [PubMed] [Google Scholar]
- 3.Scott SM, Watterberg KL. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr Res. 1995;37(1):112–116. doi: 10.1203/00006450-199501000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Huysman MW, Hokken-Koelega AC, De Ridder MA, Sauer PJ. Adrenal function in sick very preterm infants. Pediatr Res. 2000;48(5):629–633. doi: 10.1203/00006450-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Watterberg KL, Gerdes JS, Cook KL. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr Res. 2001;50(2):190–195. doi: 10.1203/00006450-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Scott SM, Cimino DF. Evidence for developmental hypopituitarism in ill preterm infants. J Perinatol. 2004;24(7):429–434. doi: 10.1038/sj.jp.7211112. [DOI] [PubMed] [Google Scholar]
- 7.Watterberg KL, Scott SM. Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics. 1995;95(1):120–125. [PubMed] [Google Scholar]
- 8.Watterberg KL, Gerdes JS, Gifford KL, Lin H-M. Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics. 1999;104(6):1258–1263. doi: 10.1542/peds.104.6.1258. [DOI] [PubMed] [Google Scholar]
- 9.Watterberg KL, Gerdes JS, Cole CH, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. 2004;114(6):1649–1657. doi: 10.1542/peds.2004-1159. [DOI] [PubMed] [Google Scholar]
- 10.Peltoniemi O, Kari MA, Heinonen K, et al. Pretreatment cortisol values may predict responses to hydrocortisone administration for the prevention of bronchopulmonary dysplasia in high-risk infants. J Pediatr. 2005;146(5):632–637. doi: 10.1016/j.jpeds.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Korte C, Styne D, Merritt TA, et al. Adrenocortical function in the very low birth weight infant: improved testing sensitivity and association with neonatal outcome. J Pediatr. 1996;128(2):257–263. doi: 10.1016/s0022-3476(96)70404-3. [DOI] [PubMed] [Google Scholar]
- 12.Lambert SWJ, Bruining HA, de Jong FH. Corticosteroid therapy in severe illness. N Engl J Med. 1997;337(18):1285–1292. doi: 10.1056/NEJM199710303371807. [DOI] [PubMed] [Google Scholar]
- 13.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348(8):727–734. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 14.Joosten KFM, DeKleija ED, Westerterp M, et al. Endocrine and metabolic responses in children with meningococcal sepsis: striking differences between survivors and nonsurvivors. J Clin Endocrinol Metab. 2000;85(10):3746–3753. doi: 10.1210/jcem.85.10.6901. [DOI] [PubMed] [Google Scholar]
- 15.Seri I, Tan R, Evans J. Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics. 2001;107(5):1070–1074. doi: 10.1542/peds.107.5.1070. [DOI] [PubMed] [Google Scholar]
- 16.Ng PC, Lee CH, Lam CW, et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F119–F126. doi: 10.1136/adc.2002.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng PC, Lee CH, Bnur FL, et al. A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics. 2006;117(2):367–375. doi: 10.1542/peds.2005-0869. [DOI] [PubMed] [Google Scholar]
- 18.Noori S, Friedlich P, Wong P, et al. Hemodynamic changes after low-dosage hydrocortisone administration in vasopressortreated preterm and term infants. Pediatrics. 2006;118(4):1456–1466. doi: 10.1542/peds.2006-0661. [DOI] [PubMed] [Google Scholar]
- 19.Anand KJS, Sipwell WG, Anysley-Green A. Randomised trial of fentanyl anesthesia in preterm babies undergoing surgery. Lancet. 1987;1(8524):62–66. doi: 10.1016/s0140-6736(87)91907-6. [DOI] [PubMed] [Google Scholar]
- 20.Anand KJS, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326(1):1–9. doi: 10.1056/NEJM199201023260101. [DOI] [PubMed] [Google Scholar]
- 21.Finer NN, Powers RJ, Ou CH, et al. Prospective evaluation of postnatal steroid administration: a 1-year experience from the California Perinatal Quality Care Collaborative. Pediatrics. 2006;117(3):704–712. doi: 10.1542/peds.2005-0796. [DOI] [PubMed] [Google Scholar]
- 22.Bolt RJ, van Weissenbruch MM, Popp-Snijders C, et al. Fetal growth and the function of the adrenal cortex in preterm infants. J Clin Endocrinol Metab. 2002;87(3):1194–1199. doi: 10.1210/jcem.87.3.8295. [DOI] [PubMed] [Google Scholar]
- 23.al Saedi S, Dean H, Dent W, Cronin C. Reference ranges for serum cortisol and 17-hydroxyprogesterone levels in preterm infants. J Pediatr. 1995;126(6):985–987. doi: 10.1016/s0022-3476(95)70229-6. [DOI] [PubMed] [Google Scholar]
- 24.Banks BA, Stouffer N, Cnaan A, et al. Association of plasma cortisol and chronic lung disease in preterm infants. Pediatrics. 2001;107(3):494–498. doi: 10.1542/peds.107.3.494. [DOI] [PubMed] [Google Scholar]
- 25.Arnold JD, Bonacruz G, Garth LI, et al. Antenatal glucocorticoids modulate the amplitude of pulsatile cortisol secretion in premature neonates. Pediatr Res. 1998;44(6):876–881. doi: 10.1203/00006450-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Yoder B, Martin H, McCurnin DC, Coalson JJ. Impaired urinary cortisol excretion and early cardiopulmonary dysfunction in immature baboons. Pediatr Res. 2002;51(4):426–432. doi: 10.1203/00006450-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Heckmann M, Hartmann MF, Kampschulte B, et al. Cortisol production rates in preterm infants in relation to growth and illness: a noninvasive prospective study using gas chromatography-mass spectrometry. J Clin Endocrinol Metab. 2005;90(10):5737–5742. doi: 10.1210/jc.2005-0870. [DOI] [PubMed] [Google Scholar]
- 28.Biswas S, Buffery J, Enoch H, et al. Pulmonary effects of triiodothyronine (T3) and hydrocortisone supplementation in preterm infants less than 30 weeks gestation: results of the THORN trial: Thyroid Hormone Replacement in Neonates. Pediatr Res. 2003;53(1):48–56. doi: 10.1203/00006450-200301000-00011. [DOI] [PubMed] [Google Scholar]